Abstract
Background
This study was performed to evaluate neck circumference (NC) and metabolic syndrome (MS) parameters in severe and non-severe (mild-moderate) obstructive sleep apnea syndrome (OSAS) patients according to apnea-hypopnea index (AHI).
Material/Method
We enrolled 44 patients diagnosed with OSAS based on overnight polysomnography. The diagnosis of OSAS was based on AHI. Apnea is a pause of airflow for more than 10 seconds. and hypopnea is a decrease of airflow for more than 10 seconds and oxygen desaturation of 4% or greater. AHI score. per hour; below 5 normal. 5–29 mild-moderate. 30 and above were grouped as severe OSAS. Height. weight. neck circumference (NC). waist circumference (WC) and body mass index (BMI) of the patients were measured. MS was diagnosed by the Adult Treatment Panel (ATP) III criteria (≥3 of the following abnormalities): 1) WC ≥94 cm for males, ≥80 cm for females; 2) arterial blood pressure ≥130/85 mmHg; 3) fasting blood glucose ≥100 mg/dl; 4) high density lipoprotein (HDL) cholesterol <40 mg/dl in man, <50 mg/dl in women; 5) triglycerides ≥150 mg/dl.
Results
Mean BMI and NC were higher in severe OSAS patients compared to non-severe patients (p=0.021. p<0.001). According to ATP III criteria. 64% of severe and 61.1% of non-severe OSAS patients were MS (p=0.847). A logistic regression model displayed an association with NC as a risk factor for severe OSAS (p=0.01). but not with MS.
Conclusions
In this study. NC in severe OSAS patients was significantly higher than in non-severe OSAS patients. The prevalence of metabolic syndrome was not correlated with OSAS severity. NC is an independent risk factor for severe OSAS.
Keywords: neck circumference, metabolic syndrome, obstructive sleep apnea syndrome, apnea-hypopnea index
Background
Obstructive sleep apnea syndrome (OSAS) is a nocturnal disorder that occurs especially in men with metabolic syndrome (MS). characterized by increased cardiovascular risk [1.2]. Recurrent pause of airflow during sleep is the characteristic feature of OSAS. ultimately leading to increased respiratory effort. oxyhemoglobin desaturation. and excessive daytime sleepiness [3]. Patients with OSAS are often overweight and they frequently have a higher incidence of arterial hypertension. coronary heart disease. and cerebrovascular disease [4]. OSAS is a risk factor for systemic hypertension and there is a strict relation between OSAS and MS [5.6]. Several studies have found increased insulin resistance and impaired glucose tolerance in OSAS patients. independent of body weight [6–11]. Intermittent hypoxia and oxidative stress may play important roles in the pathogenesis of OSAS [12.13].
On physical examination very few features have been helpful in defining the risk for OSAS and the response to therapy. Several reports have emphasized that a thick neck or a large neck is an important variable [14]. However. neck circumference (NC) and body mass index (BMI) are highly correlated. whereas gain in waist circumference (WC) over adult life has a stronger association than neck size with sleep-disordered breathing severity [15–17]. The National Cholesterol Educational Program (NCEP). Adult Treatment Panel (ATP)-III proposed a clinically practical approach that establishes the diagnosis of MS when an individual has 3 of these 5 characteristics: increased WC. high blood pressure. increased fasting glucose. increased triglycerides. and decreased high=density lipoprotein (HDL) cholesterol [18].
The aim of the present study was to evaluate the MS in patients with severe and non-severe OSAS (AHI <30 non-severe. ≥30 severe OSAS) according to NCEP-ATP-III criteria [18].
Material and Methods
Sample population
This study enrolled of 44 patients (25 males. 19 females) at the Istanbul Yedikule Chest Diseases Training and Research Hospital Sleep Disorders Unit. Clinical and biochemical observations were performed with all patients. The study protocol was approved by the local ethics committee and informed consent was obtained from every patient. Subjects with heart. neurological. autoimmune. chronic renal and liver diseases were excluded.
Measurements
Blood pressure was measured twice with a mercury sphygmomanometer from the right arm of patients in a sitting position after 5 minutes of rest. and average value was calculated. Height (m). weight (kg). NC. and WC (cm) were measured. Weight was measured with light clothing and without shoes. BMI was calculated as weight (kg) divided by height (m2). NC was measured at the middle of the neck between the mid-cervical spine and superior line of the cricothyroid membrane. in a standing position. WC was measured between the lowest rib and the crista iliaca superior. MS was diagnosed according to the National Cholesterol Education Program. Adult Treatment Panel (ATP)-III criteria by presence of at least 3 of 5 criteria: (1) WC ≥94 cm for male. ≥80 cm for female; (2) arterial blood pressure ≥130/85 mmHg or presence of drug treatment for hypertension; (3) fasting blood glucose ≥100 mg/dl or drug treatment for hyperglycemia; (4) HDL Cholesterol <40 mg/dl in man. <50 mg/dl in women; (5) triglycerides ≥150 mg/dl or drug treatment for elevated triglyceride levels. Fasting blood glucose. urea. creatinine. hemoglobin A1c. insulin. high sensitive C-reactive protein (hsCRP). cholesterol levels (HDL. LDL. triglyceride. total cholesterol) by using Abbot Architech Analisator system (IL. USA). The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated with the following formula: fasting blood glucose (mmol/l) × [insulin (mU/l)/22.5] [19].
Polysomnography
An overnight polysomnography (Embla Flaga Inc.. Iceland) was performed for all patients at the sleep laboratory. and electroencephalography. electrooculography. oral-nasal airflow. pulse oximetry. chest and abdominal movement. body position. and snoring noise were recorded. Apnea is a pause of airflow more than 10 seconds. and hypopnea is a decrease of airflow more than 10 seconds and oxygen desaturation of 4% or greater. The apnea/hypopnea index (AHI) was measured to evaluate the severity of OSAS. AHI ≥30 was described as severe OSAS. and AHI <30 was described as non-severe (mild and moderate) OSAS. Patients were divided into 2 comparable groups as severe and non-severe OSAS.
Statistical analysis
Statistical analysis was carried out by using SPSS for Windows version 16.0. Results are expressed as mean ± standard deviation. Kolmogorov Smirnov Z test was performed to determine the distribution of variables for each patients group. Regular variances were assessed with t test and irregulars with Mann-Whitney U test. The Pearson and the Spearman tests were performed to analyze the correlation between variables. Logistic regression modeling was performed to identify relationship between severe OSAS; independent variables were metabolic parameters and NC. Chi square test was used to evaluate categorical variables. A P value <0.05 was statistically significant.
Results
The mean age. AHI. BMI. and WC of OSAS patients did not differ among women and men. There was a statistical significance between men and women patients in mean waist/hip ratio and NC (p=0.006 and p<0.001). Twenty-eight patients (63.6%) were hypertensive. and 7 patients (15.9%) were diabetic and under drug treatment. Mean total and LDL cholesterol. insulin. and hs CRP levels of OSAS patients were not different between women and men. Mean triglyceride level of male OSAS patients was significantly higher and HDL cholesterol was significantly lower than in female patients (p=0.007 and p=0.005). There were no differences in mean HOMA-IR and percentage of MS between sexes. The mean hemoglobin level of men was higher than in women (p<0.001).
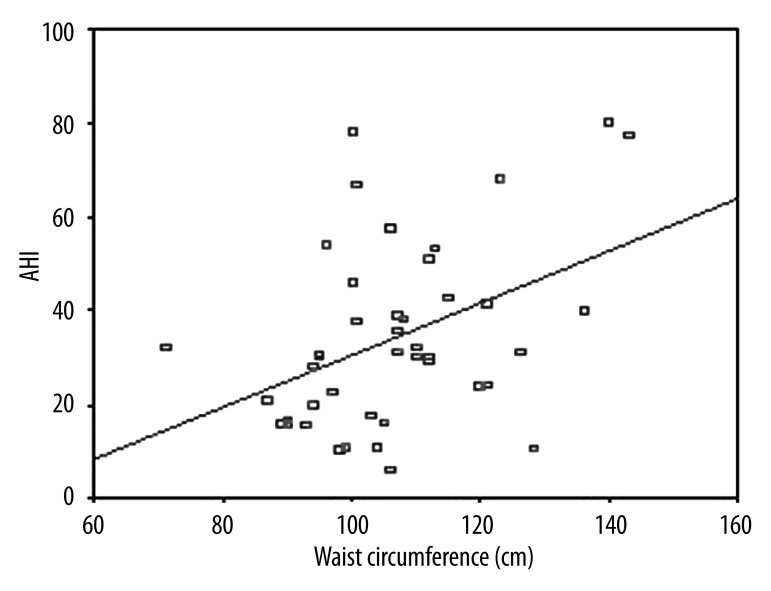
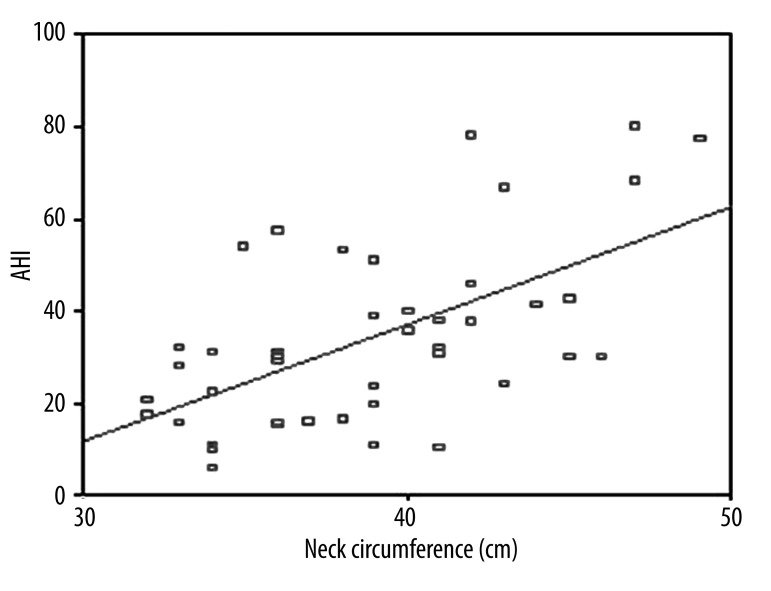
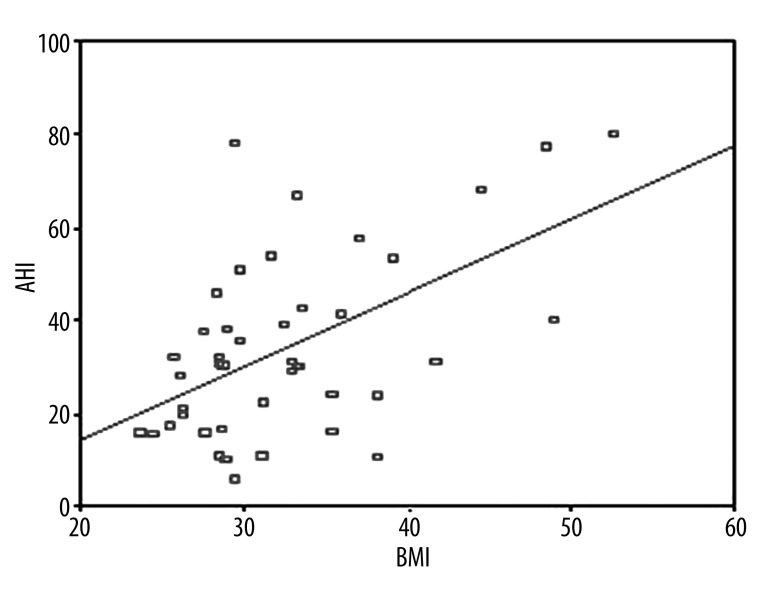
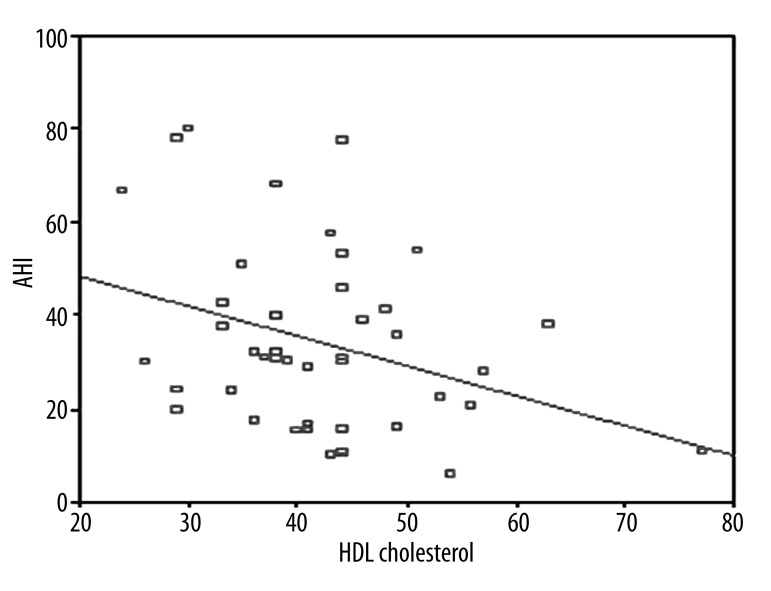
The clinical and laboratory findings of OSAS patients are given in Table 1. Twenty-five (56.8%) were severe and the remaining (19/44) were non-severe OSAS patients according to AHI criteria. Mean BMI and NC were higher in severe OSAS patients as compared to non-severe patients (p=0.021. p<0.001). NC was correlated with BMI and waist circumference (Figures 1 and 2). A logistic regression model was performed to identify the relationship between severe OSAS and independent variables as metabolic parameters and NC (Table 2). As an important result. NC was an independent risk factor for severe OSAS (odds ratio [OR]: 1.55. 95% confidence intervals [CI]: 1.09–2.21. p=0.01). MS parameters were not statistically different among OSAS patients. Mean age. WC. waist/hip ratio. systolic and diastolic blood pressure. triglycerides. cholesterol levels. hemoglobin A1c. and hs CRP values were not statistically significant in OSAS patients. We found that 68% of severe and 55.6% of non-severe OSAS patients were hypertensive. Twenty-four percent of severe and 5.6% of non-severe OSAS patients were diabetic. There were a positive correlation between AHI and BMI (r=0.545, p<0.01, Figure 3) and a negative correlation between AHI and HDL cholesterol (r=–0.333, p=0.02, Figure 4).
Table 1.
Mean clinical and laboratory findings of OSAS patients.
| Women (n=19) | Men (n=25) | Total (n=44) | p value | |
|---|---|---|---|---|
| Age (years) | 48.84±6.04 | 51.44±11.22 | 50.32±9.34 | 0.331 |
| AHI | 27.99±15.38 | 39.09±21.20 | 34.19±19.46 | 0.074 |
| BMI | 33.16±6.12 | 32.11±7.09 | 32.56±6.64 | 0.607 |
| Waist circumference(cm) | 104.47±15.73 | 108.56±13.60 | 106.80±14.53 | 0.361 |
| Waist/hip ratio | 0.94±0.08 | 1.00±0.05 | 0.97±0.07 | 0.006 |
| Neck circumference | 35.68±2.65 | 41.36±4.02 | 38.91±4.48 | <0.001 |
| Hypertension (%) | 63.2 | 64 | 63.6 | 0.954 |
| Diabetes mellitus (%) | 15.8 | 16 | 15.9 | 0.985 |
| Triglycerides (mg/dl) | 116.58±36.92 | 168.96±81.47 | 146.34±70.45 | 0.007 |
| Total cholesterol (mg/dl) | 201.95±28.19 | 213.17±36.74l | 208.32±33.43 | 0.276 |
| LDL cholesterol (mg/dl) | 133.00±24.56 | 140.84±30.99 | 137.45±28.35 | 0.370 |
| HDL cholesterol (mg/dl) | 46.58±10.00 | 38.29±8.64 | 41.87±10.04 | 0.005 |
| Hb A1c (%) | 5.56±0.38 | 6.00±0.68 | 5.81±0.61 | 0.009 |
| Insulin (IU/l) | 7.85±3.64 | 13.60±12.61 | 11.20±10.21 | 0.335 |
| hs CRP (mg/l) | 0.78±0.93 | 0.65±0.90 | 0.70±0.90 | 0.545 |
| Hemoglobin (gr/dl) | 12.95±0.72 | 14.06±0.97 | 13.59±1.02 | <0.001 |
| HOMA-IR | 1.91±0.95 | 3.43±3.37 | 2.80±2.72 | 0.242 |
| MS (%) | 68.4 | 60 | 63.6 | 0.565 |
AHI – Apnoea-hypopnoea index; hs CRP – high sensitive C-reactive protein; HOMA-IR – homeostasis model assessment for insulin resistance; MS – Metabolic Syndrome.
Figure 1.
Correlation between AHI and waist circumference (r=0.421. p=0.005).
Figure 2.
Correlation between AHI and neck circumference (r=0.592. p<0.01).
Table 2.
A logistic regression model for the associations of metabolic syndrome (MS) components with neck circumference (NC) in the presence of severe OSAS.
| VARIABLES | OR | 95% CI | P VALUE |
|---|---|---|---|
| Age (year) | 1.05 | 0.95–1.16 | 0.37 |
| Gender (male / female) | 1.86 | 0.12–28.02 | 0.66 |
| Waist circumference (cm) | 0.66 | 0.08–5.29 | 0.69 |
| Neck circumference (cm) | 1.55 | 1.09–2.21 | 0.01 |
| Fasting triglyceride (≥150 mg/dl) | 0.33 | 0.05–2.33 | 0.26 |
| Fasting blood glucose (mg/dl) | 0.28 | 0.04–2.08 | 0.21 |
| Hypertension (≥130/85 mmHg) | 1.45 | 0.25–8.33 | 0.67 |
| HDL cholesterol (mg/dl) | 2.31 | 0.36–2.34 | 0.38 |
| Metabolic syndrome (%) | 0.31 | 0.07–2.21 | 0.30 |
OSAS – obstructive sleep apnoea syndrome; HDL – high density lipoprotein; OR – odds ratio; 95% CI – 95% confidence interval; Significant value indicated in bold.
Figure 3.
Correlation between AHI and BMI (r=0.545. p<0.01).
Figure 4.
Correlation between AHI and HDL cholesterol (r=–0.333 p=0.02).
Mean HOMA-IR was not statistically significant between severe and non-severe OSAS patients. Sixty-four percent of severe and 61.1% of non-severe patients were MS according to ATP III criteria. but this difference was not statistically significant at α=0.05. The comparisons of OSAS patients according to AHI are given in Table 3. Comparison of OSAS patients for the presence of MS are indicated in Table 4. Mean BMI (p=0.042). WC (p=0.026). systolic blood pressure (p=0.023). hypertension (p=0.001). fasting blood glucose (p=0.001). and HbA1c levels were significantly higher in the patients with MS. There were no statistically significant differences at α=0.05 (p=0.84) between patients with and without MS. mean age. diastolic blood pressure. triglycerides. cholesterol. and hs CRP values.
Table 3.
Comparisons of OSAS patients’ mean results according to AHI scores.
| Non-severe OSAS AHI <30 (n=19) | Severe OSAS AHI ≥30 (n=25) | P value | |
|---|---|---|---|
| Age (years) | 49.89±7.47 | 51.16±10.42 | 0.661 |
| BMI | 29.83±4.53 | 34.55±7.39 | 0.021 |
| Waist circumference(cm) | 101.67±11.96 | 110.20±15.58 | 0.059 |
| Waist/hip ratio | 0.96±0.07 | 0.98±0.07 | 0.06 |
| Neck circumference (cm) | 36.11±3.18 | 40.84±4.34 | <0.001 |
| Systolic blood pressure (mm/Hg) | 126.67±17.82 | 134.80±23.47 | 0.224 |
| Diastolic blood pressure (mm/Hg) | 79.44±9.37 | 84.80±12.62 | 0.136 |
| Hypertension (%) | 55.6 | 68 | 0.405 |
| Diabetes Mellitus (%) | 5.6 | 24 | 0.106 |
| Triglycerides (mg/dl) | 142.00±56.04 | 143.60±75.84 | 0.940 |
| Total cholesterol (mg/dl) | 209.17±23.69 | 205.80±38.77 | 0.726 |
| LDL cholesterol (mg/dl) | 136.72±22.13 | 137.04±32.70 | 0.972 |
| HDL cholesterol (mg/dl) | 45.11±11.49 | 39.76±8.55 | 0.087 |
| Hb A1c (%) | 5.69±0.51 | 5.95±0.63 | 0.169 |
| hs CRP (mg/l) | 0.50±0.49 | 0.86±1.09 | 0.238 |
| HOMA-IR | 3.82±4.32 | 2.40±1.80 | 0.340 |
| MS (%) | 61.1 | 64 | 0.847 |
AHI – Apnoea-hypopnoea index; hs CRP – high sensitive C-reactive protein; HOMA-IR – homeostasis model assessment for insulin resistance; MS – Metabolic Syndrome.
Table 4.
Comparisons of OSAS patient’s mean results for the presence of MS.
| Patients with MS (n=23) | Patients without MS (n=21) | P value | |
|---|---|---|---|
| Age (years) | 51.61±8.78 | 48.06±10.14 | 0.230 |
| AHI | 35.08±19.45 | 32.68±20.01 | 0.701 |
| BMI | 34.09±7.22 | 29.89±4.53 | 0.042 |
| Waist circumference (cm) | 110.43±14.05 | 100.44±13.48 | 0.026 |
| Systolic blood pressure (mmHg) | 136.79±23.10 | 121.88±13.28 | 0.023 |
| Diastolic blood pressure (mmHg) | 83.57±12.54 | 81.25±9.57 | 0.525 |
| Hypertensive (%) | 81.5 | 31.3 | 0.001 |
| Diabetic (%) | 22.2 | 6.3 | 0.170 |
| Fasting blood glucose (mg/dl) | 102.57±11.44 | 90.00±9.23 | 0.001 |
| Triglycerides (mg/dl) | 155.39±68.57 | 130.50±73.11 | 0.264 |
| Total cholesterol (mg/dl) | 209.82±37.86 | 205.69±24.74 | 0.664 |
| LDL cholesterol (mg/dl) | 138.43±31.17 | 135.75±23.46 | 0.767 |
| HDL cholesterol (mg/dl) | 40.89±10.16 | 43.56±9.90 | 0.403 |
| HbA1c (%) | 5.98±0.63 | 5.53±0.45 | 0.017 |
| hs CRP (mg/l) | 0.76±0.91 | 0.62±0.92 | 0.643 |
AHI – Apnoea-hypopnoea index; hs CRP – high sensitive C-reactive protein; MS – Metabolic Syndrome.
Discussion
Some recent studies analyzed the associations of OSAS patients with obesity and MS components. There is a relationship between MS and OSAS [20]. Prevalence of OSAS increases with age and it is most prominent in middle-aged people [21]. Tufik et al. identified AHI ≥15 and BMI as independent and strong associated factors for the presence of OSAS among overweight and obese subjects of both sexes [22]. This study was performed to assess NC. BMI and MS in severe and non-severe (mild-moderate) OSAS patients according to AHI (AHI <30 non-severe. ≥30 severe OSAS). The study group consisted of 25 severe and 19 non-severe OSAS patients. In our study. mean BMI and WC were higher in patients with severe OSAS than in non-severe patients. Mean BMI was 34.55±7.39 and WC was 110.20±15.58 cm in patients with OSAS. There was a statistically significant different between severe and non-severe OSAS patients for BMI (p=0.02).
Papadavid et al. suggested that presence of MS was not a significant risk factor for OSAS in patients with psoriasis [23]. There was statistically significant evidence that only obesity and hypertension were associated with increased risk of OSAS. adjusting for psoriasis characteristics. metabolic parameters. age. and sex [24]. In our study. MS was identified in 64% of severe and 61% of non-severe OSAS patients. The prevalence of MS was not statistically different between the severe and non-severe OSAS patients. MS can develop as a result of sleep disorders [25]. Prevalence of MS was 5 times higher in OSAS patients than in normal individuals [26]. In our study. MS parameters were evaluated and 23 patients with OSAS had MS. Mean WC. BMI. systolic blood pressure. and fasting blood glucose values were significantly higher in OSAS patients with MS. In addition. 81.5% of all patients were hypertensive (p=0.001). According to a logistic regression model with independent variables. MS was not an independent risk factor for severe OSAS (OR: 0.31. 95% CI: 0.07–2.21. p=0.30).
Neck circumference (NC) is a newly identified clinical feature that may be associated with OSAS. NC is greater among man [27]. Higher NC and BMI in OSAS patients may be correlated with aerobic capacity. physical inactivity. and excess body fluid [28]. NC is a more effective factor in determining OSAS [29]. Onat et al. found that NC was a marker of central obesity. BMI. OSAS. blood pressure. and HOMA-assessed insulin resistance. In our study. we discovered that the NC in male patients was greater than in females (p<0.001). Also. in the severe OSAS patients group. mean NC value was significantly greater than in the non-severe OSAS group (p<0.001). There was a positive correlation between AHI and BMI (r=0.545. p<0.01). Higher NC value was determined to be associated with severity of OSAS. is an independent risk for severe OSAS (p=0.01). and is an independent risk factor for severe OSAS (OR: 1.55. 95% CI: 1.09–2.21. p=0.01).
Among patients with OSAS. from 1/2 to 2/3 had hypertension; these patients should be identified and treated. In a study by Lavie et al. hypertension was found in 45.3% of 2677 OSAS patients [30]. and Nieto et al. found hypertension in 62.6% of 6132 patients with OSAS [31]. In this study. 63.3% of all OSAS patients and 68% of severe OSAS patients were hypertensive. Hypertension is an important cardiovascular condition and an independent risk factor for OSAS [32.33]. Severity of OSAS may have an impact on displacement of HDL cholesterol. In The Sleep Heart Health Study. a relationship was determined between lower HDL cholesterol levels and severity of OSAS [34]. Coughlin et al. demonstrated that HDL cholesterol was lower in OSAS patients compared to a control group [35]. Triglycerides. HDL. LDL. and total cholesterol levels were not significantly different between groups in our study. but there was a negative correlation between AHI and mean HDL cholesterol level (r=–0.333. p=0.029).
The cross sectional design of this study to some extent limits the interpretation of obtained associations. Measurement of NC is a novel parameter for use in OSAS patients. In this respect. assessment of NC might be better in a large sample population to generate significant relations. The other limitation is the lack of a control group without OSAS.
Conclusions
In conclusion. NC and BMI measurements were higher in severe OSAS patients compared to non-severe patients. Increased NC may be a greater risk factor for severe OSAS than are MS parameters.
Footnotes
Source of support: Departmental sources
References
- 1.Angelico F, Ben M, Augeletti T, et al. Obstructive sleep apnoea syndrome and the metabolic syndrome in an internal medicine setting. Eur J Int Med. 2010;21:191–95. doi: 10.1016/j.ejim.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Zirlik S, Hauck T, Fuchs F, et al. Leptin. Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17(3):CR159–64. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanas N, Steiropoulos P, Nena E, et al. Predictors of obstructive sleep apnoea in males with metabolic syndrome. Vasc Health Risk Manag. 2010;6:281–86. doi: 10.2147/vhrm.s7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korostovtseva LS, Sviryaev YV, Zvartau NE, et al. Prognosis and cardiovascular morbidity and mortality in prospective study of hypertensive patients with obstructive sleep apnea syndrome in St Petersburg. Russia Med Sci Monit. 2011;17(3):CR146–53. doi: 10.12659/MSM.881448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sipilä K, Moilanen L, Nieminen T, et al. Metabolic syndrome and carotid intima media thickness in the Health 2000 Survey. Atherosclerosis. 2009;204(1):276–81. doi: 10.1016/j.atherosclerosis.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Patla M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Peled N, Kassirer M, Shitrit D, et al. The association of OSA with insulin resistance. inflammation and the metabolic syndrome. Resp Med. 2007;101:1696–701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 8.Barcelò A, Barbé F, de la Pegna M, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–50. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- 9.Makino S, Handa H, Suzukawa K, et al. Obstructive sleep apnoea syndrome. plasma adiponectin levels and insulin resistance. Clin Endocrinol. 2006;64(1):12–19. doi: 10.1111/j.1365-2265.2005.02407.x. [DOI] [PubMed] [Google Scholar]
- 10.Harsh IA, Hahn EG. Insulin resistance and other metabolic aspects of the obstructive sleep apnea syndrome. Med Sci Monit. 2005;11(3):RA70–75. [PubMed] [Google Scholar]
- 11.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):562–63. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 12.Kono M, Tatsumi K, Saibara T, et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest. 2007;131(5):1387–92. doi: 10.1378/chest.06-1807. [DOI] [PubMed] [Google Scholar]
- 13.Volna J, Kemlink D, Kalousova M, et al. Biochemical oxidative stress-related markers in patients with obstructive sleep apnea. Med Sci Monit. 2011;17(9):CR491–97. doi: 10.12659/MSM.881935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra P, Nugent C, Afendy A, et al. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int. 2008;28(8):1080–86. doi: 10.1111/j.1478-3231.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoffstein V, Szalai JP. Predictive value of clinical features in diagnosing obstructive sleep apnea. Sleep. 1993;16(2):18–22. [PubMed] [Google Scholar]
- 16.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154(2):279–89. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 17.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–52. [PubMed] [Google Scholar]
- 18.Carmelli D, Swan GE, Bliwise DL. Relationship of 30-year changes in obesity to sleep-disordered breathing in the Western Collaborative Group Study. Obes Res. 2000;8(9):632–37. doi: 10.1038/oby.2000.81. [DOI] [PubMed] [Google Scholar]
- 19.Reaven G. Metabolic syndrome: Pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–88. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- 20.Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages. limitations. and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 21.Bouloukaki I, Kapsimalis F, Mermigkis C, et al. Prediction of obstructive sleep apnea syndrome in a large Greek population. Sleep Breath. 2011;15(4):657–64. doi: 10.1007/s11325-010-0416-6. [DOI] [PubMed] [Google Scholar]
- 22.Gami AS, Somers VK. Obstructive sleep apnoea. metabolic syndrome and cardiovascular outcomes. Eur Heart J. 2004;25:709–11. doi: 10.1016/j.ehj.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Papadavid E, Vlami K, Dalamaga M. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venerol. 2012 doi: 10.1111/j.1468-3083.2012.04580.x. [DOI] [PubMed] [Google Scholar]
- 24.Dalamaga M, Papadavid E, Vlami K. Unmasking the Janus face of the association between psoriasis. metabolic syndrome and obstructive sleep apnea. Sleep Breath. 2012 doi: 10.1007/s11325-012-0749-4. [DOI] [PubMed] [Google Scholar]
- 25.Coughlin SR, Mawdsley L, Mugarza, et al. Obstructive sleep apnoea is independent association with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176(4):401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tufik S, Silva RS, Taddei JA, et al. Obstructive sleep apnoea syndrome in the sao paulo epidemiolgic sleep study. Sleep Med. 2010;11:441–46. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Ucok K, Aycicek A, Sezer M, et al. Resting metabolic rate and anthropometric measurements in male sleep apnea patients. Intern Med. 2011;50(8):833–38. doi: 10.2169/internalmedicine.50.4779. [DOI] [PubMed] [Google Scholar]
- 29.Soylu AC, Levent E, Sariman N, et al. Obstructive sleep apnea syndrome and anthropometric obesity indexes. Sleep Breath. 2012;16(4):1151–58. doi: 10.1007/s11325-011-0623-9. [DOI] [PubMed] [Google Scholar]
- 30.Hla KM, Young TB, Bidwell T, et al. Sleep apnoea and hypertention: A population based study. Ann Intern Med. 1994;120(5):382–88. doi: 10.7326/0003-4819-120-5-199403010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertention: population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onat A, Hergenc G, Yüksel H, et al. Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnoea syndrome beyond waist circumference. Clin Nutr. 2009;28:46–51. doi: 10.1016/j.clnu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Paris JM, Somers VK. Obstructive sleep apnoea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–46. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 34.Nieto FJ, Young TB, Lind BK, et al. Association of sleep disordered breathing sleep apnoea and hypertention in large community based study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 35.Newman AB, Nieto FJ, Guidry U, et al. Relationship of sleep disordered breathing to cardiovascular risk factors. The Sleep Heart Health Study. Am J Epidemiol. 2001;154:50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]