Abstract
Bacillus cereus causes food poisoning and serious non-gastrointestinal-tract infections. Non-hemolytic enterotoxin (Nhe), which is present in most B. cereus strains, is considered to be one of the main virulence factors. However, a B. cereus ΔnheBC mutant strain lacking Nhe is still cytotoxic to intestinal epithelial cells. In a screen for additional cytotoxic factors using an in vitro model for polarized colon epithelial cells we identified B. cereus sphingomyelinase (SMase) as a strong inducer of epithelial cell death. Using single and double deletion mutants of sph, the gene encoding for SMase, and nheBC in B. cereus we demonstrated that SMase is an important factor for B. cereus cytotoxicity in vitro and pathogenicity in vivo. SMase substantially complemented Nhe induced cytotoxicity in vitro. In addition, SMase but not Nhe contributed significantly to the mortality rate of larvae in vivo in the insect model Galleria mellonella. Our study suggests that the role of B. cereus SMase as a secreted virulence factor for in vivo pathogenesis has been underestimated and that Nhe and SMase complement each other significantly to cause full B. cereus virulence hence disease formation.
Introduction
Bacillus cereus is a spore forming Gram-positive bacterium that is found in natural habitats like soil and plants. Their spores can easily enter the food processing chain due to heat and dryness resistance. B. cereus is commonly accepted as a food borne pathogen causing mostly mild food borne gastroenteritis. Nevertheless, fatal outbreaks of B. cereus food poisoning [1], [2] and local and systemic non-gastrointestinal-tract infections (e.g. endophthalmitis, pneumonia, sepsis) in humans have been reported [3]. Two types of gastrointestinal diseases can be distinguished. Ingestion of the emetic toxin cereulide, a cyclic dodecadepsipeptide, causes nausea and vomiting, whereas the diarrheal syndrome has been associated mainly with the enterotoxins cytotoxin K (CytK), hemolysin BL (Hbl) and non-hemolytic enterotoxin (Nhe) [4], [5].
In contrast to food borne diseases, far less is known about non-gastrointestinal-tract infections resulting in severe septicemias. In recent years B. cereus has been increasingly recognized as an opportunistic human pathogen [6], [7]. Since B. cereus is also an insect pathogen, insect models have been widely used to assess B. cereus virulence in vivo. Especially lepidopteran larvae of the great wax moth Galleria mellonella have emerged as a popular insect model for evaluating overall microbial pathogenicity in vivo [8]–[11]. Comparable virulence data have been obtained in G. mellonella and the obviously very different mouse model [12]. B. cereus proteins like hemolysin II and the immune inhibitor A family have been associated with virulence in vivo in the insect host [13], [14], whereas major B. cereus enterotoxins such as Nhe, Hbl and CytK were poorly examined.
Expression of the secreted enterotoxins Nhe, Hbl and CytK is controlled by the PlcR regulon via a quorum sensing system (for review see [15]). Deletion of plcR abolishes in vitro cytotoxicity and reduces significantly in vivo pathogenicity in mice and insects [12]. PlcR regulates more than 40 genes including degradative enzymes, proteases and phospholipases such as phosphatidylinositol-specific phospholipase C (PI-PLC), phosphatidylcholine-specific phospholipase C (PC-PLC) and sphingomyelinase (SMase) [15]. Synergistic interaction of PC-PLC and SMase for complete lysis of human erythrocytes has been demonstrated and therefore, both enzymes have been proposed to form a cytolytic unit, named cereolysin AB [16]. B. cereus sphingomyelinase is structurally related to Staphylococcus aureus beta toxin, Clostridium perfringens alpha toxin and the sphingomyelinase of the intracellular pathogen Listeria ivanovii [16], [17]. Sphingomyelinase is a metal ion-dependent phospholipase that hydrolyzes sphingomyelin in the plasma membrane to phosphocholine and ceramide. Recently gangliosides, especially NeuAcα2-3Galβ1-4Glcβ1-1ceramide (GM3), have been shown as a direct binding site for SMase. The sialic acid of GM3 serves as a cell receptor for binding the β-hairpin region of SMase [18].
Oda et al. demonstrated that B. cereus SMase is an important virulence factor for septicemia. B. cereus strains displaying SMase activity are able to grow in vivo in mice after intraperitoneal injection in contrast to strains without SMase activity [19]. Earlier work by Beecher et al. suggested that SMase acts in connection with the hemolytic enterotoxin complex Hbl [20]. However, hbl genes are restricted to certain strains whereas the nhe operon encoding the so called non-hemolytic enterotoxin complex Nhe has been found in all strains tested so far [21]–[23]. Nhe is a pore-forming toxin that requires the expression of its individual enterotoxin components Nhe A, B and C in a certain ratio (10∶10:1) [24] and specific binding order for full in vitro cytotoxicity [25]. At least two or three Nhe components are necessary for the formation of functional membrane pores depending on the target cell type [26]. After binding of NheC and NheB to the cell surface a conformational change might occur that allows subsequent binding of NheA, resulting in cell lysis [25]. It is assumed that Nhe is the major enterotoxin playing a key role in B. cereus induced diarrhea [27] but a synergistic interaction between SMase and Nhe in B. cereus virulence has not been studied so far. Therefore, we examined the interaction of Nhe and SMase for in vitro cytotoxicity and in vivo pathogenicity. In this work, we provide evidence that B. cereus SMase enhances Nhe cytotoxicity in an in vitro model for polarized colon epithelial cells. SMase in vitro cytotoxicity depends on the presence of Nhe. Furthermore, in the insect model Galleria mellonella deletion of the SMase gene sph but not nheBC significantly reduced larvae mortality. Inactivation of both gene loci indicated that SMase and Nhe cooperate in B. cereus pathogenicity in vivo.
Materials and Methods
Bacterial Strains, Plasmids and Culture Conditions
Bacillus cereus NVH 0075-95 [28], its isogenic mutant strains and E. coli strain were routinely grown in Luria-Bertani (LB) broth or on LB agar plates at 30°C or 37°C, respectively. For cytotoxicity screening bacterial supernatants were collected from 100 ml cultures in LB broth (37°C, 150 rpm) 8 h after inoculation with 103 colony-forming units (CFU)/ml from a 16-hour pre-culture (corresponding to early stationary phase). When required, bacterial supernatants were concentrated up to 30-fold at 4°C using Amicon® centrifugal filter devices (10 kDa, Millipore). Supernatants were stored at –80°C. Unless stated otherwise, cultures were grown in 200 ml flasks and optical density at 600 nm (OD600) was recorded. Samples exceeding an OD600 of 1 were diluted 1∶10 and extrapolated.
Where appropriate a single or a combination of the following antibiotics was added to the media: ampicillin (100 µg/ml), kanamycin (50 µg/ml), spectinomycin (100 µg/ml), polymyxin B (50 µg/ml), erythromycin (3 µg/ml), chloramphenicol (5 µg/ml) or tetracycline (10 µg/ml). All bacterial strains and plasmids constructed and used in this study are listed in supplemental Table S1 and S2.
For phenotypic characterization of B. cereus wild type (WT) and mutant strains MYP agar (mannitol egg yolk polymyxin; Oxoid) and Columbia agar (containing 5% sheep blood, Oxoid) was used. Therefore, small amounts of colony material were transferred to the respective agar plate surface using the tip of a needle and incubated at 30°C for 24 h. To test for extracellular cross-complementation of hemolytic activity, B. cereus NVH 0075-95 WT, ΔnheBC, Δsph and ΔnheBCΔsph mutants were stricken out crosswise on sheep blood agar plates as described before [16] similar to diagnostic CAMP test and incubated at 30°C for 24 h.
General Molecular Methods
For cloning, genomic DNA of B. cereus NVH 0075-95 served as a template. DNA isolation, manipulation and transformation in E. coli was carried out in accordance with standard protocols [29]. All oligonucleotides used in this study are listed in Table S2. A high-fidelity Platinum® Taq DNA polymerase (Invitrogen) was used for DNA amplification and DNA manipulations were verified by sequencing.
Epithelial Cell Cytotoxicity
Ptk6 null epithelial cells (Ptk6) from mouse colonic mucosa were cultured as outlined previously [30]. In cytotoxicity assays, 1×106 Ptk6 cells were seeded onto 6-well plates and grown until confluence. For in vitro cytotoxicity tests Ptk6 cell monolayers were washed twice with DPBS and treated with either vegetative cells of various B. cereus strains (Table S3) at an MOI of 1 (2×106 bacteria) or sterile bacterial supernatant at 37°C/5% CO2 atmosphere. IEC Changes in intestinal epithelial cell (IEC) morphology, cell rounding and % cell detachment was monitored over time using light microscopy.
Identification of Cytotoxic Proteins Secreted by B. cereus
Concentrated bacterial supernatant of B. cereus NVH 0075-95 ΔnheBC was dialyzed against PBS (pH 7.4) and total protein concentration was determined using a BCA protein assay (Thermo Scientific Pierce). 1.64 mg of secreted proteins was fractionated by gel filtration using a Superdex-75 10/300 GL column in a ÄKTA Purifier system (GE) with a flow rate of 0.2 ml/min. Proteins were detected by their absorbance at 280 nm. Fractions were tested for cytotoxic activity on Ptk6 cell monolayers. After SDS-gel separation, silver stained protein bands were analyzed by LC/MS/MS on a ThermoFisher LTQ Orbitrap XL mass spectrometer by NextGen Sciences, Ann Arbor, USA. Protein identifications were accepted according to NextGen Science’s guidelines (greater than 90.0% probability, at least 2 identified peptides.) Protein probabilities were assigned by the Protein Prophet algorithm [31].
Construction of B. cereus NVH 0075-95 sph Deletion Mutants
Sphingomyelinase null mutants and complemented strains were generated as described before [32]. In brief, DNA regions of approximately 1200 bp flanking the sph gene were amplified by PCR using primers shown in Table S2, primarily inserted into the cloning vector pCR 2.1 TOPO flanking a chloramphenicol resistance cassette. The construct was cut with SacI/HindIII and inserted in the similarly digested conjugative suicide vector pAT113. The resulting vector pAT113Δsph/cm was transferred into B. cereus NVH 0075-95 WT and ΔnheBC via conjugation using the donor strain E. coli JM83/pRK24 as described elsewhere [33]. Transconjugants were screened for chloramphenicol resistance and erythromycin sensitivity. To confirm gene deletion and integration of the resistance cassette, immunoblotting, PCR and sequencing using the primer pair sph_Sac_F/sph_Hind_R, Cm_F/Cm_R_Not or InCm2_F/sph_downstream_R were carried out. The sph deletion mutants were designated B. cereus NVH 0075-95 Δsph and ΔnheBCΔsph.
Complementation of sph Deletion Mutants
To complement sph deletion mutants, plasmids containing one of the two putative plc-sph operon promoter regions Pplc and Psph directly upstream of the sph coding sequence were constructed. Using the primer pair Pplc_for/Pplc_rev a 538 bp region upstream of the plc gene (promoter Pplc-sph of the plc-sph operon) was amplified from B. cereus NVH 0075-95 genomic DNA. The PCR product was digested with EcoRI and SacI and was ligated in the similarly digested pAD123 vector containing an additional tetracycline resistance cassette (pAD/tet). In a second PCR reaction the promoterless sph coding sequence was amplified using the primer pair sph_Sac_F/sph_Hind_R. After amplification the PCR product was cloned into the SacI/HindIII restriction site of the same plasmid leading to the shuttle-vector pAD/sph/Pplc/tet. As control the sph gene including a 499 bp upstream region (putative promoter Psph described before [34], [35]) was PCR amplified using the primer pair Psph1_for/sph_Hind_R. After restriction digest the PCR product was inserted into the EcoRI-HindIII site of pAD/tet giving rise to a plasmid designated pAD/sph/Psph/tet. Both resulting plasmids were propagated in non-methylating E. coli INV110 and introduced into the B. cereus NVH 0075-95 sph single and triple mutant strains by electroporation as described previously [36]. PCR analysis with Tetr-specific primers confirmed the introduction of the plasmids into the generated mutant strains B. cereus NVH 0075-95 Δsph comPplc, Δsph comPsph, ΔnheBCΔsph comPplc and ΔnheBCΔsph comPsph. Expression of SMase was confirmed by immunoblotting and SMase activity assay.
Flow cytometric Analysis of B. cereus Cytotoxicity
For flow cytometric analysis, 1×106 Ptk6 cells were seeded onto 6-well plates and grown until confluence. For the assessment of bacterial cytotoxicity, Ptk6 cell monolayers were washed twice with DPBS, treated with serial dilutions of bacterial supernatant in RPMI 1640. After 4 h at 37°C/5% CO2, epithelial cells were washed, trypsinized and stained with Propidium iodide (Biozol) to detect necrotic cells. Samples containing 1–2×106 cells were analyzed for total and dead cell numbers using a Gallios flow cytometer (Beckman Coulter). To decipher the effect of SMase for B. cereus virulence in vitro, confluent Ptk6 cells were treated with sublethal dilutions (1∶16) of supernatant of B. cereus NVH 0075-95 Δsph or ΔnheBCΔsph supplemented with 0, 0.05, 0.1 and 0.2 U/ml of recombinant SMase (Sigma, S9396). To neutralize Nhe activity in the supernatant of Δsph, a monoclonal anti-NheB antibody (1E11) kindly provided by Richard Dietrich [27] was added to wells (10 µg/well) supplemented with 0, 0.1 and 0.2 U/ml of recombinant SMase. After 4 h incubation at 37°C/5% CO2, cells were analyzed by flow cytometry as described above.
PC-PLC and SMase Activity Assay
For enzyme activity assays supernatants of B. cereus wild type, the nheBC and sph mutants and their complemented derivatives cultivated in LB broth at 37°C were harvested (6500 g, 4 min, 4°C) at an OD600 of 4 and 7 corresponding to transition and early stationary growth phases, respectively. Using an Amplex® Red Sphingomyelinase Assay Kit (Invitrogen/Molecular Probes) production of sphingomyelinase was determined, while synthesis of phosphatidylcholine-specific phospholipase C was quantified with the EnzChek Direct Phospholipase C Assay Kit (Invitrogen/Molecular Probes) following the manufacturer‘s protocol. PC-PLC activity assay was carried out as described before [37] and sphingomyelinase assay was done in accordance except for the following changes. For SMase activity the reactions were developed for 60 min at 37°C in a light-protected microplate thermo-shaker and fluorescence was measured with a Wallac 1420 Victor2 multilabel plate reader (Perkin Elmer) at excitation/emission wavelengths of 530/585 nm. Samples of three independent cultures were examined for their phospholipolytic activities. To account for different protein concentrations of the samples enzyme activity was normalized to protein concentration of the respective supernatant using a Bradford-based protein assay (Roti-Quant, Carl Roth GmbH), resulting in mU per µg of protein.
Immunoblotting of SMase
Protein samples were prepared from bacterial supernatants of B. cereus NVH 0075-95 WT, isogenic mutant and complemented strains. 4 µg of total B. cereus protein were separated via 10% SDS/PAGE as described before [38]. After transfer to a PVDF membrane (Roche) B. cereus SMase was detected using a rabbit polyclonal anti-BcSMase antibody (1∶1000) kindly provided by Jun Sakurai [39]. An Alkaline-Phosphatase-conjugated goat-anti-rabbit IgG (1∶30000) (dianova) was used as second antibody for chromogenic SMase detection.
Insect Injection Experiments
Galleria mellonella (G. mellonella) larvae were purchased from Kerf (Unna, Germany) and kept at 15°C. Thirty last-instar G. mellonella larvae per condition, weighting 200–400 mg were kept in groups of 10 larvae per box at 15°C. Injection of 5 µl suspensions of vegetative bacteria was done intrahemocoelically into the base of the last left proleg of the larvae. 1.2–2.2×105 CFU per larva for B. cereus NVH 0075-95 WT, ΔnheBC, Δsph, ΔnheBCΔsph and the complementation mutant ΔnheBCΔsph comPplc and 7.0×105 CFU per larva for E. coli DH10B were injected, respectively. Bacterial cell counts of the injected suspension were determined for all strains by plating serial dilutions of the inocula on appropriate medium. G. mellonella survival was determined daily over 7 days at 15°C. Larvae were considered dead if they failed to respond to stimulation with a forceps. All tests were run in duplicates with 30 larvae per experiment and condition and they gave similar results for each experiment.
To determine the time-course of bacterial multiplication within G. mellonella larvae, 5 larvae of each condition (different injected bacterial strains) were homogenized individually at time-points indicated in the diagram and serial dilutions of the homogenate were plated on LB agar plates for determination of CFU per larva. Larvae were surface-cleaned with 70% Ethanol prior to homogenizing. Bacterial growth monitoring was done in two independent experiments.
Software and Statistical Analysis
Mean values and S.E. were calculated from at least three independent experiments. For statistical analysis, 2-tailed Student’s t-test was used, where applicable, to determine statistically significant differences as indicated in figure legends (GraphPad Prism, Graph Pad Software). Figures were assembled with Photoshop CS and Illustrator CS (Adobe Systems). Raw data of the insect experiments were analyzed using GraphPad Prism (version 5.0) software and plotted according to Kaplan-Meier. Curves were compared for statistical differences using log-rank analysis, which generates a P value testing the null hypothesis that the survival curves are identical. P values of 0.05 or less were considered significantly different from the null hypothesis.
Results
PlcR-mediated Cell Cytotoxicity in Intestinal Epithelial Cells
In order to analyze the role of cytotoxicity factors in B. cereus infection, 14 different B. cereus strains, which vary in their known toxin gene profile (Tab. S1), were tested for cell cytotoxicity in polarized intestinal epithelial cells (IECs) in vitro. As a cell culture model for polarized IEC we used colon epithelial cells derived from Ptk6 null mice [30].
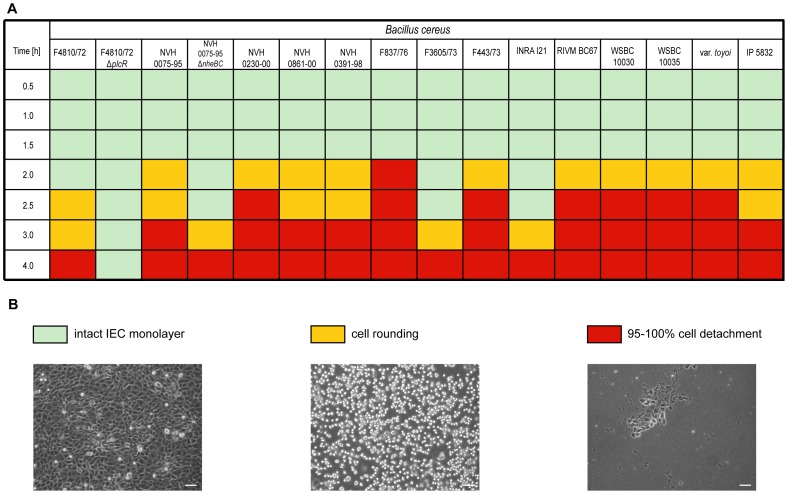
Infection of Ptk6 IEC with various B. cereus strains at an MOI of 1 caused rapid cell death with a complete detachment of cells from the tissue culture plate within 4 h (Fig. 1). Although the beginning of cellular rounding and detachment varied between 2 and 4 h there was not a gross difference between strains tested. Even two B. cereus strains isolated from two different probiotic formulas used in humans and piglet feeding [40]–[43], B. cereus var. toyoi and IP5832, revealed similar cytotoxicity compared to B. cereus NVH 0391-98 isolated from a food borne outbreak associated with high patient mortality [1] or the nhe reference strain B. cereus NVH 0075-95 (Fig. 1). Cellular cytotoxicity was mediated by a secreted B. cereus factor as rapid rounding and detachment in IEC was induced by bacterial supernatants alone. (Fig. S1). Infection of two different human colonic epithelial cell lines Caco-2 and T84 showed similar results (data not shown).
Figure 1. Cytotoxic effects of various B. cereus strains on intestinal epithelial cells (IEC).
Ptk6 cells were treated with 14 different B. cereus strains and two isogenic mutants, morphological changes were monitored over time using light microscopy. A. At an MOI of 1 all strains tested caused epithelial cell rounding (yellow) and detachment (red) within 2–4 h after infection except for the plcR deletion mutant. Intact monolayer (green). B. Representative images of Ptk6 cell monolayers. Bar, 20 µm.
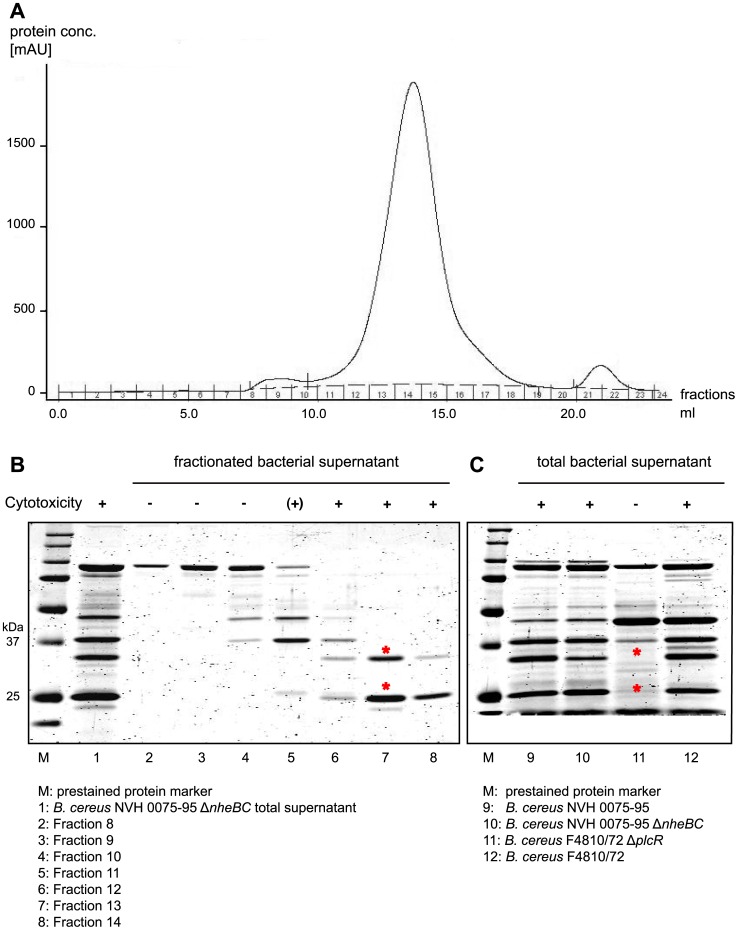
Common to all B. cereus strains tested is the presence of the nhe operon genes (Tab. S1). A B. cereus NVH 0075-95 nheBC mutant (ΔnheBC; kindly provided by Simon P. Hardy [44]) as well as its supernatant caused a delay of onset of cellular rounding by 1 h compared to wild type, but IEC still detached completely after 4 h and 6 h, respectively (Fig. 1 and S1). Due to nheB truncation and nheC deletion B. cereus NVH 0075-95 ΔnheBC is unable to produce a functional Nhe toxin, since maximal cytotoxic activity of Nhe depends on at least two Nhe components [24]. Cytotoxicity was completely abolished using a B. cereus mutant strain lacking the PlcR regulon (B. cereus F4872/10 ΔplcR) [32]. These data indicate that additional PlcR regulated proteins contribute to IEC cytotoxicity. As B. cereus NVH 0075-95 does not express enterotoxins like Hbl or CytK, we screened for other cytotoxic components in the B. cereus NVH 0075-95 ΔnheBC supernatant using fast protein liquid chromatography (FPLC). Proteins contained in the bacterial supernatant were separated according to their size on a Superdex-75 10/300 GL gel filtration column (Fig. 2A). Fractions 11–18 contained most of the extracellular bacterial proteins. IEC cytotoxicity was confined to three fractions by infecting polarized epithelial monolayers with each of the fractions of the bacterial supernatant of B. cereus NVH 0075-95 ΔnheBC. Only fractions 12–14 conveyed complete epithelial cell detachment. SDS gel electrophoresis analysis of fractions 8–14, as well as unfractionated supernatant is shown in Fig. 2B and 2C. Comparison of proteins contained in the cytotoxic fractions 12–14 with unfractionated mutant and wild type B. cereus supernatants revealed a 34 kDa and a 25 kDa protein band (indicated in Fig. 2B by red asterisk) that were absent in the non-cytotoxic supernatant of B. cereus F4810/72 ΔplcR mutant (Fig. 2C). Protein identification of the two silver stained protein bands was carried out by NextGen Sciences (AnnArbor, USA) using LC/MS/MS on a ThermoFisher LTQ Orbitrap XL mass spectrometer. Mass spectrometry analysis of the 34 kDa protein identified sphingomyelin phosphodiesterase (sphingomyelinase) of B. cereus (NCBI accession number YP_002336808) by two unique peptide sequences, (K)DHANPSFVENK(V) and (K)VQYVFANGCGPDNLSNK(G), and a sequence coverage of 74%. The 25 kDa protein band was identified as hybrid cereolysin AB (NCBI accession number CAA45501). Hybrid cereolysin AB was chosen by Gilmore et al. for the functional unit of phospholipase C (5′-terminal region) and sphingomyelinase (3′-terminal region) proposed to work together as the hemolytic determinant hybrid cereolysin AB of B. cereus with an expected molecular mass of 67 kDa [34]. The peptides identified by mass spectrometry are unique for the 3′-terminal part of hybrid cereolysin AB, which is identical to the sphingomyelinase (SMase) polypeptide. Therefore, since the smaller protein band at approximately 25 kDa is co-detected with the 34 kDa SMase protein band by immunoblot using anti-BcSMase antibody (data not shown), we propose that the 25 kDa protein might be an isoform or degradation product of sphingomyelinase.
Figure 2. Identification of cytotoxic protein from B. cereus supernatant.
A. Bacterial supernatant of B. cereus NVH 0075-95 ΔnheBC was separated on a Superdex-75 10/300 GL gel filtration column. Chromatogram of fractionated bacterial proteins is shown (fraction1–24). Protein fractions were tested on Ptk6 cells for cytotoxicity as described in Experimental procedures. Protein fractions obtained from gel filtration were analyzed by SDS-PAGE. B. Gel filtration fractions 12–14 transferred cytotoxicity to Ptk6 cells and contained two distinct proteins migrating at 34 kDa and 25 kDa (red asterisks). C. Comparing total extracellular proteins of WT and mutant B. cereus strains, two potential cytotoxic proteins (red asterisks) were absent in the supernatant of avirulent ΔplcR strain.
Construction of B. cereus sph Gene Deletion Mutant
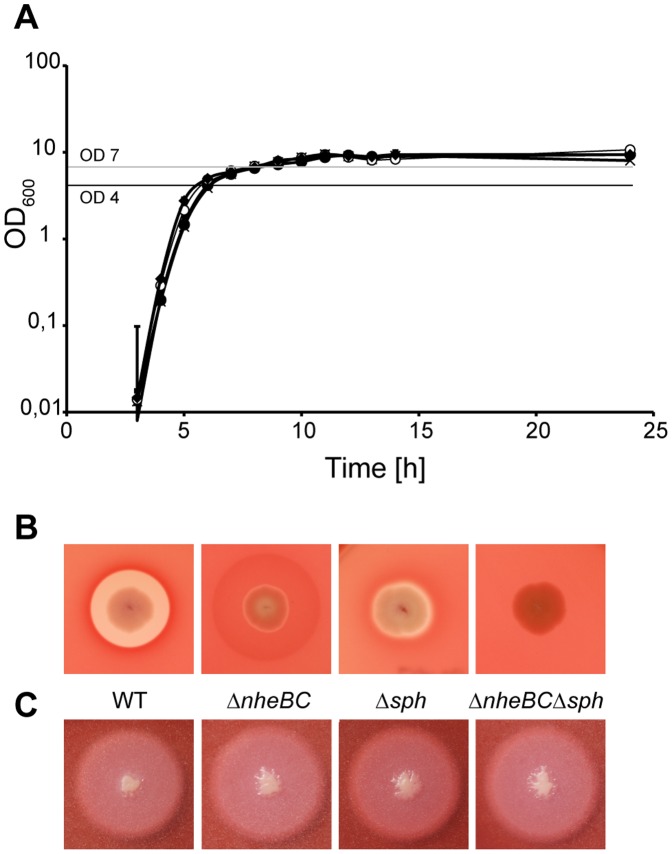
In order to address the question to which extent SMase contributes to cellular cytotoxicity in vitro, sphingomyelinase null mutants (Δsph) were constructed. Allelic gene replacement of the sph ORF by a chloramphenicol resistance cassette resulted in the B. cereus strains NVH 0075-95 Δsph and NVH 0075-95 ΔnheBCΔsph (sph::cm). B. cereus NVH 0075-95 WT and the isogenic mutants ΔnheBC, Δsph and ΔnheBCΔsph grew identical in LB medium at 37°C (Fig. 3A). On sheep blood agar, B. cereus WT showed strong beta-hemolytic activity indicated as clear zones around colonies (Fig. 3B). The hemolysis zone in the nhe mutant was markedly reduced in clearance albeit not in diameter compared to WT, while sph deletion abolished most but not all beta-hemolytic activity. The additional sph deletion resembled a ΔplcR-like hemolytic-negative phenotype in B. cereus NVH 0075-95 ΔnheBCΔsph suggesting that in Hbl-, HlyII- B. cereus strains like NVH 0075-95 the hemolytic phenotype is conveyed by Nhe and SMase.
Figure 3. Growth behavior and colony phenotypes of parental and mutant B. cereus strains.
B. cereus NVH 0075-95 (WT, •), isogenic nheBC (ΔnheBC, ×), sph (Δsph, ♦) and nheBC sph (ΔnheBCΔsph, <$>\raster="rg1"<$>) null mutants. Strains were cultivated in LB at 37°C and growth was monitored by measuring optical density at 600 nm (OD600) (A). Error bars represent standard errors derived from n = 3 independent experiments. Colony morphology on (B) Columbia Blood agar (5% sheep blood, Oxoid) and (C) MYP agar (mannitol egg yolk polymyxin agar, Oxoid) was used for specific detection of beta-hemolytic (visible as cleared zones around colonies) and PC-PLC enzyme activity (lecithin precipitation zone), respectively.
Sph Null Mutation does not Affect PC-PLC Activity
Since plc and sph are arranged in one operon (cereolysin AB operon, Fig. 4), the 5′ adjacent plc gene might have been affected by incorrect double crossover during allelic replacement of sph. To test for any polar effects, the mutant strains were cultivated on MYP agar. Typically, phosphatidylcholine-specific phospholipase C (PC-PLC) activity resulted in peripheral zones of egg yolk precipitation on MYP agar (Fig. 3C). This phenotype was not affected by inactivation of nhe or sph. In order to quantify phosphatidylcholine-specific phospholipase C activity of WT and of isogenic sph null strains we determined the lipolytic activity of culture supernatants using a substrate-specific assay for PC-PLC detection. To correct for putative differences in protein concentrations of the strain specific secretomes, enzyme activities were normalized to the protein content of each sample. Supernatant samples were taken from cells at OD600 = 4, when PC-PLC activity peaked during growth in LB medium at 37°C (data not shown). With 121.6±25.6 mU/µg protein and 120.5±25.2 mU/µg protein, respectively, phospholipolytic activity of B. cereus NVH 0075-95 WT and ΔnheBC was almost identical. After sph deletion PC-PLC activity was still high for B. cereus NVH 0075-95 Δsph (173.1±12.8mU/µg protein) and ΔnheBCΔsph (168.9±28.4mU/µg protein) and not significantly different from WT (P = 0.146) and ΔnheBC (P = 0.271), respectively.
Figure 4. Genomic organization of the chromosomal plc – sph gene cluster.
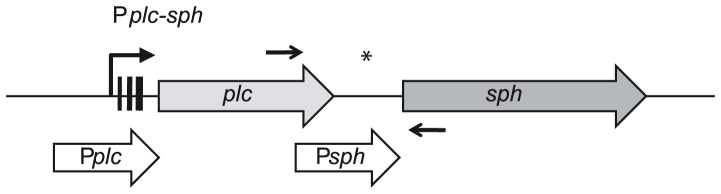
The operon comprises phosphatidylcholine-specific phospholipase C (plc) and sphingomyelinase (sph). Pplc-sph refers to the putative promoter region of the plc-sph operon. Three black bars in the promoter region represent the identified −35, −10 boxes and the ribosome-binding site. Pplc (white thick arrow) designates a 538-bp fragment spanning the operon promoter region Pplc-sph used for successful complementation, while the inter-genic region is covered by Psph (499bp; white thick arrow). The position of inplc_for and insph_rev primer binding for co-transcription analysis are indicated by two small black arrows. *putative promoter region directly upstream of sph start codon (Psph).
Sph is co-transcribed with plc from a Common Promoter
For complementation of sph null mutants we sought to reconstitute SMase protein expression under its own sph promoter in both sph knockout strains. Yamada et al. [34] and Pomerantsev et al. [35] have described a putative promoter region Psph directly upstream of the sph start codon, although sph and plc are known to be arranged in one operon (cereolysin AB operon) probably transcribed from the common promoter Pplc-sph (Fig. 4).
Because of this discrepancy, we used reverse transcriptase PCR to confirm that the plc-sph gene cluster was co-transcribed from the operon promoter region Pplc-sph (data not shown). As expected from co-transcription analysis the putative promoter region Psph directly upstream of the sph start codon failed to reconstitute SMase expression in B. cereus NVH 0075-95 Δsph comPsph and ΔnheBCΔsph comPsph (Fig. S2C).
Therefore, in trans complementation of sph null mutants was achieved by introducing the plasmid pAD/sph/Pplc/tet driving sph expression from the operon promoter Pplc-sph. SMase expression in complemented strains Δsph comPplc and ΔnheBCΔsph comPplc was confirmed by immunoblotting (Fig. S2C). Re-introduction of sph restored beta-hemolytic enzyme activity, while lipolytic activity was not affected (Fig. S2A and S2B). SMase enzyme activity was significantly enhanced in complemented strains restoring wild type activity by 321–558% due to the multicopy nature of the complementation vector pAD (Fig. 5).
Figure 5. Effect of sph deletion and complementation in trans on synthesis of SMase enzyme by B. cereus NHV 0075-95 and deduced mutant strains.
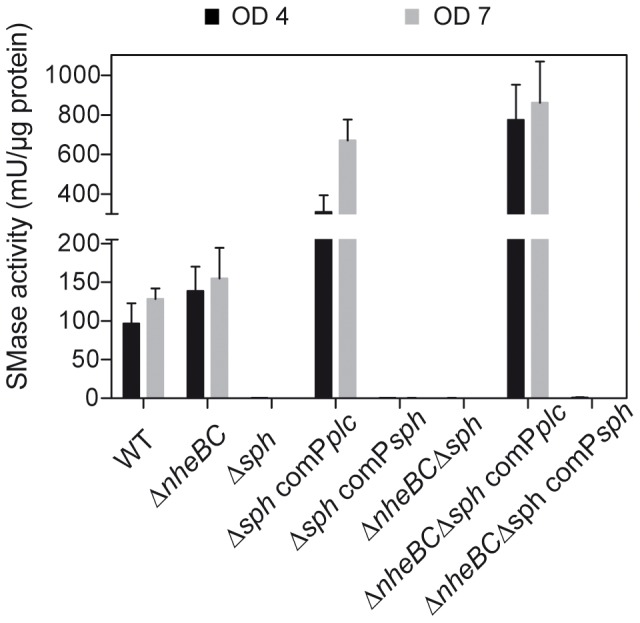
SMase activity was normalized to the protein concentration of prepared supernatant samples. Sph deletion completely abolished SMase activity, which could be restored by in trans expression of sph driven from the operon promoter region Pplc at different growth phases; OD600 = 4, black bar and OD600 = 7, grey bar. Data represent mean values ± SEM (n ≥3).
SMase adds to Nhe Induced B. cereus Cytotoxicity in vitro
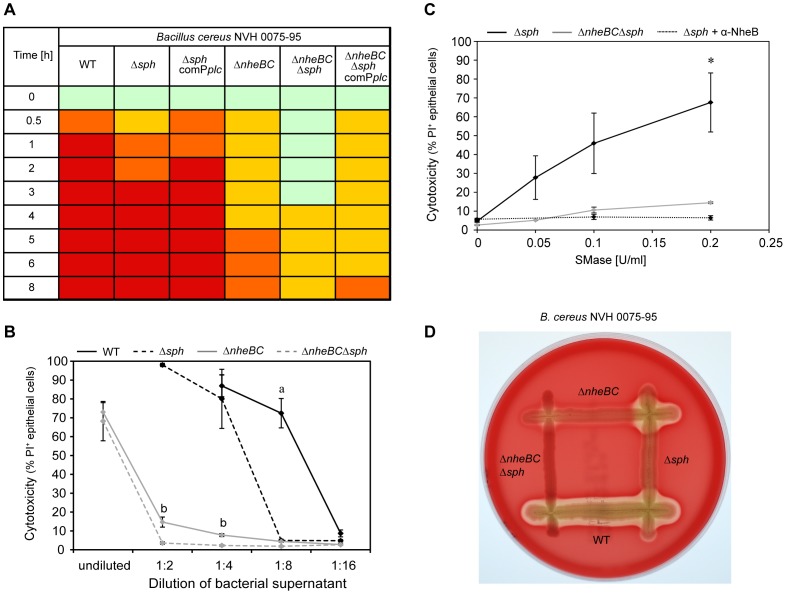
To elucidate the role of SMase to cellular cytotoxicity in vitro, we tested sph deletion mutants in our initial screen for colon epithelial cell cytotoxicity based on the morphological characteristics cell rounding and detachment. This analysis revealed that SMase as a single factor contributed little to B. cereus cytotoxicity compared to WT (Δsph). However, SMase had a significant effect on IEC cytotoxicity in combination with Nhe (ΔnheBCΔsph). Re-expression of SMase in complemented strains Δsph comPplc and ΔnheBCΔsph comPplc reversed the effect (Fig. 6A).
Figure 6. Sph deletion effected B. cereus virulence in vitro.
A. Cytotoxic effect of sterile B. cereus supernatants (1∶4 diluted) on IEC. Intact monolayer (green), cell rounding ≤50% (yellow), cell rounding >50% (orange) and 95–100% cell detachment (red) are indicated. B. Cytotoxic effects of B. cereus supernatant on IEC were analyzed using flow cytometry. Ptk6 cells were treated with various dilutions of bacterial supernatant of B. cereus NVH 0075-95 WT (black line), ΔnheBC mutant (grey line), Δsph mutant (black dashed line) and ΔnheBCΔsph mutant (grey dashed line). Samples were stained with Propidium iodide (PI) for dead epithelial cells and cytotoxicity is expressed in % of PI positive cells as determined by flow cytometric analysis. Cytotoxicity of the Δsph mutant was strongly reduced at a dilution of 1∶8 compared to WT (a, P<0.05). Sph deletion in addition to Nhe inactivation significantly reduced cytotoxicity compared to Nhe inactivation alone (b, P<0.05). Data plotted represent mean values ± SEM (n = 3). C. Cooperative cytotoxic interaction of SMase and Nhe. Addition of various concentrations of recombinant SMase (0.05, 0.1 and 0.2 U/ml) to diluted (1∶16) bacterial supernatants caused significantly higher cytotoxicity against Ptk6 cells when subtoxic Nhe concentrations were present (Δsph supernatant, black line) compared to supernatant without Nhe (ΔnheBCΔsph supernatant, grey line) (*P<0.05). Addition of anti-NheB (1E11) antibody (10 µg/well) neutralizes Nhe activity (Δsph supernatant+α-NheB, black dotted line). Cytotoxicity is expressed in % of PI positive cells and data represent mean values ± SEM (n ≥3). D. CAMP-like test on sheep blood agar demonstrated complementation of extracellular hemolytic activity between B. cereus NVH 0075-95 Δsph and ΔnheBC. Beta-hemolytic activity appeared as cleared zone around the colonies.
In order to quantify the influence of SMase on cell cytotoxicity, polarized IEC were incubated for 4 h with serial dilutions of bacterial supernatant of B. cereus WT and isogenic mutant strains (Fig. 6B). After staining for dead epithelial cells using Propidium iodide (PI), PI positive and total cell numbers were counted using flow cytometry. Mock (LB medium, 1∶2) treated IEC had 1.5%±0.1 PI positive cells. Undiluted supernatants of B. cereus NVH 0075-95 WT and sph deletion mutant showed high cytotoxicity against IEC resulting in complete detachment and disintegration of epithelial cells rendering them inaccessible for flow cytometric analysis. Wild type cytotoxicity did not drop below 50% before a dilution of 1∶16 (Fig. 6B). Deletion of sph significantly reduced B. cereus cytotoxicity compared to WT. PI positive cells decreased to 5.0%±0.5 in B. cereus NVH 0075-95 Δsph at a 1∶8 dilution compared to 72.4%±7.8 in WT.
Treatment of epithelial cells with supernatant of the B. cereus NVH 0075-95 ΔnheBC mutant strain resulted in 73.0%±5.0 PI positive cells for undiluted and 14.7%±2.7 for 1∶2 diluted samples. The additional sph deletion reduced significantly the number of dead cells to 3.6%±0.6 in B. cereus NVH 0075-95 ΔnheBCΔsph (P<0.05) (Fig. 6B). Our results emphasize the dominant role of the non-hemolytic enterotoxin Nhe for B. cereus cytotoxicity against polarized intestinal epithelial cells and at the same time hint to a separate effect of SMase adding to Nhe cytotoxicity.
SMase Supplements Nhe Cytotoxicity in vitro
To analyze the concerted action of SMase and Nhe for epithelial cell cytotoxicity, we treated polarized IECs with subtoxic dilutions (1∶16) of supernatants of B. cereus NVH 0075-95 WT and mutant strains and compared the cytotoxic effect of added purified recombinant SMase. Addition of 0.05, 0.1 and 0.2 U/ml SMase to supernatant of B. cereus NVH 0075-95 ΔnheBCΔsph consistently increased its cytotoxicity against IECs: 0.2 U/ml SMase increased cell death by 5.4-fold (14.5%±0.5 with compared to 2.7%±0.4 without (P<0.0001); Fig. 6C). In contrast when added to supernatant of B. cereus NVH 0075-95 Δsph, the addition of 0.05, 0.1 and 0.2 U/ml SMase caused a much greater increase in cytotoxicity in the presence of a non-toxic level of Nhe in the supernatant of Δsph: 0.2 U/ml SMase increased cytotoxicity by 14.4-fold (67.6%±15.6 with compared to 4.7%±0.6 without (P<0.001); Fig. 6C).
These results suggest a cooperative mechanism of enterotoxin Nhe and SMase activity in epithelial cell death in vitro. To ensure that the increase in cytotoxicity after addition of SMase was due to the remaining Nhe activity in the supernatant of Δsph, we blocked Nhe activity by using a monoclonal anti-NheB antibody (MAb 1E11) [27], which has been shown to block Nhe activity [45], in the supernatant of the sph deletion strain. Neutralization of Nhe activity resulted in a strong decrease of SMase cell cytotoxicity in a way similar to the double-knockout ΔnheBCΔsph (Fig. 6C) revealing the synergistic effect of Nhe and SMase.
To confirm the synergy of Nhe and SMase proteins a test similar to the classical Christie, Atkins, Munch-Peterson (CAMP) reaction was performed [46]. Neither B. cereus single deletion mutants Δsph and ΔnheBC, nor the double mutant ΔnheBCΔsph showed significant hemolysis of erythrocytes on sheep blood agar (Fig. 6D). However, in the area, where the streaks of B. cereus Δsph and B. cereus ΔnheBC cross, the merging of Nhe (B. cereus Δsph) and SMase (B. cereus ΔnheBC) restored the hemolytic activity comparable to B. cereus WT (Fig. 6D).
Synergistic Interaction of SMase and Nhe for Full B. cereus Virulence in vivo
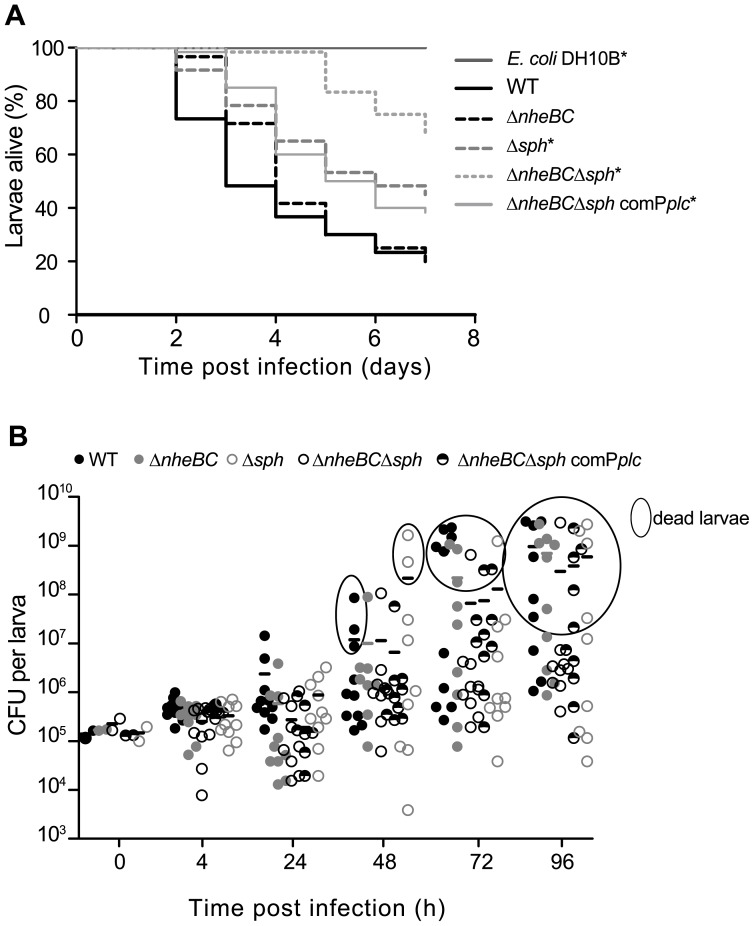
To assess the contribution of SMase and Nhe to B. cereus pathogenicity in vivo, Galleria mellonella larvae were used as a model system for bacterial infection. After intrahemocoelic injection of B. cereus NVH 0075-95 WT, isogenic mutant strains and E. coli DH10B as mock control larvae survival was monitored over seven days (Fig. 7A). All larvae injected with mock control survived the monitoring period. Following injection of B. cereus NVH 0075-95 WT mortality rate of G. mellonella larvae was >50% after 3 and >75% after 7 days (Fig. 7A). Surprisingly, survival of B. cereus NVH 0075-95 ΔnheBC injected larvae was not different to WT suggesting that Nhe contributes little to larvae mortality. Deletion of sph alone (Δsph) resulted in a significant delay of G. mellonella mortality to 22.7%±5.3 after 3 days and 55.0%±6.4 after 7 days. Additional nheBC inactivation (ΔnheBCΔsph) reduced larvae death further to 1.7%±1.6 dead larvae after 3 days and 31.7%±6.0 after 7 days. Re-expression of SMase in the complemented strain ΔnheBCΔsph comPplc restored most but not all in vivo pathogenicity.
Figure 7. Sph deletion strongly reduced the pathogenicity of B. cereus NVH 0075-95 in a Galleria mellonella in vivo model.
A. Larvae were infected by intrahemocoelic injection of 105 CFU of vegetative B. cereus NVH 0075-95 (WT), the isogenic nheBC mutant (ΔnheBC), the isogenic sph null mutant (Δsph), the nheBC sph mutant strain (ΔnheBCΔsph) or its complemented strain (ΔnheBCΔsph comPplc) as described in Materials and Methods. Larvae infected with the non-insecticidal E. coli strain DH10B served as control group. G. mellonella survival data are plotted as Kaplan-Meier plots. Data are retrieved from two independent infection experiments with a total of 60 larvae per condition. Both experiments showed very similar results. Statistical significance was determined using log-rank analysis. An asterisk indicates treatment groups with a survival distribution function statistically different from B. cereus WT (P<0.001). B. Survival and multiplication of B. cereus WT and isogenic mutant strains in G. mellonella after intrahemocoelic injection of 105 vegetative cells. Bacterial growth in two independent infection assays was monitored at indicated time points after infection (t = 0) by counting individual homogenates of five larvae per condition. CFUs recovered from dead larvae are indicated as encircled data points. Paired Student’s t-test was used to determine statistical differences between bacterial cell counts of the five treatment groups. In living or dead Galleria larvae cell counts of the sph mutant strains did not differ significantly from WT and ΔnheBC mutant.
To test whether differences in larvae mortality were attributed to a defect in bacterial growth in vivo, the bacterial load in infected larvae was quantified over time. Increasing colony-forming units (CFU) were counted 4–24 h post injection for all strains, suggesting that WT as well as mutant strains are capable of growing in the hemocoel (Fig. 7B). Bacterial load in living larvae increased up to 107 CFU. After larvae death bacterial growth was further enhanced up to 109 CFU (data points encircled, Fig. 7B). We could not detect statistical differences between colony-forming units of WT or mutant strains within living (P>0.05) or dead larvae (P>0.05) indicating that in our study reduced pathogenicity associated with sph deletion was not caused by differences in growth in vivo.
Our study shows that B. cereus SMase is an important virulence factor for in vivo pathogenesis in non-gastrointestinal-tract infections. Furthermore, it demonstrates a cooperative interaction of the major enterotoxin Nhe and phospholipase SMase in B. cereus virulence.
Discussion
B. cereus infections in humans are usually mild causing brief episodes of gastrointestinal symptoms and are probably highly underreported [4]. Considering the strong cytotoxic effect on eukaryotic cells in cell culture experiments in vitro it is fortunate that the bacteria must lack certain qualities that e.g. their close relative B. anthracis seem to possess. Even B. cereus strains isolated from probiotic formulas are cytotoxic to eukaryotic cells in vitro at a very low MOI of 1, whereas usually high doses of 105–108 enterotoxigenic B. cereus cells or spores are required for food poisoning [4], [47]. Once the protective barrier of the mucosa or dermis is overcome, B. cereus causes serious and often-fatal non-gastrointestinal-tract infections characterized by extensive tissue destruction [3]. Although B. cereus infections in the past have been rarely detected as the causative agent of infectious diseases, recent events demonstrate that new highly virulent pathogens can emerge due to the plasticity of bacterial genomes causing outbreaks with serious outcomes for affected patients [48].
Much of B. cereus virulence is mediated by secreted enterotoxins. Three cytotoxins, CytK, Hbl and Nhe, are currently considered to be responsible for B. cereus diarrheal disease but of those three only Nhe is present in most B. cereus strains currently known [4]. In contrast, enterotoxin genes hbl and cytK are often absent even from B. cereus strains isolated from disease outbreaks, which argues against a prominent role of these two toxins in disease formation [22], [23]. Nhe is considered to be the major factor in B. cereus diarrheal disease, because in vitro B. cereus cytotoxicity correlates well with Nhe concentration in the bacterial supernatant [49]. Neutralizing antibodies against Nhe abolished almost completely cytotoxicity and a B. cereus nheBC mutant NVH 0075-95 lost all cytotoxicity in cell culture based assays [27], [44], [49].
In our study we used the previously described B. cereus Nhe mutant strain NVH 0075-95 ΔnheBC [44] to identify B. cereus sphingomyelinase as an additional virulence factor. Incubation of polarized colon epithelial cells (Ptk6) with the supernatant of the ΔnheBC mutant strain for 4 h resulted in nearly 90% of dead cells revealing that other secreted cytotoxins must contribute to epithelial cell cytotoxicity as well. A major difference to the previous study, in which the lack of Nhe in B. cereus NVH 0075-95 (ΔnheBC) abolished the entire cytotoxicity of the wild type strain, was time of incubation [44]. Fagerlund et al. described that the uptake of Propidium iodide into Vero and Caco-2 cells as an indicator for cell death was abolished in the absence of Nhe in the first 15–30 minutes after adding bacterial supernatant [44]. Our observation that the onset of cell rounding and detachment was delayed in B. cereus NVH 0075-95 ΔnheBC compared to WT supports the finding that Nhe plays a significant role in the early phase of cell cytotoxicity. However, both strains caused cell death and complete detachment of epithelial cells 4 h after adding bacterial supernatants to cells.
Our study confirmed the pivotal role of the PlcR regulon in B. cereus mediated cytotoxicity and we identified sphingomyelinase as an important factor contributing to B. cereus cytotoxicity. SMase has been described before as a metal ion dependent phospholipase showing hemolytic activity against sheep erythrocytes [50]. The capacity of B. cereus to detach host cells in vitro correlated positively with the genomic presence of the sph gene [51]. Protein homologues of SMase in S. aureus (β-toxin) and Cl. perfringens (α-toxin) are well-established virulence factors involved in cytotoxicity against host cells [17], [52]. Walev et al. demonstrated cytotoxicity of S. aureus β-toxin, Streptomyces sp. SMase and B. cereus SMase against human monocytes [53]. B. cereus SMase has been shown to inhibit neurite outgrowth in PC12 cells and to induce membrane damage in host neural cells [54]. However, the role of B. cereus SMase as a virulence factor is still under debate, because SMase was nontoxic against Vero cells and in an in vitro retinal toxicity model [55], [56]. Differences in cytotoxicity observed between various cell lines may be attributed to variations in phospholipid composition and sphingomyelin content of host cell membranes as seen for red blood cells of different species varying in their sphingomyelin content ranging from 25–53.1% (human – sheep) [20], [57].
In our current study we demonstrate that SMase is a cytotoxic factor in vitro and its effect is significantly enhanced in cooperation with Nhe. The significance of SMase to overall B. cereus pathogenicity was demonstrated in sph mutants in wild type and NVH 0075-95 ΔnheBC. In vitro, sph deletion alone had little effect on epithelial cell cytotoxicity. But it added significantly to Nhe mediated cell cytotoxicity, suggesting a synergistic mode of action for the pore-forming enterotoxin Nhe and SMase. In the absence of the Nhe membrane-binding components B and C (supernatant of ΔnheBCΔsph) the addition of recombinant sphingomyelinase resulted in a small but significant increase of epithelial cell cytotoxicity. However, Nhe in the B. cereus supernatant (Δsph) enhanced the cytotoxic effect of recombinant SMase to a much higher degree indicating a cooperative interaction of Nhe and SMase via a yet unknown mechanism.
The phenomenon of hemolytic synergy of bacterial phospholipases C and pore-forming toxins has been reported before [20], [57], [58]. Beecher and Wong, 2000, demonstrated interaction of SMase and the hemolytic enterotoxin Hbl for erythrocyte hemolysis [20]. To our knowledge the results presented in this study are the first to demonstrate a complementary effect of the non-hemolytic enterotoxin Nhe and SMase. Nhe in vitro cytotoxicity requires a specific binding order of the enterotoxin components Nhe A, B and C [25]. For the formation of functional membrane pores either two or three Nhe components are necessary depending on the target cell type [26]. The secreted sphingomyelinase might adsorb to the epithelial cell surface as demonstrated for erythrocytes [59], thereby modifying membrane structure and fluidity by cleaving the membrane constituent sphingomyelin (SM). This could disrupt membrane integrity followed by moderate levels of epithelial cell lysis. Since the cytotoxic effect of SMase addition was severely enhanced in the presence of Nhe at a non-toxic concentration, Nhe might function as a gatekeeper. This is further supported by the fact that addition of anti-NheB antibody 1E11 reduced SMase induced cytotoxicity in a way similar to the double-knockout mutant ΔnheBCΔsph. The antibody 1E11 has been demonstrated to inhibit the association of Nhe A with cell-bound NheB via binding to the C-terminal part of NheB, thereby neutralizing Nhe activity [45].
Once the Nhe complex has formed functional transmembrane pores into the epithelial cell membrane, SMase could enter cells more easily hence reach otherwise inaccessible substrate pools in the inner membrane leaflet. SM hydrolysis could result in cell membrane destabilization as well as cell apoptosis via the ceramide intracellular signaling pathway [60]. Another conceivable mechanism of SMase and Nhe synergism might be that the enzymatic breakdown of membrane sphingomyelin to ceramide by SMase provides access to converted ceramide or other membrane components that can act as binding sites for the active Nhe enterotoxin complex. Such a mechanism has been demonstrated for several bacterial secreted factors such as S. aureus β-toxin and the classical S. agalactiae CAMP factor or Propionibacterium acnes and a host acidic SMase which synergistically enhance lysis of erythrocytes [61], [62]. The CAMP factor of S. agalactiae has been similar to B. cereus Nhe characterized as an oligomeric pore-forming toxin [63]. Therefore, it is tempting to speculate that B. cereus Nhe could be a CAMP-like factor.
The importance of B. cereus SMase as a virulence factor is strongly supported by in vivo observations in various insects. B. cereus SMase caused significant mortality of German cockroaches, cut- and silkworms when applied as a purified enzyme expressed in B. cereus or E. coli [64], [65]. Insect models in general and larvae of the great wax moth Galleria mellonella in particular are widely used for the assessment of bacterial pathogenicity in vivo, since the invertebrate immune system functionally resembles the mammalian innate immune response to bacterial infections [8], [10], [14], [66], [67]. In the G. mellonella model we demonstrated that sph expression is important for the pathogenic phenotype of B. cereus NVH 0075-95 suggesting that SMase is a significant virulence factor in vitro and in vivo, necessary to establish a severe course of infection. These data confirm recent results published by Oda et al. demonstrating that SMase is essential for B. cereus induced mouse mortality [19]. They showed that SMase mediates bacterial growth of clinical isolates in vivo and evasion from the host innate immune system in contrast to environmental B. cereus isolates. B. cereus strains isolated from soil did not cause lethality in mice due to their lack of SMase, but addition of SMase to B. cereus environmental strains injected into the peritoneum reconstituted virulence of clinical isolates causing them to grow in vivo [19]. However, in G. mellonella SMase may convey mortality in a different way than in mice, because our sph deletion mutants did not show differences in growth.
Given the strong role of Nhe for in vitro cell cytotoxicity, we were surprised to see that the B. cereus NVH 0075-95 ΔnheBC mutant strain behaved similar to wild type in G. mellonella larvae suggesting that Nhe’s sole contribution to B. cereus pathogenicity in vivo is maybe less than what is predicted from in vitro results. The mortality of G. mellonella larvae was significantly reduced in the B. cereus sph deletion mutant. The additional inactivation of nheB/nheC reduced larvae mortality even further, supporting our in vitro observation that SMase and Nhe cooperatively determine B. cereus virulence.
In summary, our data show that the contribution of SMase to B. cereus virulence has been underestimated in the past and our results obtained in cell culture and the Galleria model using deletion mutants of B. cereus confirm that B. cereus SMase contributes in cooperation with Nhe significantly to in vitro cytotoxicity and in vivo pathogenicity. Therefore, it is tempting to speculate that this is also the case in the human host. Secreted factors like SMase and enterotoxins may interact synergistically to cause tissue destructive effects in human B. cereus infections.
Supporting Information
Cytotoxic effects of sterile B. cereus supernatants on IEC. Ptk6 cells were treated with B. cereus F4810/72 and NVH 0075-95 WT and isogenic mutant strains. Morphological changes of Ptk6 cells were monitored over time using light microscopy. All diluted supernatants (1∶2) caused immediate epithelial cell rounding and detachment except for the plcR deletion mutant. Intact monolayer (green), cell rounding <50% (yellow), cell rounding >50% (orange) and 100% cell detachment (red) are indicated.
(DOC)
Characterization of sph deletion mutants, complemented and parental B. cereus strains. A. Hemolytic activity of B. cereus WT and isogenic mutant strains on Columbia agar (5% sheep blood, Oxoid). B. Colony morphology of WT and isogenic mutants on MYP (mannitol egg yolk polymyxin) agar indicating PC-PLC enzyme activity. C. Western blot analysis of SMase expression using a polyclonal anti-BcSMase antibody (1∶1000). Cells were grown in LB at 37°C and supernatants were harvested at similar OD600. Identical amounts (4 µg) of total protein preparations were separated on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. Lanes: 1, B. cereus NVH 0075-95 (WT); 2, nheB truncation and nheC deletion mutant strain of NVH 0075-95 (ΔnheBC); 3, sph deletion mutant of NVH 0075-95 (Δsph); 4, NVH 0075-95 Δsph comPsph, sph deletion harboring pAD/sph/Psph/tet; 5, NVH 0075-95 Δsph comPplc, sph deletion complemented via pAD/sph/Pplc/tet driving sph transcription from the operon promoter region Pplc-sph; 6, nheBC inactivation and sph deletion mutant of NVH 0075-95 (ΔnheBCΔsph); 7, NVH 0075-95 ΔnheBCΔsph comPsph and 8, NVH 0075-95 ΔnheBCΔsph comPplc.
(DOC)
Bacterial strains used in this study.
(DOC)
Plasmids and oligonucleotides used in this study.
(DOC)
Characteristics and toxin gene profiles of B. cereus strains used for cytotoxicity screening.
(DOC)
Acknowledgments
We thank Dr. Jun Sakurai and Dr. Masataka Oda for the very generous gift of the anti-SMase antibody. Special thanks go to Dr. Richard Dietrich and Dr. Erwin Märtlbauer for the kind gift of the anti-NheB antibody 1E11. We are grateful to Dr. Simon P. Hardy for providing the B. cereus NVH 0075-95 nheBC mutant, Dr. Genia Lücking for the B. cereus F4810/72 plcR deletion mutant and Dr. Elrike Frenzel, Romy Wecko, Christian Bolz, Andreas Schlitzer and Alexander Heiseke for their kind assistance with PC-PLC and SMase assay, reverse transcriptase PCR, FPLC and flow cytometric analysis. We thank all members of the Vogelmann lab and Ehling-Schulz’s Bacillus group for helpful suggestions and discussion.
Funding Statement
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) grant GRK 1482, in part by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn), project AiF 17506 N, the TUM Graduate School at Technische Universität München, Germany and the generous financial support from Roland M. Schmid, 2nd Dept. of Internal Medicine, Klinikum rechts der Isar, Munich and Siegfried Scherer, Lehrstuhl für Mikrobielle Ökologie, Department für Grundlagen der Biowissenschaften, Technische Universität München, Weihenstephan, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lund T, De Buyser ML, Granum PE (2000) A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol Microbiol 38: 254–261. [DOI] [PubMed] [Google Scholar]
- 2. Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, et al. (2005) Fatal family outbreak of Bacillus cereus-associated food poisoning. J Clin Microbiol 43: 4277–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bottone EJ (2010) Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev 23: 382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenfors Arnesen L, Fagerlund A, Granum P (2008) From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev 32: 579–606. [DOI] [PubMed] [Google Scholar]
- 5. Ehling-Schulz M, Fricker M, Scherer S (2004) Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res 48: 479–487. [DOI] [PubMed] [Google Scholar]
- 6. Christenson JC, Byington C, Korgenski EK, Adderson EE, Bruggers C, et al. (1999) Bacillus cereus infections among oncology patients at a children's hospital. Am J Infect Control 27: 543–546. [DOI] [PubMed] [Google Scholar]
- 7. Kelley JM, Onderdonk AB, Kao G (2012) Bacillus cereus septicemia attributed to a matched unrelated bone marrow transplant. Transfusion DOI 10 (1111/j.1537-2995.2012.03723) x. [DOI] [PubMed] [Google Scholar]
- 8. Walters JB, Ratcliffe NA (1983) Studies on the in vivo cellular reactions of insects: fate of pathogenic and non-pathogenic bacteria in Galleria mellonella nodules. J Insect Physiol 29: 417–424. [Google Scholar]
- 9. Fedhila S, Buisson C, Dussurget O, Serror P, Glomski I, et al. (2010) Comparative analysis of the virulence of invertebrate and mammalian pathogenic bacteria in the oral insect infection model Galleria mellonella . J Invertebr Pathol 103: 24–29. [DOI] [PubMed] [Google Scholar]
- 10. Mukherjee K, Altincicek B, Hain T, Domann E, Vilcinskas A, et al. (2010) Galleria mellonella as a model system for studying Listeria pathogenesis. Appl Environ Microbiol 76: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramarao N, Nielsen-Leroux C, Lereclus D (2012) The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J Vis Exp: e4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salamitou S, Ramisse F, Brehélin M, Bourguet D, Gilois N, et al.. (2000) The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology (Reading, England) 146 2825–2832. [DOI] [PubMed] [Google Scholar]
- 13. Tran SL, Guillemet E, Ngo-Camus M, Clybouw C, Puhar A, et al. (2011) Hemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell Microbiol 13: 92–108. [DOI] [PubMed] [Google Scholar]
- 14. Guillemet E, Cadot C, Tran SL, Guinebretière MH, Lereclus D, et al. (2010) The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J Bacteriol 192: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gohar M, Faegri K, Perchat S, Ravnum S, Okstad OA, et al. (2008) The PlcR virulence regulon of Bacillus cereus. PLoS One 3: e2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilmore MS, Cruz-Rodz AL, Leimeister-Wächter M, Kreft J, Goebel W (1989) A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: nucleotide sequence and genetic linkage. J Bacteriol 171: 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huseby M, Shi K, Brown C, Digre J, Mengistu F, et al. (2007) Structure and Biological Activities of Beta Toxin from Staphylococcus aureus . J Bacteriol 189: 8719–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oda M, Fujita A, Okui K, Miyamoto K, Shibutani M, et al. (2013) Bacillus cereus sphingomyelinase recognizes ganglioside GM3. Biochem Biophys Res Commun 431: 164–168. [DOI] [PubMed] [Google Scholar]
- 19. Oda M, Hashimoto M, Takahashi M, Ohmae Y, Seike S, et al. (2012) Role of Sphingomyelinase in Infectious Diseases Caused by Bacillus cereus . PLoS One 7: e38054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beecher DJ, Wong AC (2000) Cooperative, synergistic and antagonistic haemolytic interactions between haemolysin BL, phosphatidylcholine phospholipase C and sphingomyelinase from Bacillus cereus. Microbiology (Reading, England) 146 3033–3039. [DOI] [PubMed] [Google Scholar]
- 21. Guinebretiere MH, Broussolle V, Nguyen-The C (2002) Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains. J Clin Microbiol 40: 3053–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ehling-Schulz M, Guinebretiere MH, Monthan A, Berge O, Fricker M, et al. (2006) Toxin gene profiling of enterotoxic and emetic Bacillus cereus. FEMS Microbiol Lett 260: 232–240. [DOI] [PubMed] [Google Scholar]
- 23. Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindback T, Andersson M, et al. (2005) Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151: 183–197. [DOI] [PubMed] [Google Scholar]
- 24. Lindbäck T, Fagerlund A, Rødland MS, Granum P (2004) Characterization of the Bacillus cereus Nhe enterotoxin. Microbiology (Reading, England) 150: 3959–3967. [DOI] [PubMed] [Google Scholar]
- 25. Lindbäck T, Hardy SP, Dietrich R, Sødring M, Didier A, et al. (2010) Cytotoxicity of the Bacillus cereus Nhe enterotoxin requires specific binding order of its three exoprotein components. Infect Immun 78: 3813–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haug TM, Sand SL, Sand O, Phung D, Granum PE, et al. (2010) Formation of very large conductance channels by Bacillus cereus Nhe in Vero and GH(4) cells identifies NheA+B as the inherent pore-forming structure. J Membr Biol 237: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dietrich R, Moravek M, Bürk C, Granum PE, Märtlbauer E (2005) Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl Environ Microbiol 71: 8214–8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lund T, Granum PE (1996) Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol Lett 141: 151–156. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, and Russell D.W. (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 30. Whitehead RH, Robinson PS, Williams JA, Bie W, Tyner AL, et al. (2008) Conditionally immortalized colonic epithelial cell line from a Ptk6 null mouse that polarizes and differentiates in vitro . J Gastroenterol Hepatol 23: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658. [DOI] [PubMed] [Google Scholar]
- 32. Lücking G, Dommel MK, Scherer S, Fouet A, Ehling-Schulz M (2009) Cereulide synthesis in emetic Bacillus cereus is controlled by the transition state regulator AbrB, but not by the virulence regulator PlcR. Microbiology (Reading, England) 155: 922–931. [DOI] [PubMed] [Google Scholar]
- 33. Pezard C, Berche P, Mock M (1991) Contribution of individual toxin components to virulence of Bacillus anthracis . Infect Immun 59: 3472–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamada A, Tsukagoshi N, Udaka S, Sasaki T, Makino S, et al. (1988) Nucleotide sequence and expression in Escherichia coli of the gene coding for sphingomyelinase of Bacillus cereus . Eur J Biochem 175: 213–220. [DOI] [PubMed] [Google Scholar]
- 35. Pomerantsev AP, Kalnin KV, Osorio M, Leppla SH (2003) Phosphatidylcholine-specific phospholipase C and sphingomyelinase activities in bacteria of the Bacillus cereus group. Infect Immun 71: 6591–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ehling-Schulz M, Vukov N, Schulz A, Shaheen R, Andersson M, et al. (2005) Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus . Appl Environ Microbiol 71: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frenzel E, Doll V, Pauthner M, Lucking G, Scherer S, et al. (2012) CodY orchestrates the expression of virulence determinants in emetic Bacillus cereus by impacting key regulatory circuits. Mol Microbiol 85: 67–88. [DOI] [PubMed] [Google Scholar]
- 38. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 39. Oda M, Takahashi M, Matsuno T, Uoo K, Nagahama M, et al. (2010) Hemolysis induced by Bacillus cereus sphingomyelinase. Biochim Biophys Acta 1798: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 40. Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, et al. (2000) Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol 66: 5241–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duc l, Hong HA, Barbosa TM, Henriques AO, Cutting SM (2004) Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 70: 2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klein G (2011) Molecular characterization of the probiotic strain Bacillus cereus var. toyoi NCIMB 40112 and differentiation from food poisoning strains. J Food Prot 74: 1189–1193. [DOI] [PubMed] [Google Scholar]
- 43. Schierack P, Wieler LH, Taras D, Herwig V, Tachu B, et al. (2007) Bacillus cereus var. toyoi enhanced systemic immune response in piglets. Vet Immunol Immunopathol 118: 1–11. [DOI] [PubMed] [Google Scholar]
- 44. Fagerlund A, Lindbäck T, Storset AK, Granum P, Hardy SP (2008) Bacillus cereus Nhe is a pore-forming toxin with structural and functional properties similar to the ClyA (HlyE, SheA) family of haemolysins, able to induce osmotic lysis in epithelia. Microbiology (Reading, England) 154: 693–704. [DOI] [PubMed] [Google Scholar]
- 45. Didier A, Dietrich R, Gruber S, Bock S, Moravek M, et al. (2012) Monoclonal Antibodies Neutralize Bacillus cereus Nhe Enterotoxin by Inhibiting Ordered Binding of Its Three Exoprotein Components. Infect Immun 80: 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christie R, Atkins NE, Munch-Petersen E (1944) A note on a lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci 22: 197–200. [DOI] [PubMed] [Google Scholar]
- 47.Ehling-Schulz M, Messelhäuser U (2012) One pathogen but two different types of food borne outbreaks - Bacillus cereus in catering facilities in Germany. In: Hoorfar J, editor. Case studies in food safety and authenticity: Lessons from real-life situations. Cambridge, UK: Woodhead Publishing Ltd. 63–70. [Google Scholar]
- 48. Rasko DA, Webster DR, Sahl JW, Bashir A, Boisen N, et al. (2011) Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med 365: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moravek M, Dietrich R, Buerk C, Broussolle V, Guinebretière MH, et al. (2006) Determination of the toxic potential of Bacillus cereus isolates by quantitative enterotoxin analyses. FEMS Microbiol Lett 257: 293–298. [DOI] [PubMed] [Google Scholar]
- 50. Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, et al. (2006) Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus . J Biol Chem 281: 16157–16167. [DOI] [PubMed] [Google Scholar]
- 51. Minnaard J, Delfederico L, Vasseur V, Hollmann A, Rolny I, et al. (2007) Virulence of Bacillus cereus: a multivariate analysis. Int J Food Microbiol 116: 197–206. [DOI] [PubMed] [Google Scholar]
- 52. McDonel JL (1980) Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol Ther 10: 617–655. [DOI] [PubMed] [Google Scholar]
- 53. Walev I, Weller U, Strauch S, Foster T, Bhakdi S (1996) Selective killing of human monocytes and cytokine release provoked by sphingomyelinase (beta-toxin) of Staphylococcus aureus . Infect Immun 64: 2974–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tamura H, Noto M, Kinoshita K, Ohkuma S, Ikezawa H (1994) Inhibition of NGF-induced neurite outgrowth of PC12 cells by Bacillus cereus sphingomyelinase, a bacterial hemolysin. Toxicon 32: 629–633. [DOI] [PubMed] [Google Scholar]
- 55. Granum PE, Nissen H (1993) Sphingomyelinase is part of the 'enterotoxin complex' produced by Bacillus cereus . FEMS Microbiol Lett 110: 97–100. [DOI] [PubMed] [Google Scholar]
- 56. Beecher DJ, Olsen TW, Somers EB, Wong AC (2000) Evidence for contribution of tripartite hemolysin BL, phosphatidylcholine-preferring phospholipase C, and collagenase to virulence of Bacillus cereus endophthalmitis. Infect Immun 68: 5269–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crowell KM, Lutz F (1989) Pseudomonas aeruginosa cytotoxin: the influence of sphingomyelin on binding and cation permeability increase in mammalian erythrocytes. Toxicon 27: 531–540. [DOI] [PubMed] [Google Scholar]
- 58. Bashford CL, Alder GM, Menestrina G, Micklem KJ, Murphy JJ, et al. (1986) Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem 261: 9300–9308. [PubMed] [Google Scholar]
- 59. Ikezawa H, Mori M, Taguchi R (1980) Studies on sphingomyelinase of Bacillus cereus: hydrolytic and hemolytic actions on erythrocyte membranes. Arch Biochem Biophys 199: 572–578. [DOI] [PubMed] [Google Scholar]
- 60. Kolesnick RN, Goñi FM, Alonso A (2000) Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol 184: 285–300. [DOI] [PubMed] [Google Scholar]
- 61. Christie R, Atkins NE, Munch-Petersen E (144) A note on a lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci 22: 197–200. [DOI] [PubMed] [Google Scholar]
- 62. Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM (2011) Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One 6: e14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lang S, Palmer M (2003) Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem 278: 38167–38173. [DOI] [PubMed] [Google Scholar]
- 64. Nishiwaki H, Ito K, Otsuki K, Yamamoto H, Komai K, et al. (2004) Purification and functional characterization of insecticidal sphingomyelinase C produced by Bacillus cereus . Eur J Biochem 271: 601–606. [DOI] [PubMed] [Google Scholar]
- 65. Usui K, Miyazaki S, Kaito C, Sekimizu K (2009) Purification of a soil bacteria exotoxin using silkworm toxicity to measure specific activity. Microb Pathogenesis 46: 59–62. [DOI] [PubMed] [Google Scholar]
- 66. Fedhila S, Nel P, Lereclus D (2002) The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J Bacteriol 184: 3296–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kavanagh K, Reeves EP (2004) Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 28: 101–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cytotoxic effects of sterile B. cereus supernatants on IEC. Ptk6 cells were treated with B. cereus F4810/72 and NVH 0075-95 WT and isogenic mutant strains. Morphological changes of Ptk6 cells were monitored over time using light microscopy. All diluted supernatants (1∶2) caused immediate epithelial cell rounding and detachment except for the plcR deletion mutant. Intact monolayer (green), cell rounding <50% (yellow), cell rounding >50% (orange) and 100% cell detachment (red) are indicated.
(DOC)
Characterization of sph deletion mutants, complemented and parental B. cereus strains. A. Hemolytic activity of B. cereus WT and isogenic mutant strains on Columbia agar (5% sheep blood, Oxoid). B. Colony morphology of WT and isogenic mutants on MYP (mannitol egg yolk polymyxin) agar indicating PC-PLC enzyme activity. C. Western blot analysis of SMase expression using a polyclonal anti-BcSMase antibody (1∶1000). Cells were grown in LB at 37°C and supernatants were harvested at similar OD600. Identical amounts (4 µg) of total protein preparations were separated on a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. Lanes: 1, B. cereus NVH 0075-95 (WT); 2, nheB truncation and nheC deletion mutant strain of NVH 0075-95 (ΔnheBC); 3, sph deletion mutant of NVH 0075-95 (Δsph); 4, NVH 0075-95 Δsph comPsph, sph deletion harboring pAD/sph/Psph/tet; 5, NVH 0075-95 Δsph comPplc, sph deletion complemented via pAD/sph/Pplc/tet driving sph transcription from the operon promoter region Pplc-sph; 6, nheBC inactivation and sph deletion mutant of NVH 0075-95 (ΔnheBCΔsph); 7, NVH 0075-95 ΔnheBCΔsph comPsph and 8, NVH 0075-95 ΔnheBCΔsph comPplc.
(DOC)
Bacterial strains used in this study.
(DOC)
Plasmids and oligonucleotides used in this study.
(DOC)
Characteristics and toxin gene profiles of B. cereus strains used for cytotoxicity screening.
(DOC)