Abstract
Background
The level of disability and endurance of back muscles have been investigated, but there is conflicting evidence following specific exercise interventions for participants with recurrent low back pain (LBP). The purpose of this study was to compare the level of disability and slope of median frequency (MF) of thoracic and lumbar erector spinae (ES) muscles following core stabilization exercise (CSE) and spinal flexibility exercise (SFE).
Material/Methods
In total, 46 individuals participated in this study. There were 25 participants in the CSE intervention group (average age of 47.7±8.9 years) and 21 participants in the SFE group (average age of 53.1±9.0 years). Each group participated in the specific exercise intervention program for 4 weeks while maintaining their current activity and/or exercise levels. The Oswestry Disability Index (ODI) was used to measure the level of disability changes. The fatigability of the ES back muscles was measured by the slope of MF, using a modified Sorensen test.
Results
The disability level decreased significantly following CSE intervention (t=2.23, p<0.05). However, there was no significant difference in muscle fatigability changes in the 4-week intervention period for either group.
Conclusions
The CSE intervention reduced disability level following the 4-week intervention period. Further studies are needed to investigate the effectiveness of specific back muscle exercises in longer intervention periods for back muscle fatigability.
Keywords: electromyography, low back pain, fatigability, therapeutic exercises, median frequency
Background
Low back pain (LBP) results in increased financial burdens and is one of the most common health problems [1,2]. LBP is a common reason for seeking medical care, and the estimates of the direct cost burden in the United States vary considerably, with the most recent estimate at $86 billion in incremental health-care costs [3,4]. People with LBP often have reduced muscle strength and endurance, which may compromise the functional capacity of spinal stabilization and flexibility. However, reports regarding back muscle fatigability and disability level are controversial [5–9].
It has been reported that recurrent LBP is associated with altered motor coordination of the trunk muscles [10–12]. Although specific exercise programs as a treatment for LBP have been effective [13–15], most researchers fail to provide evidence based on the level of disability and fatigability, favoring one exercise over another, and few explain how exercise programs work. According to the Philadelphia Panel [16], this lack of evidence suggests the need for randomized, controlled trials to establish the effectiveness of exercise intervention. Understanding the efficacy of interventions might enhance the quality of treatments by using specific exercise interventions such as core stabilization exercises (CSE) and spinal flexibility exercise (SFE).
Core strengthening of trunk muscles includes the paraspinal and gluteal muscles in the back and the pelvic floor and hip girdle musculature [17,18]. The SFE stretch the low back muscles, increasing flexibility and decreasing stiffness in subjects with chronic LBP [19]. However, a controversy has arisen regarding the benefits of stretching, because of the perception that it prevents injury and may enhance performance [20].
Several studies also have identified a difference between easily fatigued erector spinae (ES) muscles and LBP based on fatigability tests [21–24]. However, no study has investigated level of disability and fatigability changes following specific therapeutic intervention in subjects with recurrent LBP. The patients with recurrent LBP possess different issues based on anatomical and functional aspects compared with chronic LBP, and often have a likelihood of reinjury as the prevalence rate of previous episodes of LBP increases [25–27].
The effect of stretching to increase hamstring flexibility in individuals with previous hamstring injury and uninjured control subjects was reported [28]; however, no support for the effectiveness of the intervention for functional improvement was found according to the results of pooled meta-analysis reports [16,29]. There was also a lack of investigation regarding back muscle endurance and trunk flexibility for the LBP group to improve function in activities of daily living.
A connection between fatigability by electromyography (EMG) and level of disability is clinically important for back muscle endurance to prevent further injuries. The difference in frequency of power spectrum for surface EMG following specific intervention would be applicable to evaluate localized muscular fatigue in a non-invasive fashion [7–9,30]. Therefore, our study was conducted in order to investigate the efficacy of exercise interventions based on the Oswestry Disability Index (ODI) and the slope of median frequency (MF) as the method for assessing muscle endurance.
Participants with LBP have less endurance and thus smaller MF during sustained muscle contractions [8,9,31,32]. The signal from surface EMG is the instantaneous algebraic summation of action potentials from muscle fibers, and its power spectrum can be estimated from a fast Fourier transform of the signal. The slope of MF of the EMG power spectrum is valuable as an alternative assessment tool to identify muscle fatigue [33,34]. However, there is a lack of research comparing this tool with randomized exercise interventions based on disability and fatigability changes.
It is important to monitor different regions of ES muscle fatigability changes for enhancing clinical applicability and specific rehabilitation strategies for participants with LBP. There is no data elucidating whether strengthening or stretching exercises are capable of altering physiologic characteristics, such as muscle fatigue, in participants with recurrent LBP. Therefore, the purpose of this study was to compare thoracic and lumbar parts of ES muscles following exercise interventions (CSE or SFE) and to investigate the level of disability and fatigability changes for participants with recurrent LBP.
Material and Methods
Selection of participants
Subjects were recruited from those who expressed interest in the study in the greater urban area of Cleveland, Ohio. The volunteers for this study were participants who presented with recurrent LBP, met study inclusion criteria, and experienced a disturbing impairment or abnormality in the functioning of the low back for more than a 2-month duration [35]. In our study, the Consolidated Standards of Reporting Trials (CONSORT) statement was used for the randomization process and allocation concealment to improve the quality of the scientific literature to avoid contamination of interventions [36].
Participants were able to participate if they: 1) were 21 years of age or older, 2) had recurrent LBP for more than 2 months without pain referral into the lower extremities, 3) reported pain as tolerated during exercise, and 4) had no structural deficits such as scoliosis, kyphosis, or spondylitis.
Participants were excluded from participation if they: 1) had a diagnosed psychological illness that might interfere with the study protocol, 2) had overt neurological signs (sensory deficits or motor paralysis), and/or 3) were pregnant. Participants were withdrawn from the study if they requested to withdraw. Those subjects who met study inclusion criteria received information about the study and signed a copy of the informed consent form approved by the Institutional Review Board at Cleveland State University.
The effect of dominance was also investigated based on the leg test, since the previous study confirmed that dominance side could be a confounding factor [37]. In the current study, the right lower extremity was regarded as the dominant side for all participants, as they preferred to use the right limb to kick a ball [38,39].
Based on the selection criteria, the participants’ demographics were compared. Forty-six participants participated in this study, and Table 1 indicates that 25 participants comprised the CSE intervention group (average age of 47.7±8.9 years); 21 participants were in the SFE group (average age of 53.1±9.1 years). Overall, there were no differences between groups in age, height, body weight or the months since pain onset.
Table 1.
Summary of subject demographics and bivariate relationship with selected demographics.
| Variable | CSE Group | SFE Group | Statistic | p |
|---|---|---|---|---|
| Number of subjects | 25 | 25 | ||
| Age (years) | ||||
| Range | 29–62 | 27–63 | t=–2.01 | p>0.05 |
| Mean ±SD | 47.7±8.9 | 53.1±9.1 | ||
| Gender | ||||
| Female | 5 | 10 | χ2=0.71 | p>0.05 |
| Male | 9 | 6 | ||
| Height (cm) | ||||
| Range | 155–187 | 159–184 | t=1.78 | p>0.05 |
| Mean ±SD | 172.8±7.5 | 167.8±7.8 | ||
| Body weight (kg) | ||||
| Range | 52–82 | 50–79 | t=1.68 | p>0.05 |
| Mean ±SD | 70.53±8.03 | 64.80±10.51 | ||
| Onset months | ||||
| Range | ||||
| Mean ±SD | 11.88±5.56 | 10.09±7.000 | t=0.96 | p>0.05 |
Data are given as Mean (± standard deviation) except where noted.
χ2 indicates Chi-square; df – degree of freedom; p – probability; N – Number of cases; CSE – core stabilization exercise; SFE – spinal flexibility exercise.
Disability level
Subject disability was inferred from self-reported scores on the Oswestry Low Back Pain Disability Index (ODI) [40]. The ODI is one of the most frequently used tools for measuring disability. A sum is calculated and presented as a percentage, where 0% represents no disability and 100% represents the worst possible disability.
EMG measurement
The EMG measurements were obtained from the participants and repeated under identical conditions after 4 weeks of intervention. In this study, the endurance of the back muscles was determined by using a modified version of the isometric fatigue test as originally introduced by Sorensen [31,37,41]. The participants, with EMG electrodes attached over the muscles of the low back, lay prone while lifting their trunks off the table and holding the position for 1 minute. The participants’ upper bodies were positioned with their iliac crests at the edge of the table; their lower bodies were secured at the ankles and hamstring level using seatbelt straps. Participants held their arms across their chests with each hand placed on the opposite shoulder while standard verbalized encouragement was given throughout the time-controlled 60-second test.
The EMG electrodes were placed bilaterally over the greatest convexity of the thoracic ES at the T10–T11 level and the lumbar ES muscles over the belly of the longissimus at the L4–L5 levels [42]. These electrode sites and the distance of the electrodes were carefully determined for each subject according to the method of Zipp [43].
The EMG signals were pre-amplified at the skin (gain 35×) and further amplified downstream (bandwidth 20–4000 Hz; model D-100 pre-amplifier and model ENG 55 driver amplifier, Therapeutics Unlimited, Iowa City, Iowa), with the total system adjusted for each subject to allow maximal amplification without saturation of the analogue-to digital converter. The digitized data were stored on computer disks for subsequent analysis.
Muscle fatigue measurements were evaluated on both the right and left sides for the ES muscles and were characterized by the MF of a fast Fourier transform (FFT) of the EMG data. Using standard FFT of the EMG data, the power spectrum for each 1-second time interval was obtained. The MF difference indicates local muscle fatigue during a sustained isometric contraction of the muscle [44]. The frequency content of the signal shifts to decreased frequencies as the amplitude of the recorded signals increases. This cross-talk across all electrode sites was carefully monitored and checked with the same methods used in our previous study [45].
EMG signals from the isometric fatigue test were transformed into their frequency spectrum using wavelet analysis. The MF was defined as the frequency that divided the spectrum into 2 equal areas [31]. The linear regression analyses were performed to calculate MF as a function of time. The initial MF was defined as the intercept of the regression line, and the slope of MF was determined as the slope of the regression line. The test method was repeated following the completion of the 4-week exercise program. In addition, this method assured that the testing conditions were the same for pre- and post-test sessions by the examiner, specifically the surface electrodes and trunk angle.
Exercise protocol
The participants were assigned to the CSE group or the SFE group and came into the lab once a week for 4 weeks, in addition to performing the exercises at home daily for 20 minutes. The randomization procedure was conducted by a computer program before recruitment began and was balanced to ensure equal allocation to either group. In addition, the examiner who collected data for all outcome measures was blinded to the participants’ group allocation. The participants were supervised in the lab in order to ensure that the exercises were performed correctly. To ensure adherence, the participants kept an exercise log and phone calls were made to each subject at least once a week. The intensity of the exercise was at the subject’s tolerance level, and the participants were encouraged to report any problems immediately.
The exercise protocol was conducted for approximately 20 minutes, and participants were required to hold each position for 5 seconds without emphasis on co-contraction of the abdominal muscles. Specific information regarding each exercise protocol was utilized to allow practical application 5 times a week for 2 sets of 15 repetitions performed as tolerated.
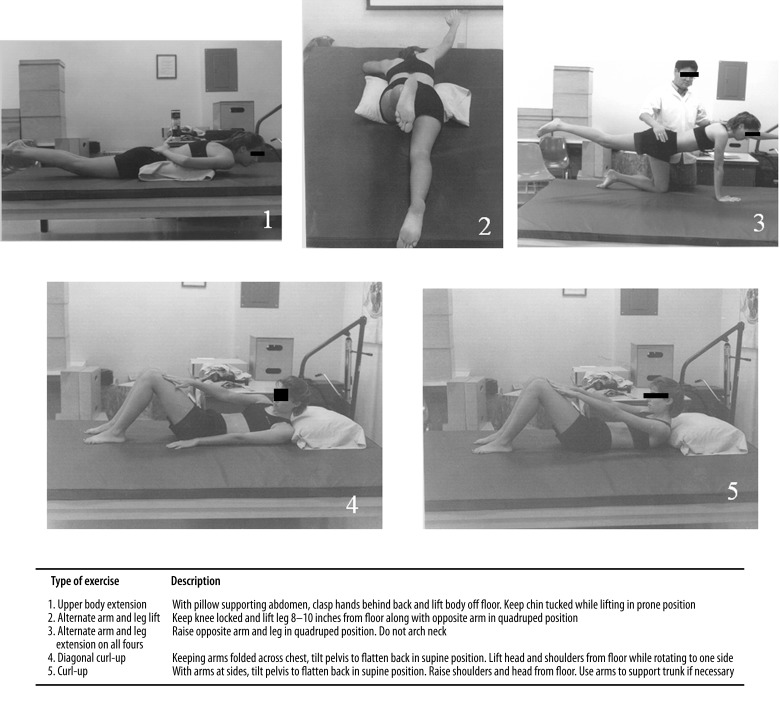
The CSE approach used in this study is commonly advocated in the rehabilitation of LBP patients [31,46]. The exercise program consisted of 5 different types of exercises (Figure 1): upper body extension in prone position, alternate arm and leg lift in quadruped position, alternate arm and leg lift in prone position, and diagonal curl-up and straight curl-up in supine positions. The quadruped exercise, for example, was performed from an all-fours position with the arms and legs extending reciprocally to recruit the trunk and hip extensors.
Figure 1.
The core stabilization exercise program.
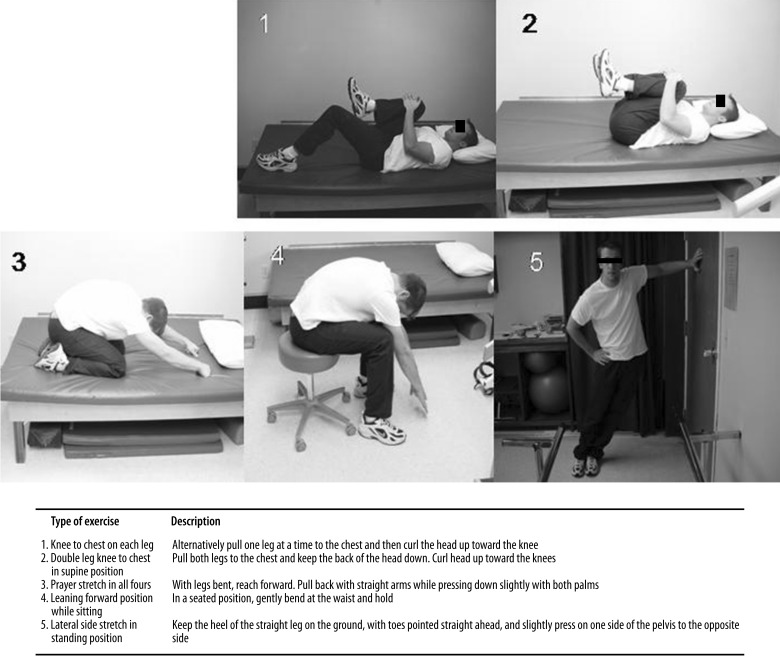
The SFE protocol included 5 exercises (Figure 2): knee to chest for each leg in supine position, double leg knee to chest in supine position, prayer stretch on all fours, leaning forward position while sitting, and lateral side stretch in standing position.
Figure 2.
The spinal flexibility exercise program.
Statistical analysis
Descriptive statistics were used to compare the mean and standard deviation of each muscle group as well as subject characteristics. The changes in slope of MF for the thoracic and lumbar ES muscles following interventions were compared by paired t-test. The differences between pre- and post-exercise interventions were also compared by the independent t-test. For all statistical tests, type I error rate was set at 0.05.
The preliminary power analyses that were conducted revealed that the sample sizes used were sufficient for analysis. Preliminary power analyses associated with comparing the 2 independent treatment groups, conducted under the assumptions of setting type I error rate at 0.05, with 2-tailed testing, and assuming effect sizes of 0.2, 0.4, 0.6, 0.7, 0.8, and 0.9, produced estimated power values of 0.10, 0.26, 0.50, 0.63, 0.75, and 0.84, respectively. Since these power estimates would be associated with follow-up tests of simple effects (e.g., exploring the nature of a treatment by time interaction for outcomes), these power estimates are potentially conservative. The analyses were conducted under the assumptions of setting a type I error rate at p<0.05.
Results
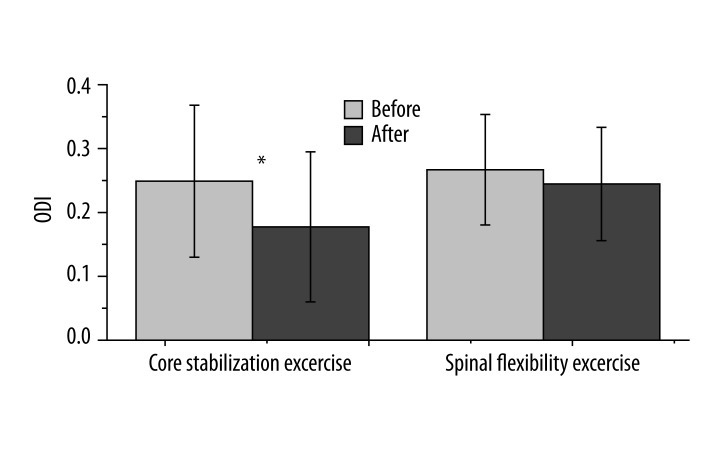
The level of disability was compared based on the ODI scores. As shown in Figure 4, the level of disability significantly decreased from 24.89±11.89 to 17.73±11.75 for the CSE group (t=2.23, p<0.05). The disability level was reduced by 28.8% following the exercise intervention. Following the SFE intervention, however, the disability level was reduced from 26.69±8.65 to 24.46±8.87 (t=–0.86, p>0.05), and the index was reduced by 8.3%.
Figure 4.
The Oswestry Disability Index (ODI) scale following two interventions. The ODI decreased significantly from 24.89±11.89 to 17.73±11.75 for the core stabilization exercise group (t=2.23, p=0.03). However, the ODI of the spinal flexibility exercise group changed from 26.69±8.65 to 24.46±8.87 following the intervention (t=–0.86, p=0.40).
In Table 2, the slopes of MF measurements were included for thoracic and lumbar ES muscles. Following CSE intervention, there were no changes on the right thoracic ES (t=0.01, p>0.05, 95% CI: −0.08 to 0.08), left thoracic ES (t=–0.25, p>0.05, 95% CI: −0.09 to 0.07), right lumbar ES (t=–0.31, p>0.05, 95% CI: −0.10 to 0.08), or left lumbar ES (t=–0.51, p>0.05, 95% CI: −0.15 to 0.09). Regarding the SFE intervention, there were no changes on the right thoracic ES (t=0.55, p>0.05, 95% CI: −0.07 to 0.12), left thoracic ES (t=–0.51, p>0.05, 95% CI: −0.08 to 0.05), right lumbar ES (t=0.67, p>0.05, 95% CI: −0.09 to 0.18), or left lumbar ES (t=–0.11, p>0.05, 95% CI: −0.16 to 0.14).
Table 2.
The slope of MF measurements for thoracic and lumbar ES muscles between pre- and post-intervention.
| CSE Pre | CSE Post | T | p | SFE Pre | SFE Post | T | p | |
|---|---|---|---|---|---|---|---|---|
| Right TE | –0.16 (0.03) | –0.17 (0.03) | 0.10 | 0.91 | –0.20 (0.03) | –0.25 (0.03) | 0.56 | 0.58 |
| Left TE | –0.20 (0.03) | –0.19 (0.03) | –0.25 | 0.80 | –0.21 (0.02) | –0.23 (0.03) | –0.52 | 0.61 |
| Right LE | –0.25 (0.04) | –0.27 (0.04) | –0.30 | 0.76 | –0.32 (0.04) | –0.36 (0.04) | 0.67 | 0.51 |
| Left LE | –0.27 (0.05) | –0.23 (0.05) | –0.51 | 0.61 | –0.33 (0.03) | –0.39 (0.04) | –0.11 | 0.91 |
Mean (± standard deviation); TE – thoracic erector spinae; LE – lumbar erector spinae; CSE – core stabilization exercise; SFE – spinal flexibility exercise.
Discussion
The purpose of this study was to compare fatigability changes for the thoracic and lumbar parts of the ES muscles following randomized trials (CSE or SFE) in participants with recurrent LBP.
The results of this study based on the ODI scale revealed that disability level decreased significantly following the CSE intervention. However, the ODI of the SFE group was reduced by 8.3% following the intervention. The minimal clinically important difference (MCID) is the smallest difference in a measured variable that signifies an important change in the management of the patient [47]. There is a real chance that a statistically significant result might be too small to be sensitive enough to detect a clinical difference. The problem of equating statistical significance with clinical importance is that it ignores the possibility that a statistically significant result may not always be clinically relevant.
In this regard, the 28.8% reduction of disability level following the CSE intervention is important for a range of commonly used back pain outcome measures. A moderate disability level was reported before the CSE intervention, and the level changed to minimal disability after the 4-week period. Although a 30% change from baseline could be a clinically meaningful improvement, this finding indicated a valuable disability reduction when comparing before and after measures for individual patients [48].
The EMG signal phenomena are referred to as myoelectric manifestations of fatigue [31]. The exercise intervention after 4 weeks was not enough to decrease back muscle fatigability; however, the part of the back muscles based on thoracic and lumbar regions should be considered carefully for future back muscle fatigability studies.
The muscle endurance following the CSE increased, and there was no significant difference between interventions. However, these changes were significantly different in the part of the low back muscles. The slope of MF measurements for the thoracic and lumbar ES muscles needs to be considered following the interventions. The MF slope decayed less in the CSE group than in the SFE group, indicating increased endurance following the CSE. The slope of MF in our study demonstrated no statistically significant difference following treatments; however, the effect may be clinically valuable.
However, during a fatiguing trunk extension exercise, an increase in the lumbar paraspinal EMG signal occurs up to approximately 55% of maximum fatigue, at which point a decrease in EMG is observed [49]. Although the results of our study indicated no differences following exercise intervention, the back muscles allow for continuation of the exercise.
Measuring different parts of the lumbar ES muscles is essential for understanding the anatomical and biomechanical characteristics of the trunk muscles [45]. The results of this interaction indicated that the combined effects based on muscles following the intervention were discernibly larger and significant among those explanatory variables. According to Janda, the ES muscles stiffen more than the abdominal muscles in participants with LBP [50]. The attachment of the lumbar ES muscles, rather than the thoracic ES muscles, results in an effective lever arm for lumbar stabilization. Therefore, the lumbar ES muscle is more effective in creating a stabilizing moment over the lumbar vertebral segments during the test.
One of the limitations in this study was the selection of the group, which included ‘volunteers’. The small sample size may have potential limitations, and increasing the number of participants would serve to increase the power of the study. Another limitation was that the individual exercise programs might be different within a selected group. It would be reasonable to provide stabilization exercises for patients who have evidence of instability, and flexibility exercises should be prescribed for patients who have limited range of motion/muscular restrictions. However, there was no evidence that flexibility or stretching exercises improve muscle fatigability, although the exercises were randomly provided to compare effectiveness of the intervention.
In addition, the variability between the results of the electrode placement might be important. The crosstalk and signal fidelity across all electrode sites was carefully monitored and checked on the back muscles with the sensitivity of the measurement. It would be beneficial to quantify inter- and intra-subject variability with subgroup analyses, which can increase efficacy of the test. In our data, differences in baseline values for dependent measures were carefully analyzed for within and between group statistical analyses. Despite these limitations, the quantification of the fatigability test was valuable.
It is important for future studies to have longer exercise intervention periods in order to differentiate the outcomes of the musculoskeletal system. Future studies should also include a larger sample size in order to better generalize the results. Follow-up, randomized controlled trials to more fully investigate treatment effects and factors that might mediate these effects should be pursued.
Conclusions
The level of disability improved following the CSE intervention, but there was no statistically significant difference in the SFE group. Since the 4-week exercise intervention was insufficient to improve back muscle fatigability, further randomized controlled studies are needed to investigate the effectiveness of exercise interventions over longer time periods for neuromuscular rehabilitation training.
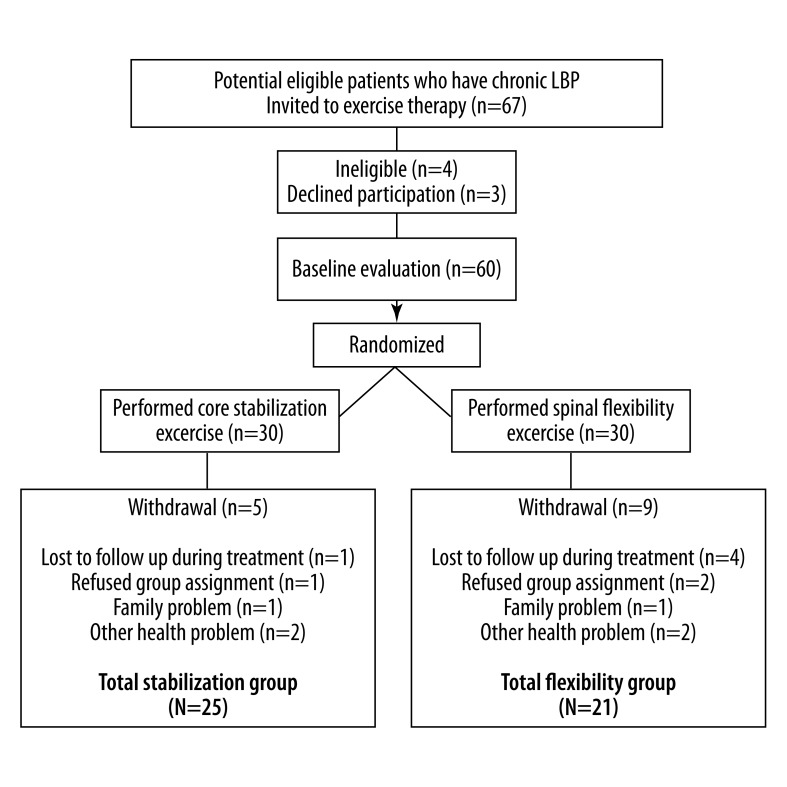
Figure 3.
Flow diagram depicting how the treatment group was formed as well as group membership and number of drop-outs throughout the course of the study.
Footnotes
Source of support: This work was supported by Cleveland State University, Korea University and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0003015)
References
- 1.Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382–96. doi: 10.2106/00004623-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Carlson C. Axial back pain in the athlete: pathophysiology and approach to rehabilitation. Curr Rev Musculoskelet Med. 2009;2(2):88–93. doi: 10.1007/s12178-009-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Ivanova JI, et al. Real-world practice patterns, health-care utilization, and costs in patients with low back pain: the long road to guideline-concordant care. Spine J. 2011;11(7):622–32. doi: 10.1016/j.spinee.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Granata KP, Slota GP, Wilson SE. Influence of fatigue in neuromuscular control of spinal stability. Hum Factors. 2004;46(1):81–91. doi: 10.1518/hfes.46.1.81.30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coorevits PL, et al. Statistical modelling of fatigue-related electromyographic median frequency characteristics of back and hip muscles during a standardized isometric back extension test. J Electromyogr Kinesiol. 2005;15(5):444–51. doi: 10.1016/j.jelekin.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Vuillerme N, Anziani B, Rougier P. Trunk extensor muscles fatigue affects undisturbed postural control in young healthy adults. Clin Biomech (Bristol, Avon) 2007;22(5):489–94. doi: 10.1016/j.clinbiomech.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Sung PS, Zurcher U, Kaufman M. Gender differences in spectral and entropic measures of erector spinae muscle fatigue. J Rehabil Res Dev. 2008;45(9):1431–39. [PubMed] [Google Scholar]
- 9.Johanson E, et al. The effect of acute back muscle fatigue on postural control strategy in people with and without recurrent low back pain. Eur Spine J. 2011;20(12):2152–59. doi: 10.1007/s00586-011-1825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao H, et al. Motor training of the lumbar paraspinal muscles induces immediate changes in motor coordination in patients with recurrent low back pain. J Pain. 2010;11(11):1120–28. doi: 10.1016/j.jpain.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Hodges PW. Core stability exercise in chronic low back pain. Orthop Clin North Am. 2003;34(2):245–54. doi: 10.1016/s0030-5898(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Hodges PW, Moseley GL. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13(4):361–70. doi: 10.1016/s1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 13.Abenhaim L, et al. The role of activity in the therapeutic management of back pain. Report of the International Paris Task Force on Back Pain. Spine (Phila Pa 1976) 2000;25(4 Suppl):1–33S. doi: 10.1097/00007632-200002151-00001. [DOI] [PubMed] [Google Scholar]
- 14.van Tulder MW, et al. Behavioral treatment for chronic low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine. 2001;26(3):270–81. doi: 10.1097/00007632-200102010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Rhee HS, Kim YH, Sung PS. A randomized controlled trial to determine the effect of spinal stabilization exercise intervention based on pain level and standing balance differences in patients with low back pain. Med Sci Monit. 2012;18(3):CR174–89. doi: 10.12659/MSM.882522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions for low back pain. Phys Ther. 2001;81(10):1641–74. [PubMed] [Google Scholar]
- 17.Nadler SF, et al. Hip muscle imbalance and low back pain in athletes: influence of core strengthening. Med Sci Sports Exerc. 2002;34(1):9–16. doi: 10.1097/00005768-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Richardson CA, et al. Therapeutic exercise for spinal segmental stabilization in low back pain. London: Harcourt Publishers; 1999. [Google Scholar]
- 19.Rainville J, et al. Exercise as a treatment for chronic low back pain. Spine J. 2004;4(1):106–15. doi: 10.1016/s1529-9430(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins J, Beazell J. Flexibility for runners. Clin Sports Med. 2010;29(3):365–77. doi: 10.1016/j.csm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.McGill SM. Low back exercises: evidence for improving exercise regimens. Phys Ther. 1998;78(7):754–65. doi: 10.1093/ptj/78.7.754. [DOI] [PubMed] [Google Scholar]
- 22.Mayer TG, et al. Lumbar myoelectric spectral analysis for endurance assessment. A comparison of normals with deconditioned patients. Spine. 1989;14(9):986–91. doi: 10.1097/00007632-198909000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Klein AB, et al. Comparison of spinal mobility and isometric trunk extensor forces with electromyographic spectral analysis in identifying low back pain. Physical Therapy. 1991;71(6):445–54. doi: 10.1093/ptj/71.6.445. [DOI] [PubMed] [Google Scholar]
- 24.Lee T, Kim YH, Sung PS. A comparison of pain level and entropy changes following core stability exercise intervention. Med Sci Monit. 2011;17(7):CR362–68. doi: 10.12659/MSM.881846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson CP, Brown MD. Is there a role for exercise in the treatment of patients with low back pain? Clin Orthop. 1983;(179):39–45. [PubMed] [Google Scholar]
- 26.Lee TR, Kim YH, Sung PS. Spectral and entropy changes for back muscle fatigability following spinal stabilization exercises. J Rehabil Res Dev. 2010;47(2):133–42. doi: 10.1682/jrrd.2009.07.0088. [DOI] [PubMed] [Google Scholar]
- 27.Stanton TR, et al. How do we define the condition ‘recurrent low back pain’? A systematic review. Eur Spine J. 2010;19(4):533–39. doi: 10.1007/s00586-009-1214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Sullivan K, Murray E, Sainsbury D. The effect of warm-up, static stretching and dynamic stretching on hamstring flexibility in previously injured subjects. BMC Musculoskelet Disord. 2009;10:37. doi: 10.1186/1471-2474-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elnaggar IM, et al. Effects of spinal flexion and extension exercises on low-back pain and spinal mobility in chronic mechanical low-back pain patients. Spine. 1991;16(8):967–72. doi: 10.1097/00007632-199108000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Hatzikotoulas K, et al. Muscle fatigue and electromyographic changes are not different in women and men matched for strength. Eur J Appl Physiol. 2004;92(3):298–304. doi: 10.1007/s00421-004-1095-4. [DOI] [PubMed] [Google Scholar]
- 31.Sung PS. Multifidi muscles median frequency before and after spinal stabilization exercises. Arch Phys Med Rehabil. 2003;84(9):1313–18. doi: 10.1016/s0003-9993(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 32.Elfving B, Dedering A, Nemeth G. Lumbar muscle fatigue and recovery in patients with long-term low-back trouble – electromyography and health-related factors. Clin Biomech (Bristol, Avon) 2003;18(7):619–30. doi: 10.1016/s0268-0033(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 33.Roy SH, et al. Classification of back muscle impairment based on the surface electromyographic signal. J Rehabil Res Dev. 1997;34(4):405–14. [PubMed] [Google Scholar]
- 34.Mannion AF, et al. The use of surface EMG power spectral analysis in the evaluation of back muscle function. J Rehabil Res Dev. 1997;34(4):427–39. [PubMed] [Google Scholar]
- 35.Klenerman L, et al. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine. 1995;20(4):478–84. doi: 10.1097/00007632-199502001-00012. [DOI] [PubMed] [Google Scholar]
- 36.Begg C, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. Jama. 1996;276(8):637–39. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 37.Sung PS, Spratt KF, Wilder DG. A possible methodological flaw in comparing dominant and nondominant sided lumbar spine muscle responses without simultaneously considering hand dominance. Spine. 2004;29(17):1914–22. doi: 10.1097/01.brs.0000137071.47606.19. [DOI] [PubMed] [Google Scholar]
- 38.Brophy R, et al. Gender influences: the role of leg dominance in ACL injury among soccer players. Br J Sports Med. 2010;44(10):694–97. doi: 10.1136/bjsm.2008.051243. [DOI] [PubMed] [Google Scholar]
- 39.Andersen TE, et al. Video analysis of the mechanisms for ankle injuries in football. Am J Sports Med. 2004;32(1 Suppl):69–79S. doi: 10.1177/0363546503262023. [DOI] [PubMed] [Google Scholar]
- 40.Fairbank JC, et al. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271–73. [PubMed] [Google Scholar]
- 41.Sung PS, Zurcher U, Kaufman M. Reliability difference between spectral and entropic measures of erector spinae muscle fatigability. J Electromyogr Kinesiol. 2010;20(1):25–30. doi: 10.1016/j.jelekin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Lariviere C, et al. Evaluation of measurement strategies to increase the reliability of EMG indices to assess back muscle fatigue and recovery. J Electromyogr Kinesiol. 2002;12(2):91–102. doi: 10.1016/s1050-6411(02)00011-1. [DOI] [PubMed] [Google Scholar]
- 43.Zipp P. Recommendations for the standardization of lead positions in surface electromyography. Eur J Appl Physiol. 1982;50:41–54. [Google Scholar]
- 44.Wilder DG, Pope MH, Frymoyer JW. The biomechanics of lumbar disc herniation and the effect of overload and instability. J Spinal Disord. 1988;1(1):16–32. [PubMed] [Google Scholar]
- 45.Sung PS, Lammers AR, Danial P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009;9(2):115–20. doi: 10.1016/j.spinee.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 46.O’Sullivan P, et al. Altered patterns of abdominal muscle activation in patients with chronic low back pain. Aust J Physiother. 1997;43(2):91–98. doi: 10.1016/s0004-9514(14)60403-7. [DOI] [PubMed] [Google Scholar]
- 47.Wu CY, et al. Responsiveness, minimal detectable change, and minimal clinically important difference of the Nottingham Extended Activities of Daily Living Scale in patients with improved performance after stroke rehabilitation. Arch Phys Med Rehabil. 2011;92(8):1281–87. doi: 10.1016/j.apmr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Ostelo RW, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33(1):90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 49.Clark BC, Manini TM, Ploutz-Snyder LL. Derecruitment of the lumbar musculature with fatiguing trunk extension exercise. Spine (Phila Pa 1976) 2003;28(3):282–87. doi: 10.1097/01.BRS.0000042227.06526.A2. [DOI] [PubMed] [Google Scholar]
- 50.Janda V. Muscles, central nervous motor regulation and back problems. In: Korr IM, editor. The Neurologic Mechanisms in Manulative Therapy. New York: Plenum Press; 1978. [Google Scholar]