Abstract
Background
Citrulline is an amino acid produced by enterocytes. Serum citrulline concentration has been proposed as a marker of enterocyte mass and function. Our study focused on evaluation of citrulline levels in patients with diarrhea related to toxic intestinal damage (mucositis), intestinal graft versus host disease (GVHD), and other etiology of diarrhea (e.g., dysmicrobia) after allogeneic stem cells transplantation (SCT).
Material/Methods
This was a prospective study in 11 adults (18 blood samples) with diarrhea developed after allogeneic SCT in 4/2011–1/2012 compared to twenty healthy control samples.
Results
The median (interquartile range) of citrulline levels was significantly lower in the transplanted patients group compared to healthy controls: 9.3 (3.62–15.38) vs. 33.3 (26.82–36.23) μmol/L, p<0.0001. The median values of citrulline in patients with post-transplant toxic intestinal mucositis (n=8, days 1–22 post-transplant) vs. intestinal GVHD (n=7, day 43–142) vs. other etiology of diarrhea (e.g., dysmicrobia) (n=3, day 120–127) were: 9.55 (2.95–12.03) vs. 5 (3.85–9.05) vs. 15.6 (15.45–18.3) μmol/L resp. Serum citrulline levels were significantly higher in other (eg, dysmicrobic) etiology of diarrhea in comparison with mucositis (p=0.0336) and GVHD (p=0.0152).
Conclusions
Citrulline levels are very low shortly after the myeloablative FLU/MEL or BuCY2 conditioning allogeneic SCT due to the toxic intestinal damage. Significantly low levels of citrulline were also in patients with intestinal GVHD later on. Other observations in larger groups of patients are necessary before any specific recommendation for citrulline levels monitoring in intestinal GVHD can be made.
Keywords: citrulline, transplantation, enterocytes, mucositis, graft versus host disease
Background
Citrulline is an amino acid produced by enterocytes of the small bowel; plasma citrulline concentration has been proposed as a marker of enterocyte mass and function [1]. Citrulline concentration appears to be a quantitative parameter that is independent of the underlying causes of epithelial cell loss such as surgical resection, cellular rejection after small bowel transplantation, and celiac and non-celiac disease, and provides an objective parameter for cancer treatment-related gut toxicity [1–6]. Very low citrulline concentrations were observed after allogeneic and autologous hematopoietic stem cells transplantation (SCT). Citrulline-based assessment of intestinal damage has been shown to be an objective and reliable marker in these patients [6–12]. Plasma citrulline is also a reliable biomarker of enterocyte functional mass in HIV patients [13]. On the other hand, citrulline plasma level monitoring has not yet become a standard approach used in oncological treatment-induced intestinal toxicity assessment and there is still little information about it in allogeneic stem cells transplantation patients, especially in relation to graft versus host disease (GVHD) [14].
Our observational study was focused on determining the feasibility of citrulline levels evaluation in patients with diarrhea related to toxic intestinal damage (mucositis), intestinal graft versus host disease (GVHD), and other etiology of diarrhea (e.g., dysmicrobia) after allogeneic stem cells transplantation with toxic myeloablative conditioning regimens.
Material and Methods
This was a single-centre prospective and observational study in 11 adult patients (total 18 blood samples collected at different time points) with diarrhea developed after allogeneic hematopoietic SCT in 4/2011-1/2012. Repeated sampling was indicated in reoccurrence of diarrhea. The median (interquartile range) age of the patients was 55 (44–62) years, male to female ratio 8/3, and the main hematological diagnoses covered were: acute myeloid leukemia (AML) 4, myelodysplasia (MDS) 3, chronic lymphocytic leukemia (CLL) 1, acute lymphoblastic leukemia (ALL) 1, multiple myeloma (MM) 1 and non-Hodgkin lymphoma (NHL) 1. This patient cohort was compared to 20 healthy controls (blood donors).
The allogeneic SCT conditioning regimens were BuCY2 (busulphan total dose 16 mg/kg, cyclophosphamide total dose 120 mg/kg) in 3/11 patients and the FLU/MEL (fludarabine total dose 120 mg/m2, melphalan 140 mg/m2) in 8/11 patients. Patients were given the standard systemic antimycotic, antibacterial and antiviral prophylaxis (fluconazole, quinolones, and acyclovir). The study was observational and study-specific signed informed consent and Ethics Committee approval was not necessary because there was no medical intervention and data were used anonymously.
The serum samples of the patients were collected prospectively, processed within 2 hours after collection and stored frozen at −80°C until batch-wise analysis was performed. For determination of citrulline, a spectrophotometric kit (L-Citrulline Kit, Immundiagnostik AG, Bensheim, Germany) was used, where the principle of determination is based on the reaction of citrulline with diacetylmonoxime. The interference of reaction byproducts is reduced by thiosemicarbazide treatment [15]. The sample preparation and analytic procedure were performed according to the instructions of the manufacturer. In comparison with the HPLC methods, this spectrophotometric kit can be widely used because of its low demands on laboratory equipment and personal experience.
Glomerular filtration rate was estimated (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation, 4 parametric and isotope dilution mass spectrometry (IDMS) traceable version [16].
The monitoring, assessment and definitions
The blood samples were collected in the morning and several characteristics and variables were recorded on the day of sample collection: age, sex, diagnosis, pretransplant conditioning regimen, number of days since the transplantation, number of diarrhea stools in the day of the sample collection, blood leukocytes count, serum creatinine levels, microbiology and Clostridium difficile toxin analysis, presence or absence of graft versus host disease (GVHD), the most likely etiology of the diarrhea (post-transplant toxicity – intestinal mucositis, GVHD or other (e.g., dysmicrobia). Dysmicrobia was defined as changes in delicate equilibrium in composition of microflora due to the administration of broad-spectrum antibiotics. Diarrhea was defined as a discharge of semisolid or fluid fecal matter from the bowel, and its severity was characterized by the total number of stool per day.
Statistics
All data were analyzed using R (R Core Team, 2012 http://www.R-project.org/; version 2.15.1), packages nlme (J. Pinheiro, D. Bates, S. DebRoy, D. Sarkar and the R Development Core Team, 2012; version 3.1-104), knitr (Y. Xie, 2012; version 0.6.3) and RStudio IDE (version 0.96.304). If not stated otherwise, descriptive statistics is expressed as median (interquartile range). Count data were compared with the Fisher’s exact test, and for univariate unpaired comparisons Wilcoxon rank sum test with continuity correction was used. Differences among serum citrulline levels in analyzed groups were computed using linear mixed effects model fit by REML. As fixed effect, diarrhea etiology was included. We checked for normality and homogeneity by visual inspections of plots of standardized residuals against fitted values. P values <0.05 were considered as statistically significant differences.
Results
There was no difference in the median age [55 years (44–62) vs. 43 years (39–54), p=0.13] and male/female ratio (8/3 vs. 18/2; p=0.32) in the transplanted patients vs. control group. Characteristics of the groups and the blood samples are shown in detail in Table 1. No patient had toxin Clostridium difficile positive enterocolitis.
Table 1.
Characteristics of the patients and blood samples. If not stated otherwise, data are expressed as median (interquartile range).
| transplanted patients (n=11) | healthy controls (n=20) | p | |
|---|---|---|---|
| No. of evaluated samples | 18 | 20 | – |
| Serum creatinine (μmol/L) | 74.00 (63.25–103.5) | – | – |
| Glomerular filtration (mL/s) | 1.48 (1.06–1.9) | – | – |
| No. of days post-transplant | 49.00 (15.00–115.0) | – | – |
| No. of stools per day | 8.50 (6.00–10.8) | – | – |
| Serum citrulline (μmol/L) | 9.30 (3.62–15.38) | 33.3 (26.8–36.2) | <0.0001 |
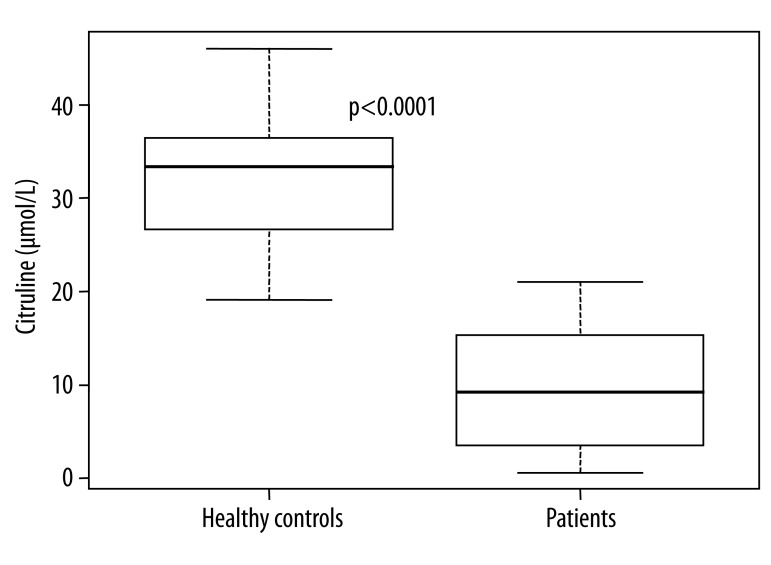
The median value of citrulline levels was significantly lower in the transplanted patients group compared to the healthy controls: 95% confidence interval of median difference was 16.5–26.2 μmol/L (Table 1 and Figure 1).
Figure 1.
Citrulline levels (μmol/L) in the transplanted patients and healthy controls.
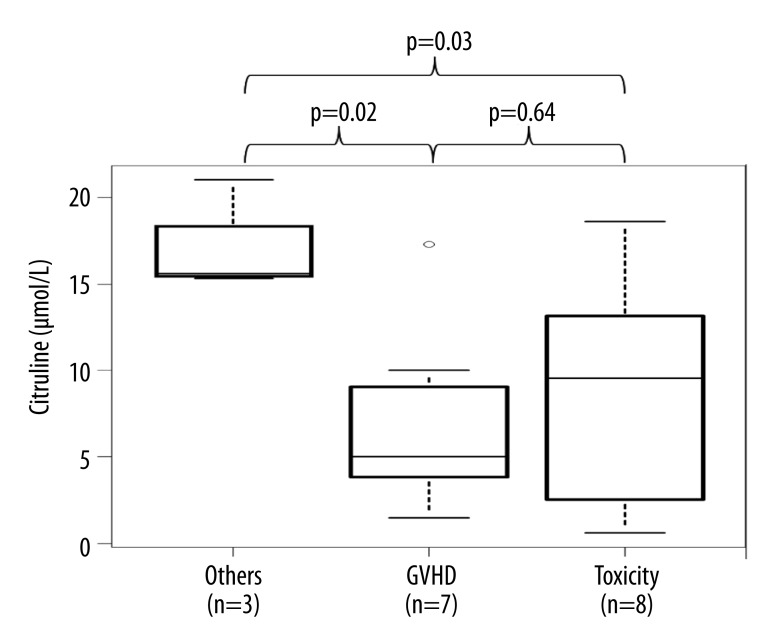
The median values of citrulline levels in patients with post-transplant toxic intestinal damage (mucositis) (n=8, day 1–22 post-transplant) vs. intestinal GVHD (n=7, day 43–142) vs. other etiology of diarrhea (dysmicrobia) (n=3, day 120–127) were: 9.55 (2.95–12.03) vs. 5 (3.85–9.05) vs. 15.6 (15.45–18.3) μmol/L resp. (Figure 2). Serum citrulline levels were significantly higher in other etiology of diarrhea (dysmicrobia) in comparison with mucositis (p=0.0336, CI=–17.6 to 0.84) and GVHD (p=0.0152, CI=–18.7 to −2.4). However, differences between serum citrulline concentration in patients with diarrhea caused by mucositis and GVHD were not significantly different (p=0.6359, CI=–4.74 to 7.46).
Figure 2.
Citrulline levels in patients with toxic intestinal damage (mucositis) vs. intestinal graft versus host disease vs. other etiology of diarrhea (dysmicrobia).
Discussion
Citrulline is an amino acid produced by enterocytes. It is considered a marker of small intestine enterocytes mass, independent of the underlying cause for epithelial cell loss [1–7,9]. Citrulline plasma level monitoring, however, has not yet become a standard approach used in oncological treatment-induced intestinal toxicity assessment. There is still little information about it in allogeneic stem cells transplantation patients, especially in relation to graft versus host disease (GVHD) [6–9,12,14]. Thus, we decided to conduct this observation focused on evaluation of the feasibility of citrulline level evaluation in patients after allogeneic stem cells transplantation, with diarrhea related to toxic intestinal mucositis shortly after the transplantation or with intestinal GVHD later on. Citrulline levels were compared between 11 patients (18 blood samples) and a control group of 20 healthy blood transfusion donors (20 samples).
In humans, citrulline plasma concentration is about 40 μmol/L and the threshold for establishing a diagnosis of intestinal failure is 10 μmol/L in villous atrophy coeliac disease – citrulline level <10 μmol/L is considered predictive of total villous atrophy, citrulline level 10–20 μmol/L is predictive of proximal only total or subtotal villous atrophy and >20 μmo/L is indicating only partial villous atrophy [2,5]. In a cohort of hemato-oncological patients after stem cells transplantation in Lutgens et al. [7], the mean level of citrulline at the start of the conditioning regimen was 25.7 μmol/L, decreased to the lowest citrulline concentration after transplantation on day 5 with the mean 8.9 μmol/L, and the concentration no longer differed from the baseline value at day 21 post-transplant. In Velden et al. [8] the myeloablative conditioning regimen led to rapid decline in citrulline levels <10 μmol/L, mean nadir of citrulline was 4.5–7.0 μmol/L and hypocitrullinemia lasted for more than one week in most patients. Herbers et al. [9] performed monitoring in 94 allogeneic or autologous haematopoietic stem-cell transplant recipients after the start of myeloablative chemotherapy until 30 days thereafter and found that significant decrease was seen immediately after the start of myeloablative therapy, with citrulline reaching 10 μmol/L around day 9, and – depending on specific toxicity of the regimen – a nadir was reached 14–17 days after the start of the conditioning regimen and citrulline <10 μmol/L lasted for 16–21 days [9].
In our patients with toxic intestinal damage and blood samples taken shortly after the transplantation on days 1–22 post-transplant, the low levels of citrulline corresponded with the findings of the above mentioned authors. Significantly low citrulline levels (<10 μmol/L) were observed also in a group of our patients considered as suffering with intestinal GVHD damage on days 43–142 post-transplant. Because citrulline levels return back to pre-transplant baseline by 21–30 days after the transplantation [7,8], we assume that our results and observation reflect the intestinal GVHD damage rather than unusually prolonged toxic intestinal post-transplant mucositis. To verify this, however, matched-pair analysis of patients with equal time after the transplantation with the same conditioning regimen and differing only with presence and absence of intestinal GVHD should be performed to verify the findings.
Lower citrulline levels were observed also in patients with other etiology of diarrhea – i.e. dysmicrobia and with laboratory samples taken late post-transplant on days 120–127 and this was statistically significantly different compared to the healthy controls: median 15.6 (15.45–18.3) vs. 33.3 (26.82–36.23) μmol/L, p=0.009 (CI=6.2–22.6).
Citrulline, as a small molecule (amino acid), is freely filtered by renal glomerulus and subsequently reabsorbed and metabolized in the proximal tubule. That is why compromised renal function is an important factor when considering plasma citrulline levels as a marker of intestinal failure, as this potentially can increase circulating citrulline values [5]. On the other hand, the biomarker is not significantly influenced by nutritional or inflammatory status [5]. In our setting, no significant correlation between eGFR and citrullin was observed (Spearman’s rank correlation, rho=0.07, p=0.79). This observation suggests that in our pilot study, eGFR was not an important factor that would add any information to the interpretation of citrulline levels.
We agree with Herbers et al. [9], who stated that for clinical purposes a scoring system on the basis of absolute citrulline values seems practical for determining intestinal mucosal barrier damage. However, the reliability of these results suffers from absence of a “gold standard” for type of intestinal involvement diagnosis and potential confounding of citrulline levels by GFR. We agree that knowledge of transplantation regimens that induce long-term citrullinaemia <10 μmol/L may help define adequate parenteral nutrition and antimicrobial medication approaches (in low-risk regimens without prolonged decreased citrullinaemia, parenteral nutrition can promote villous atrophy, increase intestinal permeability, and enhance bacterial translocation [9]. In our study, there was prolonged recovery of citrulline plasma levels in 1 individually and repeatedly monitored patient with toxic intestinal damage and later on with intestinal GVHD, and his citrulline levels were: 3.4 μmol/L on day 1 post-transplant (mucositis); 1.6 μmol/L on day 20 (mucositis); 5.0 μmol/L on day 89 (GVHD); 15.6 μmol/L on day 127 (other etiology of diarrhea [dysmicrobia]) and 19.5 μmol/L on day 189 post-transplant (without diarrhea).
Conclusions
The citrulline serum level monitoring is feasible. Citrulline levels are very low shortly after the myeloablative FLU/MEL or BuCY2 conditioning allogeneic stem cell transplantation due to toxic intestinal damage. Low levels of citrulline were also observed later on in patients with intestinal GVHD. Other observations in larger groups of patients are necessary before any specific recommendation for citrulline level monitoring in intestinal GVHD can be made.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Supported by the Ministry of Health, Czech Republic for conceptual development of research organization 00669806 – Faculty Hospital in Pilsen, Czech Republic
References
- 1.Crenn P, Coudray C, Thuillier F, et al. Postabsorptive plasma citrulline concentration is a marker of absorptive enterocyte mass and intestinal failure in humans. Gastroenterology. 2000;119:1496–505. doi: 10.1053/gast.2000.20227. [DOI] [PubMed] [Google Scholar]
- 2.Crenn P, Vahedi K, Lavergne Slove A, et al. Plasma citrulline: a marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology. 2003;124:1210–19. doi: 10.1016/s0016-5085(03)00170-7. [DOI] [PubMed] [Google Scholar]
- 3.Gondolesi G, Fishbein T, Chehade M, et al. Serum citrulline is a potential marker for rejection of intestinal allografts. Transplant Proc. 2002;34:918–20. doi: 10.1016/s0041-1345(02)02669-6. [DOI] [PubMed] [Google Scholar]
- 4.Pappas PA, Saudubray JM, Tzakis AG, et al. Serum citrulline as a marker of acute cellular rejection for intestinal transplantation. Transplant Pro. 2002;34:915–17. doi: 10.1016/s0041-1345(02)02668-4. [DOI] [PubMed] [Google Scholar]
- 5.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–39. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Blijlevens NM, Lutgens LC, Schattenberg AV, Donnelly JP. Citrulline: a potentially simple quantitative marker of intestinal epithelial damage following myeloablative therapy. Bone Marrow Transplant. 2004;34:193–96. doi: 10.1038/sj.bmt.1704563. [DOI] [PubMed] [Google Scholar]
- 7.Lutgens LC, Blijlevens NM, Deutz NE, et al. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer. 2005;103:191–99. doi: 10.1002/cncr.20733. [DOI] [PubMed] [Google Scholar]
- 8.van der Velden WJ, Herbers AH, Feuth T, et al. Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation. PLoS One. 2010;5:e15156. doi: 10.1371/journal.pone.0015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbers AH, Feuth T, Donnelly JP, Blijlevens NM. Citrulline-based assessment score: first choice for measuring and monitoring intestinal failure after high-dose chemotherapy. Ann Oncol. 2010;21:1706–11. doi: 10.1093/annonc/mdp596. [DOI] [PubMed] [Google Scholar]
- 10.Herbers AH, Blijlevens NM, Donnelly JP, et al. Bacteraemia coincides with low citrulline concentrations after high-dose melphalan in autologous HSCT recipients. Bone Marrow Transplant. 2008;42:345–49. doi: 10.1038/bmt.2008.170. [DOI] [PubMed] [Google Scholar]
- 11.Blijlevens NM, Donnelly JP, DePauw BE. Inflammatory response to mucosal barrier injury after myeloablative therapy in allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:703–7. doi: 10.1038/sj.bmt.1705118. [DOI] [PubMed] [Google Scholar]
- 12.Derikx J, Blijlevens N, Donnelly J, et al. Loss of enterocyte mass is accompanied by diminished turnover of enterocytes after myeloablative therapy in haematopoietic stem-cell transplant recipients. Ann Onc. 2009;20:337–42. doi: 10.1093/annonc/mdn579. [DOI] [PubMed] [Google Scholar]
- 13.Crenn P, Truchis P, Neveux N, et al. Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr. 2009;90:587–94. doi: 10.3945/ajcn.2009.27448. [DOI] [PubMed] [Google Scholar]
- 14.Merlin E, Minet-Quinard R, Doré E, et al. Citrullinaemia is a helpful marker of gastro-intestinal GvHD in children. Bone Marrow Transplant. 2010;45(Suppl 2):128. [Google Scholar]
- 15.Knipp M, Vasák M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem. 2000;15:257–64. doi: 10.1006/abio.2000.4805. [DOI] [PubMed] [Google Scholar]
- 16.Tidman M, Sjöström P, Jones I. A Comparison of GFR estimating formulae based upon s-cystatin C and s-creatinine and a combination of the two. Nephrol Dial Transplant. 2008;23:154–60. doi: 10.1093/ndt/gfm661. [DOI] [PubMed] [Google Scholar]