Abstract
We describe the cloning and function of the human XRCC1 gene, which is the first mammalian gene isolated that affects cellular sensitivity to ionizing radiation. The CHO mutant EM9 has 10-fold-higher sensitivity to ethyl methanesulfonate, 1.8-fold-higher sensitivity to ionizing radiation, a reduced capacity to rejoin single-strand DNA breaks, and a 10-fold-elevated level of sister chromatid exchange compared with the CHO parental cells. The complementing human gene was cloned from a cosmid library of a tertiary transformant. Two cosmid clones produced transformants that showed approximately 100% correction of the repair defect in EM9 cells, as determined by the kinetics of strand break repair, cell survival, and the level of sister chromatid exchange. A nearly full-length clone obtained from the pcD2 human cDNA expression library gave approximately 80% correction of EM9, as determined by the level of sister chromatid exchange. Based on an analysis of the nucleotide sequence of the cDNA insert compared with that of the 5' end of the gene from a cosmid clone, the cDNA clone appeared to be missing approximately 100 bp of transcribed sequence, including 26 nucleotides of coding sequence. The cDNA probe detected a single transcript of approximately 2.2 kb in HeLa polyadenylated RNA by Northern (RNA) blot hybridization. From the open reading frame and the positions of likely start sites for transcription and translation, the size of the putative XRCC1 protein is 633 amino acids (69.5 kDa). The size of the XRCC1 gene is 33 kb, as determined by localizing the endpoints on a restriction endonuclease site map of one cosmid clone. The deduced amino acid sequence did not show significant homology with any protein in the protein sequence data bases examined.
Full text
PDF



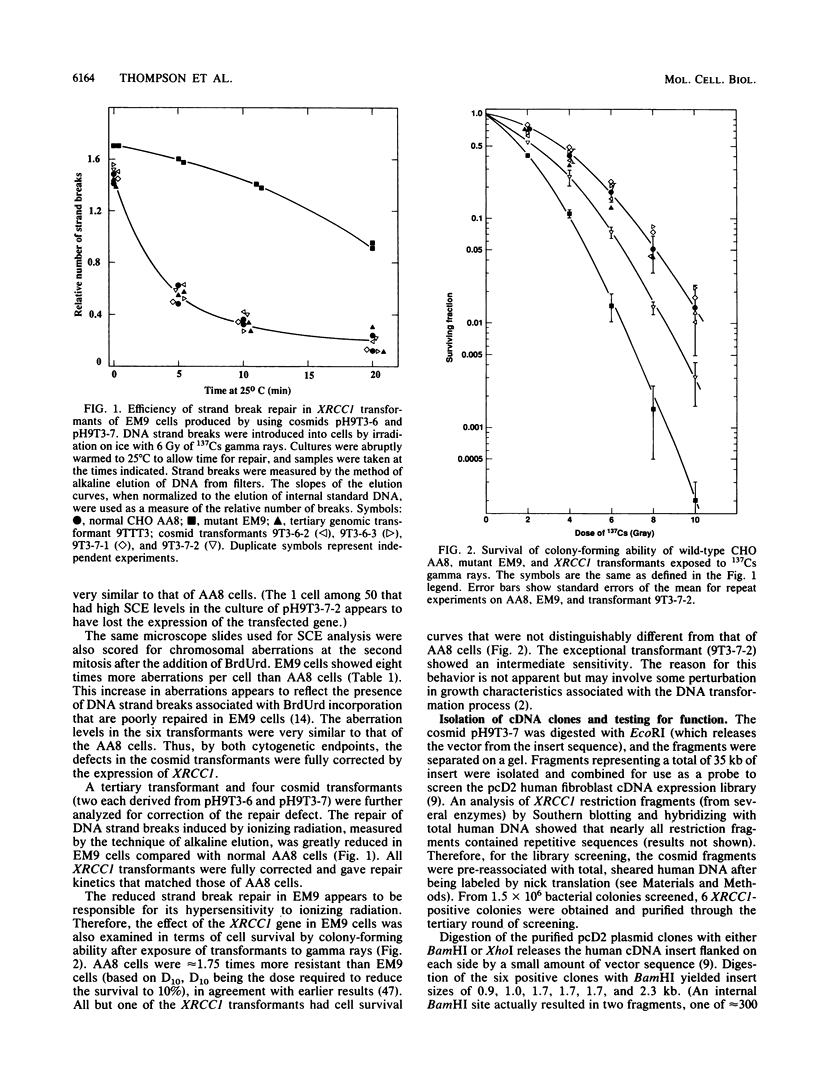

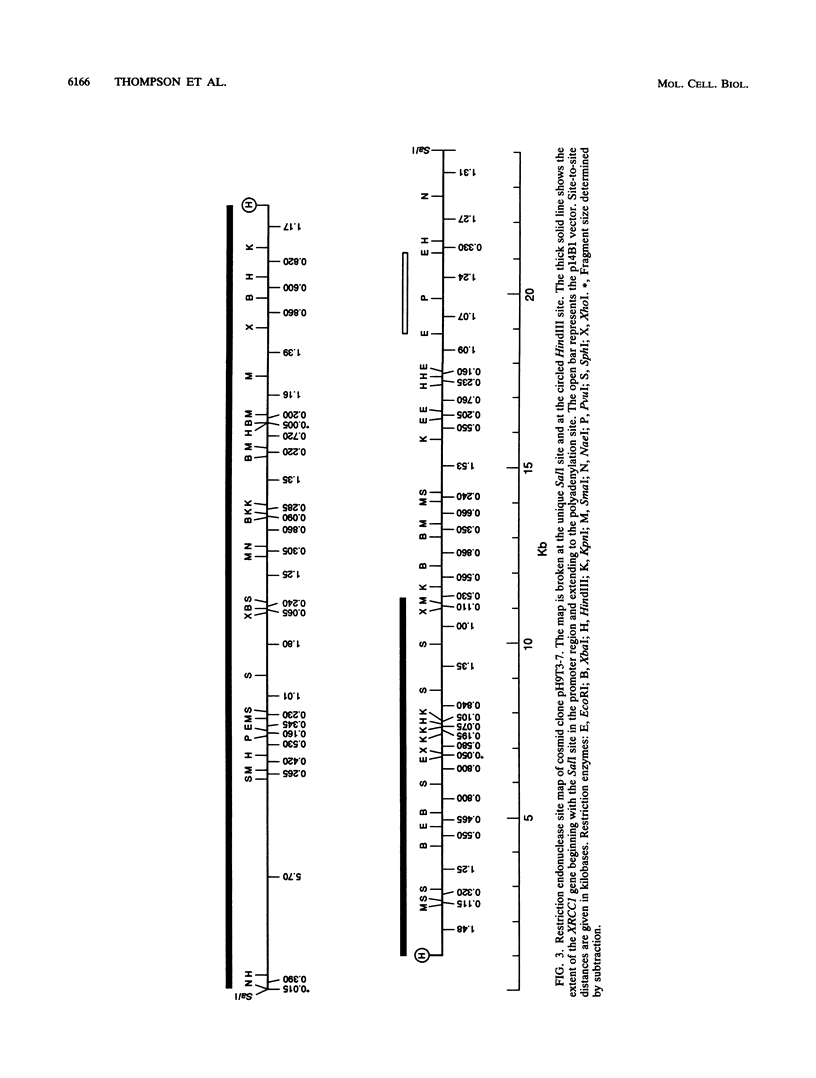


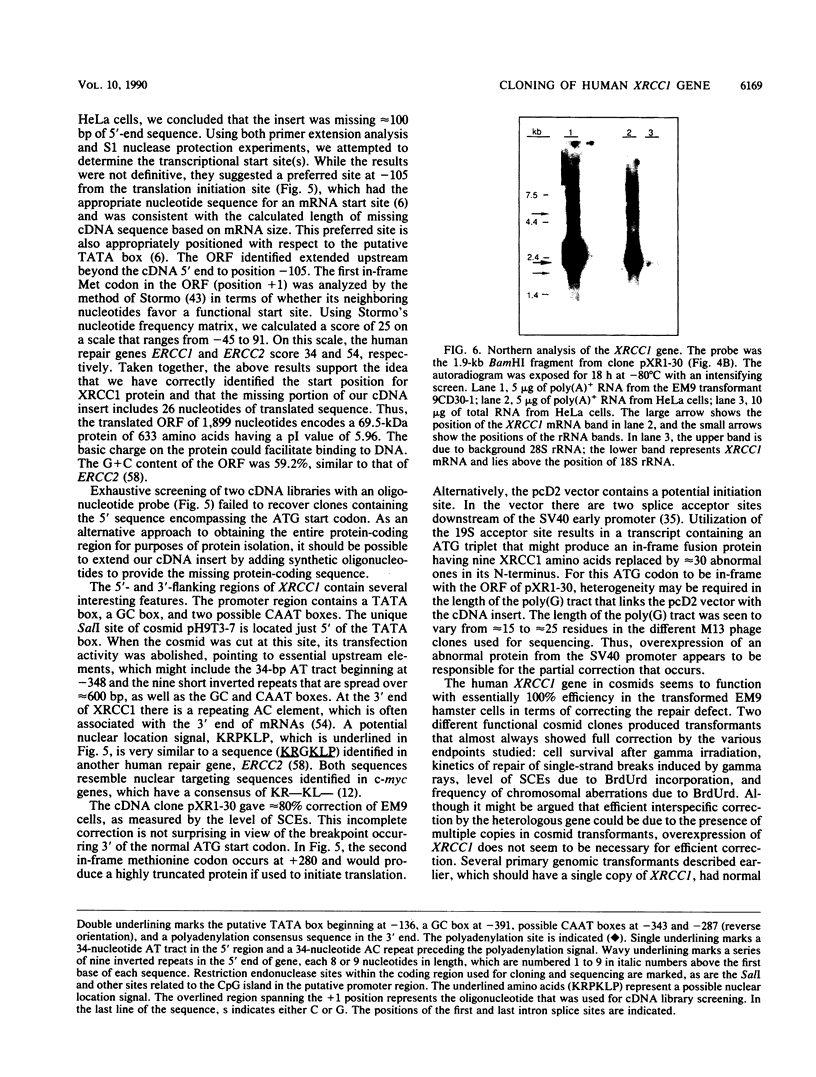


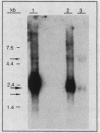
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell L. The mutagenic and carcinogenic effects of gene transfer. Mutagenesis. 1989 Jul;4(4):245–253. doi: 10.1093/mutage/4.4.245. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Boyd J. B., Mason J. M., Yamamoto A. H., Brodberg R. K., Banga S. S., Sakaguchi K. A genetic and molecular analysis of DNA repair in Drosophila. J Cell Sci Suppl. 1987;6:39–60. doi: 10.1242/jcs.1984.supplement_6.3. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brown W. M. Polymorphism in mitochondrial DNA of humans as revealed by restriction endonuclease analysis. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3605–3609. doi: 10.1073/pnas.77.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano A. V., Minkler J. L., Dillehay L. E., Thompson L. H. Incorporated bromodeoxyuridine enhances the sister-chromatid exchange and chromosomal aberration frequencies in an EMS-sensitive Chinese hamster cell line. Mutat Res. 1986 Sep;162(2):233–239. doi: 10.1016/0027-5107(86)90090-4. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaro C. M., Pearson M. L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Genet. 1981 Sep;7(5):603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988 Oct;8(10):4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debenham P. G., Jones N. J., Webb M. B. Vector-mediated DNA double-strand break repair analysis in normal, and radiation-sensitive, Chinese hamster V79 cells. Mutat Res. 1988 May;199(1):1–9. doi: 10.1016/0027-5107(88)90224-2. [DOI] [PubMed] [Google Scholar]
- Dillehay L. E., Thompson L. H., Carrano A. V. DNA-strand breaks associated with halogenated pyrimidine incorporation. Mutat Res. 1984 Mar-Apr;131(3-4):129–136. doi: 10.1016/0167-8817(84)90052-x. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller L. F., Painter R. B. A Chinese hamster ovary cell line hypersensitive to ionizing radiation and deficient in repair replication. Mutat Res. 1988 Mar;193(2):109–121. doi: 10.1016/0167-8817(88)90041-7. [DOI] [PubMed] [Google Scholar]
- Giaccia A., Weinstein R., Hu J., Stamato T. D. Cell cycle-dependent repair of double-strand DNA breaks in a gamma-ray-sensitive Chinese hamster cell. Somat Cell Mol Genet. 1985 Sep;11(5):485–491. doi: 10.1007/BF01534842. [DOI] [PubMed] [Google Scholar]
- Hoy C. A., Fuscoe J. C., Thompson L. H. Recombination and ligation of transfected DNA in CHO mutant EM9, which has high levels of sister chromatid exchange. Mol Cell Biol. 1987 May;7(5):2007–2011. doi: 10.1128/mcb.7.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy C. A., Salazar E. P., Thompson L. H. Rapid detection of DNA-damaging agents using repair-deficient CHO cells. Mutat Res. 1984 Oct;130(5):321–332. doi: 10.1016/0165-1161(84)90018-9. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Jones N. J., Cox R., Thacker J. Isolation and cross-sensitivity of X-ray-sensitive mutants of V79-4 hamster cells. Mutat Res. 1987 May;183(3):279–286. doi: 10.1016/0167-8817(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Jones N. J., Cox R., Thacker J. Six complementation groups for ionising-radiation sensitivity in Chinese hamster cells. Mutat Res. 1988 Mar;193(2):139–144. doi: 10.1016/0167-8817(88)90044-2. [DOI] [PubMed] [Google Scholar]
- Jones N. J., Stewart S. A., Thompson L. H. Biochemical and genetic analysis of the Chinese hamster mutants irs1 and irs2 and their comparison to cultured ataxia telangiectasia cells. Mutagenesis. 1990 Jan;5(1):15–23. doi: 10.1093/mutage/5.1.15. [DOI] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Sedgwick S. G., Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984 Nov-Dec;132(5-6):189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- MacInnes M. A., Mudgett J. S. Cloning of the functional human excision repair gene ERCC-5: potential gene regulatory features conserved with other human repair genes. Prog Clin Biol Res. 1990;340A:265–274. [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Carrano A. V., Fertitta A., Perry B., Thompson L. H., Tucker J. D., Weber C. A. Refined mapping of the three DNA repair genes, ERCC1, ERCC2, and XRCC1, on human chromosome 19. Cytogenet Cell Genet. 1989;52(1-2):11–14. doi: 10.1159/000132829. [DOI] [PubMed] [Google Scholar]
- Myles G. M., Sancar A. DNA repair. Chem Res Toxicol. 1989 Jul-Aug;2(4):197–226. doi: 10.1021/tx00010a001. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkel D., Thompson L. H., Gray J. W., Vanderlaan M. Measurement of sister chromatid exchanges at very low bromodeoxyuridine substitution levels using a monoclonal antibody in Chinese hamster ovary cells. Cancer Res. 1985 Nov;45(11 Pt 2):5795–5798. [PubMed] [Google Scholar]
- Potter H., Dressler D. A 'Southern Cross' method for the analysis of genome organization and the localization of transcription units. Gene. 1986;48(2-3):229–239. doi: 10.1016/0378-1119(86)90081-8. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Harris A. L., Hickson I. D. Defective repair of DNA single- and double-strand breaks in the bleomycin- and X-ray-sensitive Chinese hamster ovary cell mutant, BLM-2. Mutat Res. 1989 Mar;217(2):93–100. doi: 10.1016/0921-8777(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Harris A. L., Hickson I. D. Isolation and characterization of Chinese hamster ovary cell lines sensitive to mitomycin C and bleomycin. Cancer Res. 1985 Nov;45(11 Pt 1):5304–5309. [PubMed] [Google Scholar]
- Siciliano M. J., Carrano A. V., Thompson L. H. Assignment of a human DNA-repair gene associated with sister-chromatid exchange to chromosome 19. Mutat Res. 1986 Aug;174(4):303–308. doi: 10.1016/0165-7992(86)90051-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Weinstein R., Giaccia A., Mackenzie L. Isolation of cell cycle-dependent gamma ray-sensitive Chinese hamster ovary cell. Somatic Cell Genet. 1983 Mar;9(2):165–173. doi: 10.1007/BF01543175. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Bachinski L. L., Stallings R. L., Dolf G., Weber C. A., Westerveld A., Siciliano M. J. Complementation of repair gene mutations on the hemizygous chromosome 9 in CHO: a third repair gene on human chromosome 19. Genomics. 1989 Nov;5(4):670–679. doi: 10.1016/0888-7543(89)90107-9. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Carrano A. V., Mazrimas J. A., Mooney C. L., Minkler J. L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res. 1982 Aug;95(2-3):427–440. doi: 10.1016/0027-5107(82)90276-7. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Mooney C. L., Carrano A. V. Hypersensitivity to mutation and sister-chromatid-exchange induction in CHO cell mutants defective in incising DNA containing UV lesions. Somatic Cell Genet. 1982 Nov;8(6):759–773. doi: 10.1007/BF01543017. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Minkler J. L., Fuscoe J. C., Henning K. A., Carrano A. V. DNA-mediated transfer of a human DNA repair gene that controls sister chromatid exchange. Mol Cell Biol. 1985 Apr;5(4):881–884. doi: 10.1128/mcb.5.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Fong S., Brookman K. Validation of conditions for efficient detection of HPRT and APRT mutations in suspension-cultured Chinese hamster ovary cells. Mutat Res. 1980 Feb;74(1):21–36. doi: 10.1016/0165-1161(80)90188-0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Salazar E. P., Brookman K. W., Collins C. C., Stewart S. A., Busch D. B., Weber C. A. Recent progress with the DNA repair mutants of Chinese hamster ovary cells. J Cell Sci Suppl. 1987;6:97–110. doi: 10.1242/jcs.1984.supplement_6.6. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Somatic cell genetics approach to dissecting mammalian DNA repair. Environ Mol Mutagen. 1989;14(4):264–281. doi: 10.1002/em.2850140409. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Inducible DNA repair systems. Annu Rev Biochem. 1985;54:425–457. doi: 10.1146/annurev.bi.54.070185.002233. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Marsh L., Dodson L. A. Genetic analyses of DNA repair: inference and extrapolation. Annu Rev Genet. 1985;19:103–126. doi: 10.1146/annurev.ge.19.120185.000535. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. ERCC2: cDNA cloning and molecular characterization of a human nucleotide excision repair gene with high homology to yeast RAD3. EMBO J. 1990 May;9(5):1437–1447. doi: 10.1002/j.1460-2075.1990.tb08260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeda G., van Ham R. C., Masurel R., Westerveld A., Odijk H., de Wit J., Bootsma D., van der Eb A. J., Hoeijmakers J. H. Molecular cloning and biological characterization of the human excision repair gene ERCC-3. Mol Cell Biol. 1990 Jun;10(6):2570–2581. doi: 10.1128/mcb.10.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Burki H. J. Repair capability and the cellular age response for killing and mutation induction after UV. Mutat Res. 1982 Aug;95(2-3):505–514. doi: 10.1016/0027-5107(82)90281-0. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Tran Q., van der Schans G. P., Simons J. W. Characterization of an X-ray-hypersensitive mutant of V79 Chinese hamster cells. Mutat Res. 1988 Nov;194(3):239–249. doi: 10.1016/0167-8817(88)90025-9. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]
- vanAnkeren S. C., Murray D., Meyn R. E. Induction and rejoining of gamma-ray-induced DNA single- and double-strand breaks in Chinese hamster AA8 cells and in two radiosensitive clones. Radiat Res. 1988 Dec;116(3):511–525. [PubMed] [Google Scholar]