Abstract
Background
The aim of this study was to evaluate atrophy rates, perfusion, and diffusion disturbances within the hippocampus, which is the site of characteristic changes in Alzheimer’s disease (AD) and mild cognitive impairment (MCI).
Material/Methods
Thirty patients with AD (mean age 71.2 yrs) – 34 with MCI (mean age 67.7 yrs) and 20 healthy controls (mean age 68.1 yrs) – underwent structural MR examination followed by perfusion and diffusion-weighted imaging on a 1.5 T scanner. Visual rating of hippocampal atrophy, planimetric measurements of hippocampal formation (HF) and perihippocampal fluid spaces (PFSs), and values of relative cerebral blood volume (rCBV) and apparent diffusion coefficient (ADC) were assessed. The results were correlated with the MMSE scores.
Results
In AD we found decreased size of HF and increased diameters of PFSs and ADC values, compared to MCI and control group. Compared to normal controls, the MCI group showed decreased HF size and increased diameters of only medial PFS. There were no differences in rCBV values among all the subject groups. Planimetric measurements of hippocampal atrophy showed the highest accuracy in diagnosing AD and MCI. In all patients, the increased rates of hippocampal atrophy correlated with the increased ADC values. In MCI, MMSE scores correlated with the HF size and ADC values.
Conclusions
In AD and MCI, hippocampal atrophy is associated with decreased tissue integrity without coexisting perfusion disturbances. Of all evaluated hippocampal measurements, atrophy rates seem to be the most useful parameters in detecting changes among AD, MCI, and control subjects.
Keywords: hippocampus, Alzheimer’s disease, mild cognitive impairment, planimetry, Scheltens’ rating scale, diffusion-weighted imaging, perfusion-weighted imaging
Background
Alzheimer’s disease (AD) is the most common type of dementia, and mild cognitive impairment (MCI) is regarded as the predementia state. Both pathologies are caused by degenerative process resulting in generalized atrophy, microscopic amyloid plaques, and neurofibrillary tangles formation [1]. In AD, the neuropathological damage of the grey matter begins in primary limbic areas such as hippocampi, and spreads to associated limbic areas such as posterior cingulate cortex, followed by the temporo-parietal, and finally frontal cortices [2].
One of the first and most striking manifestations of AD is memory impairment. The hippocampus and adjacent structures are crucial for memory; their bilateral destruction leads invariably to the loss of this essential intellectual function [3]. Atrophy of the medial temporal lobe (MTA), including the hippocampus and entorhinal cortex, is a sensitive marker for AD [4–8]. MTA occurs early in the disease process and is also found in MCI [5,7,9–12]. MTA can be assessed with visual inspection [4,8,10], or using either planimetric or, more recently, volumetric measurements performed on CT and MRI images [11–15]. Volumetric assessment of the hippocampal region, though very accurate, is difficult to apply in routine clinical practice. By contrast, the assessment of MTA using a standardized visual rating scale is a quick and easy measurement, with a comparable accuracy [4,8,10] and results similar to volumetric assessments of MTA [16].
Recent advances in MR techniques give opportunities to look not only at anatomy and atrophy of the medial temporal lobe, but also at microstructural alterations or perfusion disturbances within this region, which may be assessed with diffusion- (DWI) and perfusion-(PWI) weighted imaging.
Diffusion-weighted imaging is a sensitive tool that allows quantifying of physiologic alterations in water diffusion that result from microscopic structural changes not detectable with anatomical MR imaging. Water diffusion can be measured with the apparent diffusion coefficient (ADC) parameter. In AD and MCI, increased ADC values were found, suggesting an expansion of extracellular space due to neuron loss and disruption of cell membranes [17–19].
Perfusion-weighted imaging is an advanced MR technique that enables measurements of cerebral hemodynamics at the capillary level. Among perfusion-weighted MR methods, the dynamic susceptibility contrast (DSC) technique is most widely used. It enables quantitative assessment of cerebral blood volumes (CBV), thus providing information similar to that obtained in PET and SPECT studies. The value of CBV is correlated with vessel density and is lower in AD and MCI patients [20–22].
To our knowledge, this is the first study evaluating correlation of MTA with both diffusion and perfusion results within the hippocampus in AD and MCI patients.
The aim of this study was to evaluate atrophy rates, as well as perfusion and diffusion disturbances, within the hippocampus in patients with AD and MCI, and to investigate whether there are any relations among these changes. Sensitivity and specificity of planimetric measurements, as well as PWI and DWI, were assessed. The results of the neuroimaging techniques were also correlated with scores of Mini-Mental State Examination (MMSE).
Material and Methods
Material
Thirty subjects diagnosed with AD (mean age 71.2 yrs, 11 men, 19 women), 34 patients diagnosed with MCI (mean age 67.7 yrs, 13 men, 21 women), and 20 cognitively normal elderly controls (mean age 67.7 yrs, 6 men, 14 women) were enrolled in the study. Subjects with AD and MCI were outpatients referred from the Department of Psychiatry, and the control subjects were volunteers recruited from hospital employees and families of the patients. All AD patients fulfilled the DSM-IV and NINCDS-ADRDA criteria for probable AD [23] and the diagnosis of MCI was determined according to Petersen’s criteria [24,25]. The exclusion criteria for all 3 subgroups were severe head injury, headache, current alcohol abuse or dependence, and loss of 25% of body weight in the past year. Subjects with cerebral vascular damage or vascular risk factors were also excluded from the study. An additional exclusion criterion for the control group was any history of major psychiatric or central nervous system illness.
All subjects underwent detailed psychiatric examination, as well as laboratory and neuropsychological tests, including Mini-Mental State Examination (MMSE) adjusted for age and education level, Clinical Dementia Rating (CDR), Clock Drawing Test, Dem-Tect, and Global Scale of Depression (GSD). The distributions of age and gender, as well as the MMSE scores, for each group are presented in Table 1.
Table 1.
The distribution of age, gender and MMSE scores within the subject groups (mean ± standard deviation).
| AD | MCI | CG | |
|---|---|---|---|
| Patient No. | 30 | 34 | 20 |
| Age (years) | 71.2±8.0 | 67.7±8.7 | 68.1±7.1 |
| Sex (Male/Female) | 11/19 | 13/21 | 6/14 |
| MMSE (points) | 17.8±4.3 | 26.1±1.7 | 29.8±0.4 |
AD – Alzheimer’s disease; MCI – mild cognitive impairment; CG – control group; MMSE – Mini-Mental State Examination (scores 0–10 severe dementia; 11–18 moderate dementia; 19–23 mild dementia; 24–26 mild cognitive impairment without dementia; 27–30 normal score),
The study was conducted in accordance with the guidelines of the University Ethics Committee for conducting research involving humans. All patients or their relative/caregiver provided signed consent to participate in the examination.
Methods
Data acquisition: All subjects underwent plain MR studies followed by DWI and PWI on a 1.5 T MR scanner (Signa Hdx, GE Medical Systems) using a 16-channel HNS (head-neck-spine) coil.
Structural MR examination
Plain MR examination included FRFSE T2-weighted coronal and sagittal images, as well as FLAIR axial images. Axial images were parallel to the anterior commissure-posterior commissure (AC-PC) line.
Diffusion-weighted imaging (DWI)
Transverse single-shot echoplanar diffusion-weighted imaging was carried out using the following parameters: TE 89.9 ms, TR 8000 ms, slice thickness – 5mm, FOV 26 cm, matrix size 128×128, NEX – 1, diffusion sensitive gradient b=1000 s/mm2 in the 3 orthogonal directions, scanning time: 42 seconds. Axial DWI images were parallel to the anterior commissure – posterior commissure (AC-PC) line.
Perfusion weighted imaging (PWI)
PWI was performed with the Dynamic Susceptibility Contrast Enhanced method (DSC) using fast echoplanar T2*-weighted gradient echo sequence with the following parameters: TR=1.900 ms, TE=80 ms, FOV=30 cm, matrix=192×128, slice thickness=8 mm without spacing, NEX – 1.0. Ten seconds after the start of image acquisition, a bolus of a 1.0 mol/l gadobutrol formula (Gadovist, Bayer Health Care, Germany) in a dose of 0.2 ml/kg of body weight was injected via a 20-gauge catheter placed in the antecubital vein. Contrast was administered with an automatic injector (Medrad) at a rate of 5 ml/s and was followed by a saline bolus (20 ml at 5 ml/s). The whole perfusion imaging lasted 1 min 26 s, in which sets of images from 13 axial slices were obtained before, during, and after contrast injection.
During the whole MR examination the subjects were instructed to keep their eyes closed. No sedation or anesthesia was used in any of the patients.
Data postprocessing
Structural imaging
To assess the rates of medial temporal lobe atrophy, hippocampi and peri-hippocampal cerebrospinal fluid (CSF) spaces were evaluated.
Visual rating and planimetric measurements of the hippocampal regions were assessed on coronal T2-weighted images on the slices including pons.
Visual assessment was performed using the method proposed by Scheltens et al, which is based on estimation of both the volume of the medial temporal lobe including the hippocampus, and the volume of the surrounding CSF spaces, in particular the temporal horn of the lateral ventricle and the choroid fissure [4]. Scores ranged from 0 (no atrophy) to 4 (severe atrophy).
Planimetric measurements consisted of the calculations of the transverse and vertical diameters of the hippocampus, transverse and vertical diameters of the whole peri-hippocampal CSF space (Figure 1A), as well as transverse diameters of medial (choroid fissure) and lateral (temporal horn) CSF space and vertical diameter of the superior CSF space (Figure 1B). Measurements were made bilaterally.
Figure 1.
(A) Measurements of the hippocampus and the perihippocampal CSF spaces. Hippocampal index = (1*2) divided by (3*4). (B)– measurements of the lateral (1), superior (2) and medial (3) CSF spaces. Lateral CSF space index = 1 divided by 4, superior CSF space index = 2 divided by 5, medial CSF space index = 3 divided by 4.
Then several ratios were calculated, including: hippocampal index (HI) – the product of transverse and vertical hippocampal diameters divided by the product of transverse and vertical diameters of the whole peri-hippocampal CSF space (Figure 1A); normalized hippocampal size (HS) – the product of transverse and vertical diameters of the hippocampus divided by the transverse diameter of the brain; medial CSF space index – width of medial CSF space divided by width of the whole peri-hippocampal CSF space; superior CSF space index – vertical diameter of the superior CSF space divided by vertical diameter of the whole peri-hippocampal CSF space; and lateral CSF space index – width of lateral CSF space divided by width of the whole peri-hippocampal CSF space (Figure 1B).
Diffusion-weighted imaging
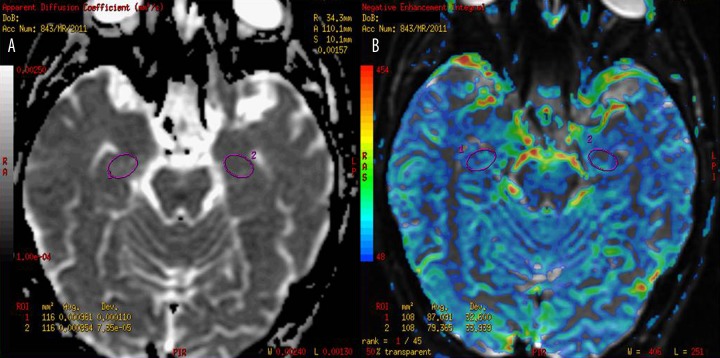
The diffusion-weighted images were post-processed using Functool software (GE Medical Systems, ADW workstation 4.4). ADC maps were computed pixel-by-pixel based on the Stejskal and Tanner equation [26]. ADC values in mm2/s were obtained using elliptical ROIs (size: 100–120 mm2) placed manually within both hippocampi on ADC maps, with special attention paid to avoid peri-hippocampal CSF spaces (Figure 2A).
Figure 2.
Placement of ROIs within hippocampi on ADC (A) and CBV perfusion maps (B).
Perfusion-weighted imaging
The dynamic images were post-processed using Functool software (GE Medical Systems, ADW workstation 4.4). CBV maps were computed on a pixel-wise basis from the first-pass data as described by Belliveau et al. [27]. Regional CBV values were obtained using elliptical ROIs (size 80–100 mm2) placed manually within hippocampi (Figure 2B). Since CBV values obtained with this methodology are not absolute measurements and must be related to the reference standard, all CBV values from hippocampi were normalized to the mean values in the cerebellar cortex (circular ROIs, size 525 mm2), which is the region less affected in AD compared to other cortical measures [28].
Statistical analysis
A group comparison of demographics was performed using analysis of variance (ANOVA) for continuous variables (age) and Pearson chi2 for categorical variables (gender).
Differences in the results of the visual and planimetric assessment, as well as ADC and rCBV values among the AD, MCI and control subgroups, were evaluated using ANOVA followed by the LSD test as a post-hoc test. Associations between planimetric measurements, as well as perfusion and diffusion results, were assessed using Pearson’s correlation coefficient.
From multiple performed measurements, only those showing the most significant differences among patient subgroups were selected to check their sensitivity and specificity in distinguishing AD and MCI. To assess accuracy of the evaluated MR methods, Youden coefficient was calculated according to the equation: (sensitivity + specificity) – 1.
Correlations between the results of visual rating, planimetric measurements, rCBV or ADC values and the results of cognitive test were estimated for AD and MCI patients using Pearson’s correlation coefficients.
Statistical computations were performed using Statistica PL software package version 4.0, and p<0.05 was set as the significance level.
Results
There were no significant differences in age (analysis of variance; p=0.19) and sex distribution (chi2=0.37, df=2, p=0.82) among the 3 groups of subjects.
AD patients showed significantly higher results of Scheltens’ visual rating scale within right and left hippocampus than the MCI and the control group (p<0.0001). The MCI group, compared to the control subjects, also showed higher rates of visually assessed atrophy within right (p=0.0007) and left (p=0.001) hippocampus. Mean results of visual rating of the right and left MTA are shown in Table 2.
Table 2.
Mean results of planimetric measurements, ADC and rCBV values from both hippocampal regions in AD, MCI and the control group with the results of analysis of variance (ANOVA) and the post hoc Tukey LSD test.
| Parameter (mean) | Subject groups (SD) | ANOVA | post hoc Tukey test, p values | ||||
|---|---|---|---|---|---|---|---|
| AD | MCI | CG | p values | AD vs CG | AD vs MCI | MCI vs CG | |
| Visual rating | 2.68 (0.9) | 1.63 (1.06) | 0.67 (0.61) | <0.0001* | <0.0001* | <0.0001* | 0.0004* |
|
| |||||||
| Hippocampal index | 0.28 (0.07) | 0.44 (0.11) | 0.51 (0.06) | <0.0001* | <0.0001* | <0.0001* | 0.004* |
|
| |||||||
| Hippocampal size | 0.054 (0.014) | 0.080 (0.019) | 0.091 (0.017) | <0.0001* | <0.0001* | <0.0001* | 0.029* |
|
| |||||||
| Medial CSF space index | 0.32 (0.08) | 0.23 (0.05) | 0.16 (0.05) | <0.0001* | <0.0001* | <0.0001* | 0.0002* |
|
| |||||||
| Superior CSF space index | 0.39 (0.06) | 0.29 (0.09) | 0.27 (0.14) | <0.0001* | <0.0001* | 0.0002* | 0.34 |
|
| |||||||
| Lateral CSF space index | 0.23 (0.07) | 0.16 (0.09) | 0.12 (0.05) | <0.0001* | <0.0001* | 0.001* | 0.1 |
|
| |||||||
| ADC | 0.97 (0.12) | 0.94 (0.10) | 0.89 (0.07) | 0.059 | 0.01* | 0.38 | 0.09 |
|
| |||||||
| rCBV | 0.92 (0.12) | 0.90 (0.09) | 0.89 (0.13) | 0.26 | 0.14 | 0.19 | 0.72 |
AD – Alzheimer’s disease; MCI – mild cognitive impairment; CG – control group; SD – standard deviation; mean (mean value for the right and left side); ANOVA – analysis of variance;
statistically significant p values less than 0.05.
The right and left HIs showed significantly lower values in AD compared to the control group (p<0.000), in AD compared to MCI (p<0.000), as well as in MCI compared to the control group (right: p=0.012, left: p=0.006). The mean results of bilateral HIs measurements are shown in Table 2.
Similarly, the right and left HSs showed significantly lower values in AD compared to the control group (p<0.000), in AD compared to the MCI group (p<0.0001), and in the MCI compared to the control group (p=0.029).
Evaluation of the values of the peri-hippocampal CSF spaces revealed the following results. In AD, compared to the control group, significantly higher values of both medial (p=0.000), lateral (p=0.000) and superior (right: p=0.049, left: p=0.000) CSF space indices were found. In AD, compared to MCI, significantly higher values of both medial (p=0.000), both lateral (right: p=0.02, left: p=0.000), and both superior (right: p=0.03, left: p=0.000) CSF space indices were noticed. On the other hand, in MCI compared to CG, only both medial CSF spaces showed significantly higher indices (right: p=0.02, left: p=0.000). Mean values of bilateral medial, superior and lateral CSF space indices are shown in Table 2.
Mean hippocampal ADC results, as well as separate ADC measures from the right and left hippocampus, showed the highest values in AD, intermediate in MCI, and the lowest in the control group. Significantly increased ADC values were found only in AD patients compared to the control group, and regarded values from the right (p=0.02) and left hippocampus (p=0.01) as well as mean hippocampal ADC value (p=0.01). Mean ADC values for both hippocampi are shown in Table 2.
No significant differences among the subject groups were found in rCBV values from both hippocampi. Mean rCBV values within both hippocampi are shown in Table 2.
In MCI and AD patients there were significant correlations between mean ADC values and several hippocampal measures such as mean results of visual rating (r=0.3, p=0.009), and mean HI (r=–0.4, p=0.000), as well as mean HS (r=–0.43, p=0.000), mean medial CSF space index (r=0.34, p=0.005), and mean lateral CSF space index (r=0.31, p=0.01).
In both patient groups rCBV values did not show any correlations with the planimetric measures or ADC values (p>0.05).
The results of sensitivity, specificity, accuracy and the values of Youden coefficient for parameters rating MTA showing the most significant differences among patient subgroups (visual rating scale, mean HI, mean HS and mean medial CSF space) and for mean ADC values are shown in Table 3. The highest accuracy in distinguishing AD from normal controls was found for mean HI, mean HS, and mean medial CSF space index, while the highest accuracy in distinguishing MCI from normal controls was revealed for the scores of visual rating and mean medial CSF space index (Table 3).
Table 3.
Accuracy of planimetric measurements as well as ADC values from the hippocampal region in distinguishing AD and MCI from healthy controls.
| Parameter (mean) | Patient subgroups | Cut point values | Sensitivity | Specificity | Youden coefficient | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean scores of visual rating | AD | ≥2.0 | 0.7 | 0.95 | 0.65 | 0.89 | ||||
|
| ||||||||||
| MCI | ≥1.5 | 0.64 | 0.85 | 0.49 | 0.77** | |||||
|
| ||||||||||
| Mean HI | AD | <0.4 | 0.9 | 0.95 | 0.85 | 0.98* | ||||
|
| ||||||||||
| MCI | <0.47 | 0.52 | 0.80 | 0.32 | 0.31 | |||||
|
| ||||||||||
| Mean HS | AD | <0.076 | 0.93 | 0.85 | 0.78 | 0.96* | ||||
|
| ||||||||||
| MCI | <0.096 | 0.85 | 0.50 | 0.35 | 0.71 | |||||
|
| ||||||||||
| Medial CSF space index | AD | >0.24 | 0.83 | 0.9 | 0.73 | 0.95* | ||||
|
|
||||||||||
| MCI | <0.2 | 0.76 | 0.7 | 0.46 | 0.77** | |||||
|
| ||||||||||
| Mean ADC | AD | >0.98 | 0.43 | 0.8 | 0.23 | 0.63 | ||||
|
| ||||||||||
| MCI | >0.98 | 0.38 | 0.8 | 0.18 | 0.61 | |||||
AD – Alzheimer’s disease; MCI – mild cognitive impairment; ADC – Apparent Diffusion Coefficient; Youden coefficient – (sensitivity + specificity) – 1;
the highest results of accuracy in distinguishing AD from healthy controls;
the highest results of accuracy in distinguishing MCI from healthy controls; HI – hippocampal index; HS – hippocampal space; CSF – cerebro-spinal fluid.
Analysis of correlations between the MMSE scores and the MR measurements within hippocampi showed several significant associations only in the group of MCI patients. The MMSE scores showed significant correlations with the mean HI (r=0.4, p=0.026), mean HS (r=0.04, p=0.02), mean medial CSF space index (r=–0.42, p=0.01), and mean ADC value (r=–0.49, p=0.006). In AD patients there were no correlations between MMSE scores and any of the hippocampal measurements.
Discussion
We observed significant differences in MTA using visual rating scale, with AD subjects showing the highest and MCI patients intermediate rate of hippocampal atrophy compared to age-matched healthy volunteers, as has been previously reported [4,10]. Though visual rating using Scheltens’ scale has been proved to be a reliable and standardized method [4], it is still prone to subjective assessment. To make our assessment of MTA more objective, we also performed planimetric measurements of the hippocampal region in a manner similar to Scheltens’ criteria. We measured hippocampal index, which reflected the size of hippocampus related to the size of the perihippocampal CSF spaces. In Scheltens’ rating scale the visual assessment of the hippocampal region also regards assessment of the height of the hippocampus and the sizes of medial, superior, and lateral CSF spaces surrounding the hippocampus. Planimetric measurements showed the smallest sizes of hippocampi in AD, and intermediate in MCI patients, compared to normal controls. The results of our planimetric measurements paralleled the results of visual rating scale, which is in agreement with other studies also reporting strong correlations of Scheltens’ scale with volumetric studies [29].
We also measured sizes of medial, superior, and lateral CSF spaces surrounding the hippocampus. We found increased diameters of all CSF spaces in AD compared to MCI and normal controls. On the other hand, in the MCI group, compared to the normal controls, only the medial CSF spaces were enlarged. This is in agreement with the Scheltens’ criteria of MTA, in which the first visible change is the increased middle choroid fissure width [4]. This finding reflects the pattern of MTA that starts in the medial aspects of the medial temporal lobe.
We also introduced a planimetric measurement of the hippocampal formation itself, independent of the size of the peri-hippocampal fluid spaces, which was called HS. This parameter was used to avoid overestimation of the MTA in patients with subcortical atrophy and subsequently enlarged CSF spaces. As expected, HS showed the lowest values in AD, intermediate in MCI, and highest in normal controls.
There is still uncertainty about the clinical value of MTA for the diagnosis of AD and MCI. Studies looking at the specificity of MTA for AD have shown results ranging from 50% to 96% [8] in moderate to severe cases of AD. In our study, 4 parameters describing the rates of MTA (including visual rating scale, HI, HS and the size of the medial CSF space) revealed significant differences between all evaluated subjects. In AD, sensitivity of planimetric measurements ranged from 0.83 to 0.93 and specificity between 0.85 and 0.95, and was higher than specificity and sensitivity of visual rating (0.7 and 0.95, respectively). On the other hand, in MCI, visual rating scale and mean medial CSF space index showed higher sensitivity and specificity (0.64 and 0.85; 0.76 and 0.7, respectively) than other planimetric measurements. Similar results were obtained by Du et al, who reported sensitivity and specificity of hippocampal volumes to be as high as 0.78 and 0.90 in distinguishing AD, and 0.52 and 0.79 in distinguishing MCI [5]. In the study by Korf et al., the visual rating of MTA was found to have a strong predictive value of conversion to AD, stronger and also independent on age, gender, education, MMSE, CDR Sum of Boxes, and Verbal Delayed Recall, as well as the presence of hypertension, depression, APOE eta 4 allele, and white matter hyperintensities [10].
Visual rating and simple linear measurements are very reliable and fast methods to estimate MTA. Volumetric assessment of the hippocampal region, though very accurate, is difficult to use in routine clinical practice. Reasons for this include the need for digital MRI data, the time consuming nature of region-of-interest analysis, and the fact that automated hippocampal volume measurement techniques are not widely available. The main advantage of visual rating and planimetric measurements is that they are fast and easy to use. Wahlund et al reported the time spent on visual rating of MTA (1–2 min) to be 10-fold shorter than time spent calculating the medial temporal lobe volume (10–12 min) in a single subject [29].
The second major aim of our work was to estimate diffusion and perfusion disturbances within hippocampal formations in AD and MCI patients, and their relation to the severity of MTA.
The diffusivity of water depends primarily on the presence of microscopic structural barriers in tissue such as membranes of cell bodies, axons, and myelin sheaths, which can alter the random motion of water molecules. Pathologic disruption of the cell membranes, and loss of myelin and axonal processes may diminish the restriction of water motion and lead to increased diffusivity measured by ADC parameter. Several DWI studies revealed higher ADC values within the posterior cingulate region, as well as occipital and parietal white matter in patients with AD, compared to normal controls [18,30,31]. On the other hand, Bozzao et al did not find any significant changes in ADC values between AD and normal controls in multiple brain locations [21]. The number of DWI studies of the hippocampal formation is limited in the literature, and their results are also contradictory. Hanyu et al and Bozzao et al did not find any differences in hippocampal ADC values between AD and normal controls [21,31], but Kantarci et al found elevated ADC values within the hippocampi in AD and MCI patients compared to normal controls [18]. Moreover, elevated ADC values in MCI patients were also able to predict conversion to AD [19]. As in those studies, we found increased ADC values within both hippocampi in AD and MCI patients compared to normal controls, but only in the AD group did the ADC values reach the level of significant difference. In our study ADC values were not able to differentiate AD from MCI, and MCI from the control group. Reaching the same specificity of 80% as Kantarci et al., we found slightly lower values of sensitivity in distinguishing AD and MCI patients from controls (0.43 and 0.38, respectively), compared to the 0.57 and 0.47 found by Kantarci [18]. Different ADC values in AD and MCI patients compared to controls indicate that DWI is sensitive to subtle microstructural changes that occur in the course of degenerative process of AD type, but showing less accuracy than visual and planimetric MTA rating in distinguishing AD and MCI from healthy controls.
The third evaluated pathological process within the hippocampus regarded disturbances of cerebral microcirculation measured with PWI using the parameter of rCBV. The studies on the use of this method in diagnosis of AD and MCI are still limited in the literature [20,21,32,33], but they parallel changes in metabolism and blood flow seen with PET and SPECT [34–37]. In AD, significant hypoperfusion was found in the posterior cingulate, temporo-parietal, and frontal sensori-motor cortices [20,22,33]. The results of perfusion studies within the hippocampus have been contradictory. Some of them revealed significant hypoperfusion within hippocampal formation in AD and MCI patients compared to controls [21,33], which was not confirmed by Luckhaus et al. [38]. In our study, we did not find any significant changes in perfusion parameters among the 3 evaluated groups. Even more interesting, we observed slightly increased rCBV values in AD patients compared to the control group and the MCI subgroup. On one hand, this fact may be explained by the technical problems we encountered in the evaluation of this region, especially in AD patients, due to small sizes of both hippocampi and close proximity of choroid plexus in temporal horns of lateral ventricles, inclusion of which, within ROIs, may lead to an overestimation of the perfusion parameters [39]. In their first study, Cavallin et al also experienced difficulties in interpreting DSC images in the temporal regions [40]. On the other hand, another explanation of relatively high perfusion parameters in hippocampi of the AD patients could be the presence of luxury perfusion in those structures. This hypothesis was drawn from the results of SPECT and PET studies of the medial temporal structures, which did not reveal significant decrease in blood flow in early AD [41] or even in mid-to-moderate AD, despite significant reduction of oxygen metabolism [42].
We also aimed to evaluate any associations between the 3 pathological processes, such as hippocampal atrophy, microstructural damage, and perfusion disturbances in AD and MCI patients. In all patients the increasing rate of MTA was correlated with the increasing water diffusivity within hippocampal formation. This result is not surprising, since the increased diffusivity in the brains of patients with AD and MCI has been attributed to the loss of neuronal cell bodies, axons, and dendrites, causing enlargement of the extracellular space where water diffusivity is faster. Loss of hippocampal neuron cell bodies, synapses and dendrites early in the pathological progression of AD leads to macroscopic atrophy of the hippocampus, which can be assessed with planimetric measurements [19]. On the other hand, we did not notice any differences in rCBV among the subject groups, despite different rates of MTA. This finding may confirm the results of previous SPECT and PET, as well as a few DSC MRI studies revealing that perfusion measurements are not related to brain atrophy [21,38,41,42]. These data also support earlier indications that perfusion and volume changes within hippocampi are at least partially dissociated in the pathogenesis of AD [38].
We also investigated correlations between pathological changes within hippocampal region and the scores of neuropsychological tests such as the MMSE. Only in the subgroup of MCI patients were the lower MMSE values significantly associated with the increased rates of MTA and the increased ADC values, but AD patients did not show any correlation between MMSE scores and hippocampal pathology. The explanation of these findings may be that in AD, brain pathology is more widespread and not only hippocampal changes influence the cognitive state in these patients, while in MCI in the early stages of dementia the crucial pathology is the most pronounced and focused in hippocampi, which has a direct influence on cognition. Correlations of MTA with MMSE, as well as several executive functions, including flexibility, inhibition, working memory and set shifting performance, have already been reported [4,5]. This implies an important role of MTA in age-related decline, and its important role in memory and learning. Hippocampi have connections with the prefrontal cortex, what may explain the correlation between MTA and MMSE. Results of previous studies show the importance of the prefrontal-hippocampal circuit with regard to working memory performance. This implies that diminished functioning of this circuit, as a consequence of MTA, results in decreased working memory performance and hence impaired executive functions [5].
Conclusions
In patients with AD we found significant rates of medial temporal atrophy with increased diffusivity of water reflecting microstructural changes with no detectable alterations in blood perfusion within the hippocampus. In MCI we also showed anatomical and microstructural changes similar to those found in AD, but less severe, which reflects the fact that involvement of the hippocampi occurs very early during the pathological progression of AD type degeneration.
Progressing atrophy of hippocampi is associated with the increasing water diffusivity, but not with the changes in perfusion, in patients with AD and MCI.
Of several hippocampal measurements, Scheltens’ visual rating scale and simple planimetric measurements of hippocampal index, hippocampal size, and medial CSF space index are the methods showing the highest accuracy in distinguishing between AD, MCI, and the control group.
Footnotes
Source of support: This work was supported by government grant number (KB/129/13422/IT1-B/U/08)
References
- 1.Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27:578–84. doi: 10.1111/j.1440-1789.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 2.Risse SC, Raskind MA, Nochlin D, et al. Neuropathological findings in patients with clinical diagnoses of probable Alzheimer’s disease. Am J Psych. 1990;147:168–72. doi: 10.1176/ajp.147.2.168. [DOI] [PubMed] [Google Scholar]
- 3.Ball MJ, Fisman M, Hachinski V, et al. A new definition of Alzheimer’s disease: a hippocampal dementia. Lancet. 1985;i:14–16. doi: 10.1016/s0140-6736(85)90965-1. [DOI] [PubMed] [Google Scholar]
- 4.Scheltens P, Leys D, Barkhof F. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal aging: diagnostic value and neuropsychological correlations. J Neurol Neurosurg Psychiatry. 1992;55:967–72. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–47. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golebiowski M, Barcikowska M, Pfeffer A, et al. Magnetic resonance-based hippocampal volumetry in patients with dementia of the Alzheimer type. Dement Geriatr Cogn Disord. 1999;10:284–88. doi: 10.1159/000017133. [DOI] [PubMed] [Google Scholar]
- 7.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–96. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 8.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132:195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- 9.Visser PJ, Verhey FR, Hofman PA, et al. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72:491–97. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korf E, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- 11.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment. Prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 12.Colliot O, Chetalet G, Chupin M, et al. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Neuroradiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- 13.Czarnecka A, Zimny A, Sasiadek M. Correlation of CT perfusion and CT volumetry in patients with Alzheimer’s disease. Pol J Radiol. 2010;75(2):16–22. [PMC free article] [PubMed] [Google Scholar]
- 14.Galton CJ, Patterson K, Graham K, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- 15.Mummery CJ, Patterson K, Price CJ, et al. A voxel based morphometry study of semantic dementia: the relation of temporal lobe atrophy to cognitive deficit. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 16.Bresciani L, Rossi R, Testa C, et al. Visual assessment of medial temporal lobe atrophy on MR films in Alzheimer’s disease: Comparison with volumetry. Aging Clin Exp Res. 2005;17:8–13. doi: 10.1007/BF03337714. [DOI] [PubMed] [Google Scholar]
- 17.Zeineh MM, Holdsworth S, Skare S, et al. Challenges of high resolution diffusion imaging of the human medial temporal lobe in Alzheimer’s disease. Top Magn Reson Imaging. 2010;21(6):355–65. doi: 10.1097/RMR.0b013e31823f6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional diffusivity of water in mild cognitive impairment and Alzheimer’s disease. Radiology. 2001;219(1):101–7. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantarci K, Petersen RC, Boeve BF, et al. DWI predicts future progression to Alzheimer’s disease in amnestic mild cognitive impairment. Neurology. 2005;64(5):902–4. doi: 10.1212/01.WNL.0000153076.46126.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris GJ, Lewis RF, Satlin A, et al. Dynamic Susceptibility Contrast MR Imaging of Regional Cerebral Blood Volume in Alzheimer Disease: A promising Alternative to Nuclear Medicine. AJNR. 1998;19:1727–32. [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzao A, Floris R, Baviera ME, et al. Diffusion and perfusion MR imaging in cases of Alzheimer’s disease: correlations with cortical atrophy and lesion load. AJNR. 2001;22:1030–36. [PMC free article] [PubMed] [Google Scholar]
- 22.Zimny A, Szewczyk P, Czarnecka A, et al. Usefulness of perfusion-weighted MR imaging in the differential diagnosis of Alzheimer’s disease and mild cognitive impairment. Med Sci Monit. 2010;16(Suppl 1):5–10. [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 24.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, Doody R, Kurz A. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 26.Stejskal EO, Tanner JE. Spin diffusion measurements: spin-echo in presence of time dependent field gradient. J Chem Phys. 1965;42:288–92. [Google Scholar]
- 27.Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990;14:538–46. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- 28.Talbot PR, Lloyd JJ, Snowden JS, et al. Choice of reference region in the quantification of single-photon emission tomography in primary degenerative dementia. Eur J Nucl Med. 1994;21:503–8. doi: 10.1007/BF00173036. [DOI] [PubMed] [Google Scholar]
- 29.Wahlund LO, Julin P, Lindqvist J, Scheltens P. Visual assessment of medial temporal lobe atrophy in demented and healthy controls: correlation with volumetry. Psychiatry Res. 1990;90:193–99. doi: 10.1016/s0925-4927(99)00016-5. [DOI] [PubMed] [Google Scholar]
- 30.Sandson TA, Felician O, Edelman RR, Warach S. Diffusion weighted magnetic resonance imaging in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1999;10:166–71. doi: 10.1159/000017099. [DOI] [PubMed] [Google Scholar]
- 31.Hanyu H, Sakurai H, Takasaki M, et al. Diffusion weighted MR imaging of the hippocampus and temporal white matter in Alzheimer’s disease. J Neurol Sci. 1998;156:195–200. doi: 10.1016/s0022-510x(98)00043-4. [DOI] [PubMed] [Google Scholar]
- 32.Fayed N, Davila J, Oliveros A, et al. Utility of different MR modalities in mild cognitive impairment and its use as a predictor of conversion to probable dementia. Acad Radiol. 2008;15:1089–98. doi: 10.1016/j.acra.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Cavallin L, Axelsson R, Wahlund LO, et al. Voxel-based correlation between coregistered single-photon emission computed tomography and dynamic susceptibility contrast magnetic resonance imaging in subjects with suspected Alzheimer disease. Acta Radiol. 2008;49:1154–61. doi: 10.1080/02841850802438512. [DOI] [PubMed] [Google Scholar]
- 34.Mielke R, Kessler J, Szelies B, et al. Normal and pathological aging: findings of positron–emission-tomography. J Neural Transm. 1998;105:821–37. doi: 10.1007/s007020050097. [DOI] [PubMed] [Google Scholar]
- 35.Jagust WJ. Neuroimaging in dementia. Neurol Clin. 2000;18:885–902. doi: 10.1016/s0733-8619(05)70231-0. [DOI] [PubMed] [Google Scholar]
- 36.Petrella JR, Coleman RE, Doraiswamy PM. Neuroimaging and early diagnosis of Alzheimer disease: a look to the future. Radiology. 2003;226:315–36. doi: 10.1148/radiol.2262011600. [DOI] [PubMed] [Google Scholar]
- 37.Chetelat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–77. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 38.Luckhaus C, Cohnen M, Flus MO, et al. The relation of regional cerebral perfusion and atrophy in mild cognitive impairment (MCI) and early Alzheimer’s dementia. Psychiatry Research: Neuroimaging. 2010;183:44–51. doi: 10.1016/j.pscychresns.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Yoshiura T, Mihara F, Kuwabara Y, et al. MR relative cerebral blood flow mapping of Alzheimer disease: correlation with Tc-99m-HMPAO SPECT. Acad Radiol. 2002;9:1383–87. doi: 10.1016/s1076-6332(03)80665-7. [DOI] [PubMed] [Google Scholar]
- 40.Cavallin L, Danielsson R, Oksengard AR, et al. Can dynamic susceptibility contrast magnetic resonance imaging replace single-photon emission computed tomography in the diagnosis of patients with Alzheimer’s disease? A pilot study. Acta Radiol. 2006;47:977–85. doi: 10.1080/02841850600965070. [DOI] [PubMed] [Google Scholar]
- 41.Kogure D, Matsuda H, Ohnishi T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–62. [PubMed] [Google Scholar]
- 42.Ishii K, Sasaki M, Yamaji S, et al. Paradoxical hippocampus perfusion in mild-to-moderate Alzheimer’s disease. J Nucl Med. 1998;39:293–98. [PubMed] [Google Scholar]