Abstract
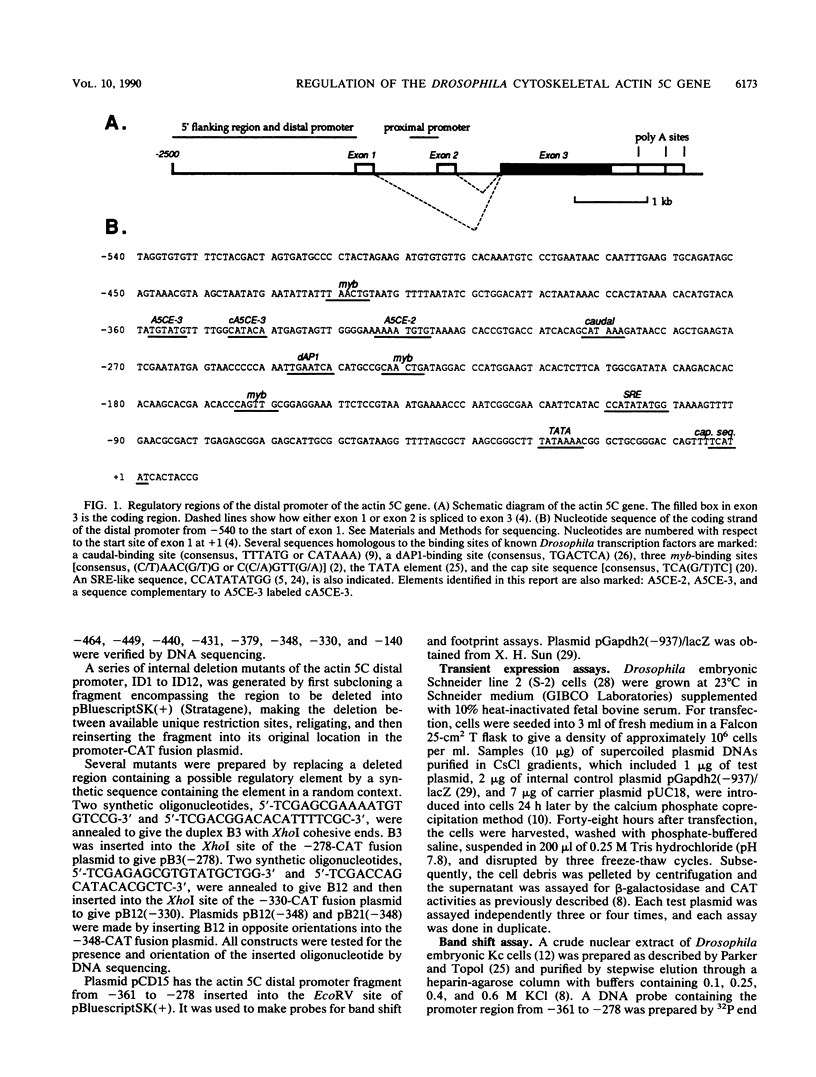
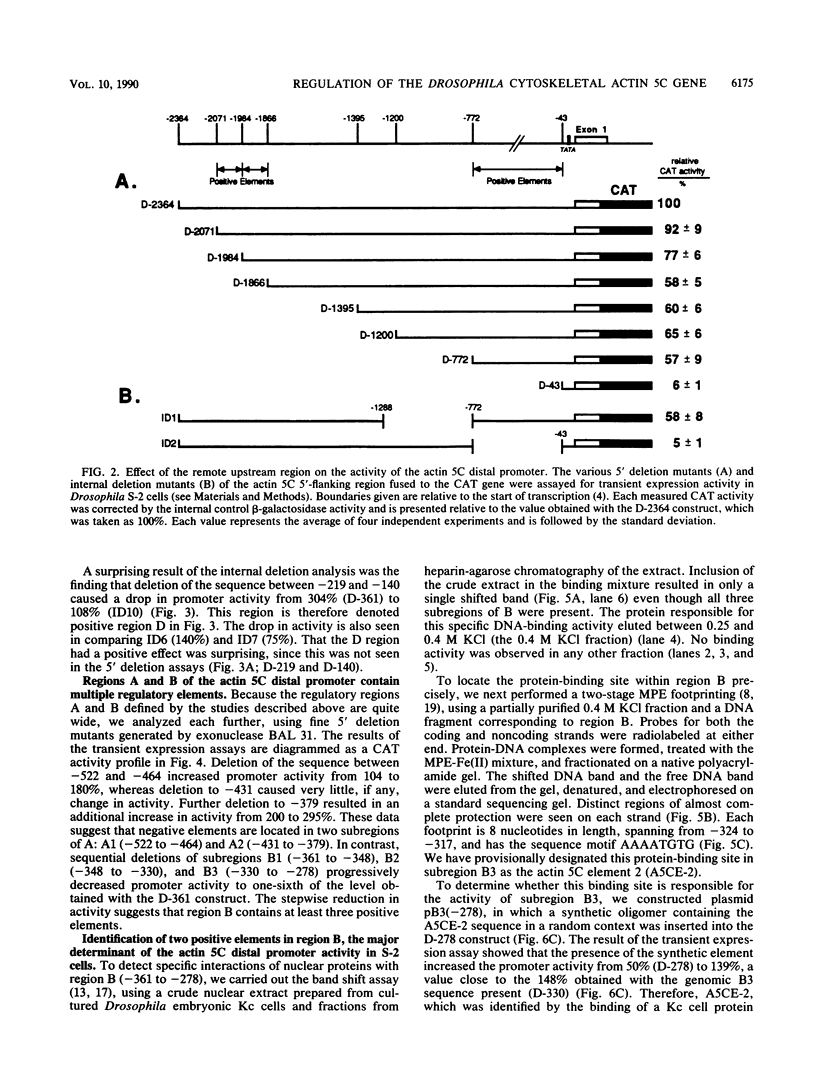
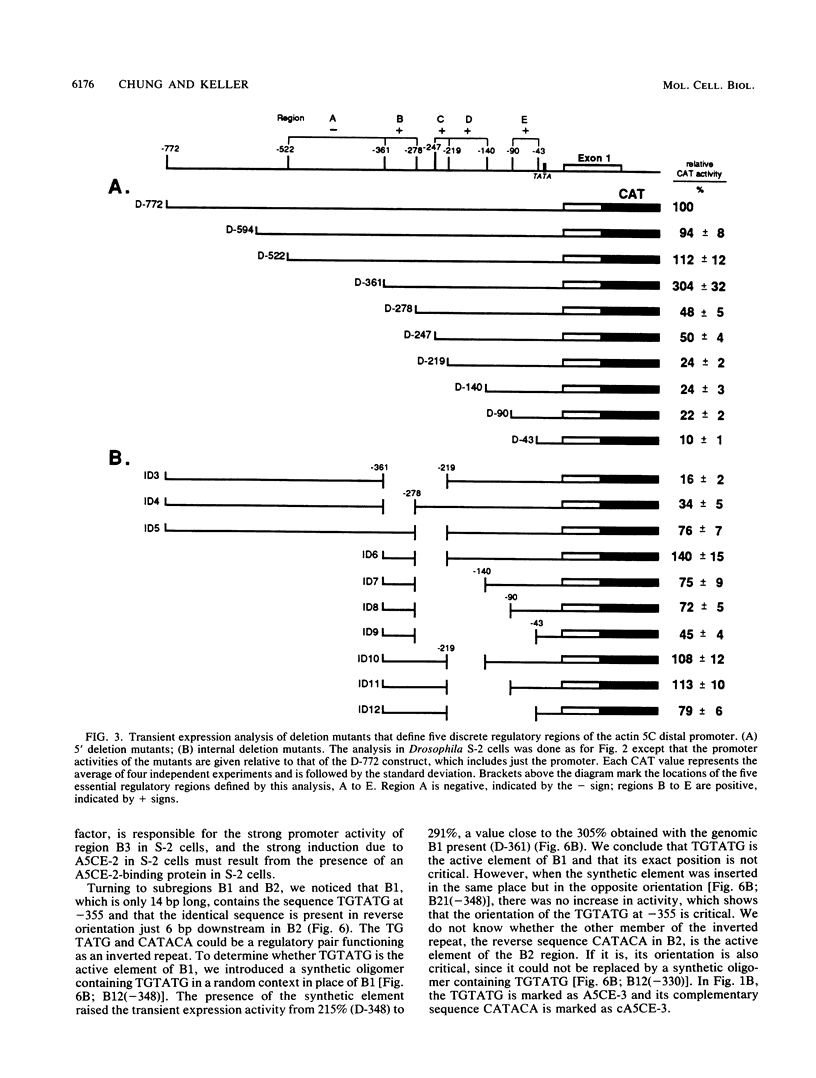
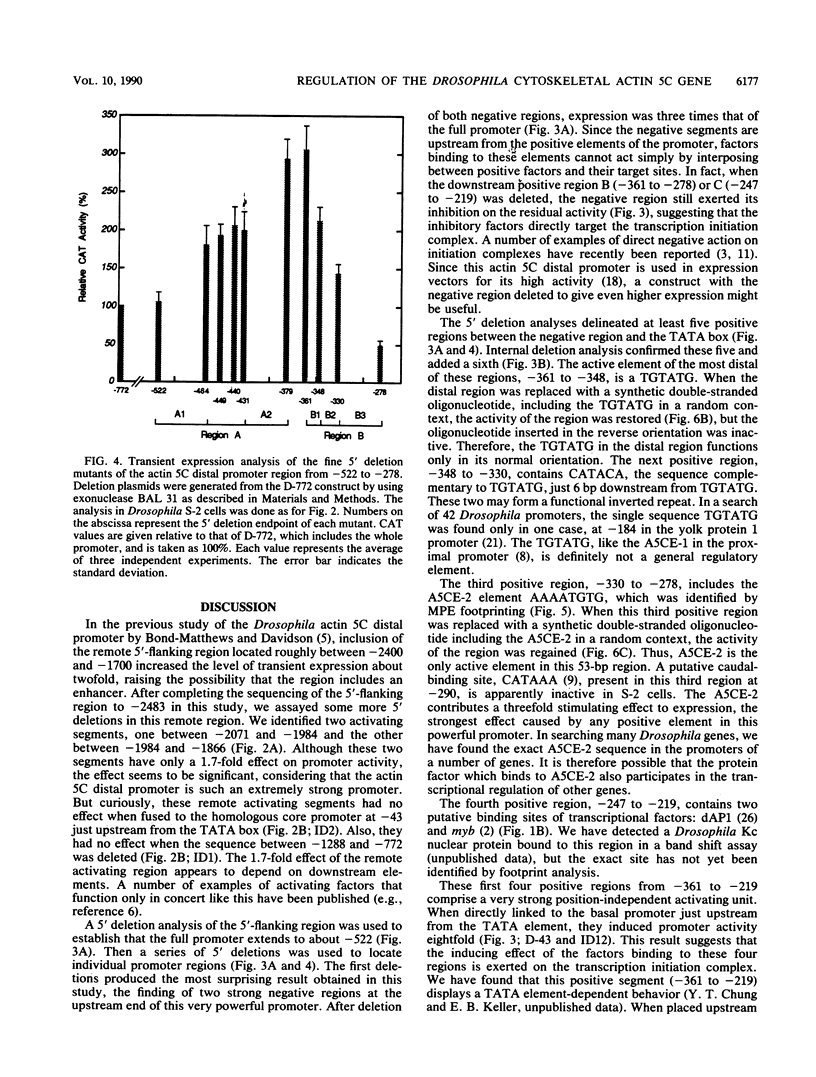
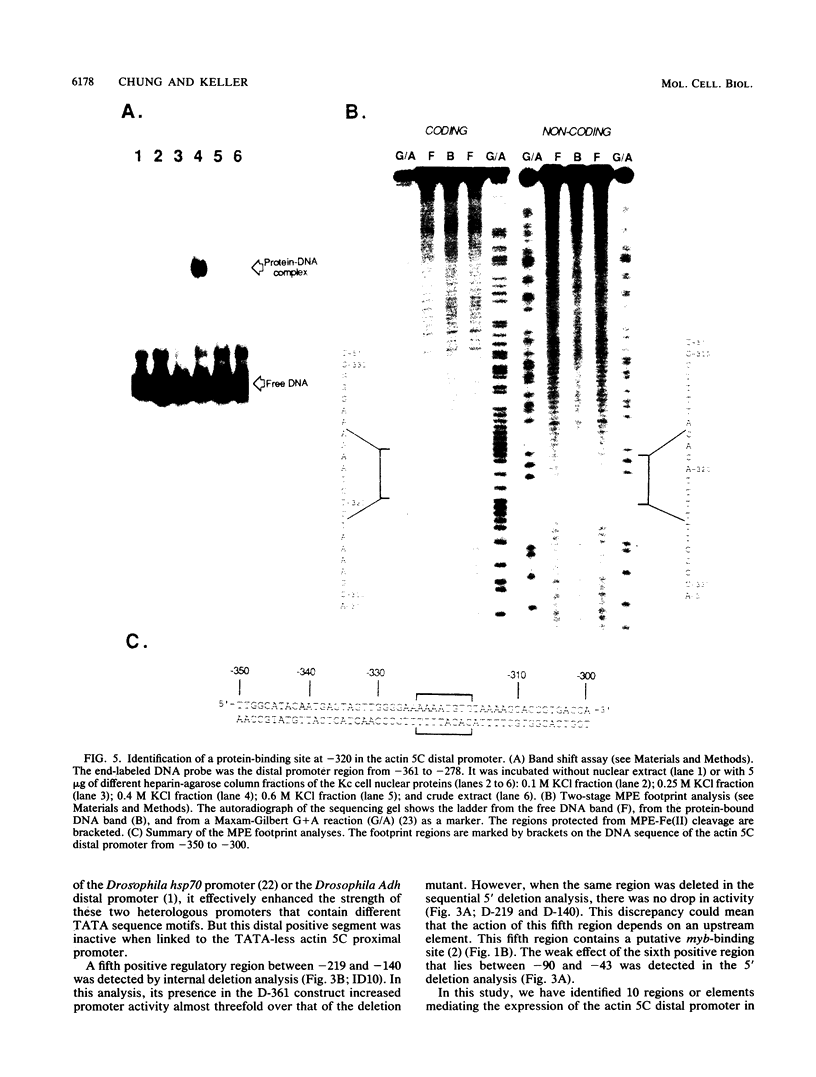
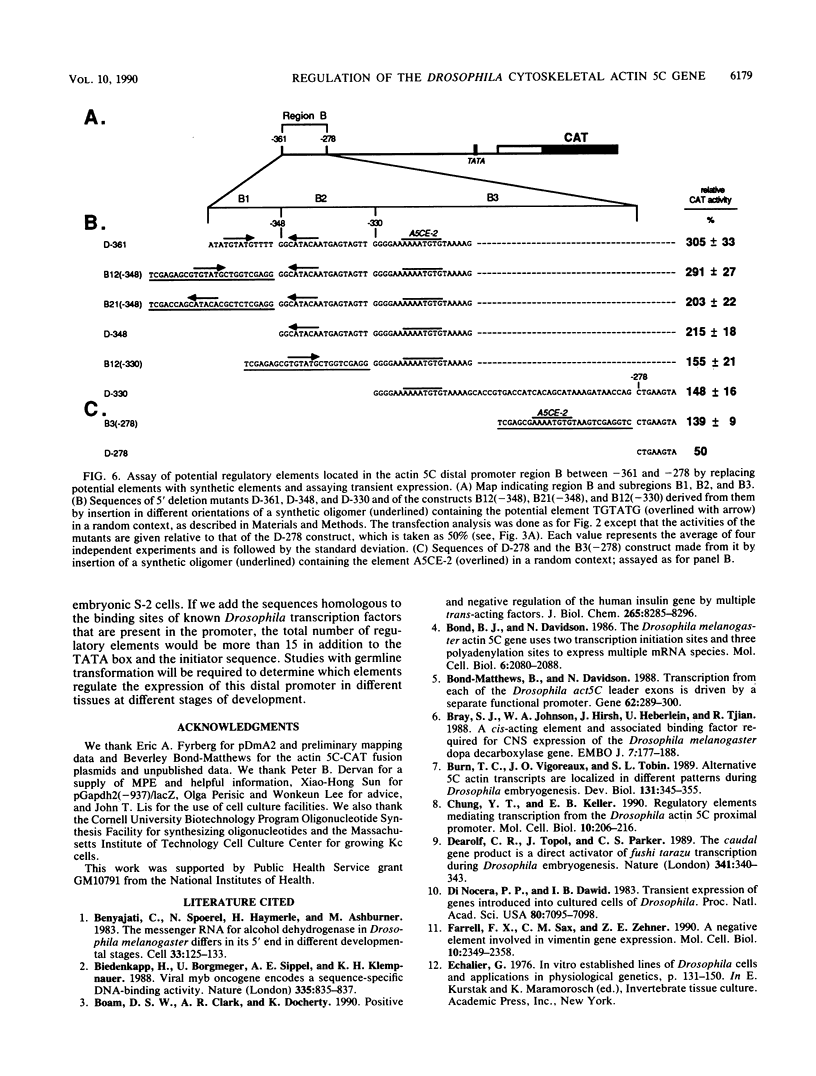
The major cytoskeletal actin gene of Drosophila melanogaster, the actin 5C gene, has two promoters, the distal one of which controls synthesis of actin in a tissue- and developmental stage-specific manner. This very strong promoter has widely been used for expression of heterologous genes in cultured cells. To locate functional regulatory elements in this distal promoter, mutants of the promoter were fused to the bacterial chloramphenicol acetyltransferase gene and assayed for transient expression activity in cultured Drosophila embryonic Schneider line 2 cells. The results showed that the upstream end of the promoter extends to 522 bp from the transcription start site. In addition, there are two remote activating regions about 2 kb upstream. Between -522 and -379 are two regions that exert a strong negative effect. Downstream from these negative regions are at least six positive regions and a TATA element. The strongest positive determinant of the promoter was identified at -320 as AAAATGTG by footprinting and by a replacement experiment. When the relevant region was replaced by a synthetic sequence containing this element in a random context, the transient expression activity was restored. The sequence TGTATG located at -355 was also identified as a positive element by a similar replacement approach. Apparently the very high activity of this promoter is the result of the combined activities of multiple factors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Boam D. S., Clark A. R., Docherty K. Positive and negative regulation of the human insulin gene by multiple trans-acting factors. J Biol Chem. 1990 May 15;265(14):8285–8296. [PubMed] [Google Scholar]
- Bond-Matthews B., Davidson N. Transcription from each of the Drosophila act5C leader exons is driven by a separate functional promoter. Gene. 1988;62(2):289–300. doi: 10.1016/0378-1119(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Bond B. J., Davidson N. The Drosophila melanogaster actin 5C gene uses two transcription initiation sites and three polyadenylation sites to express multiple mRNA species. Mol Cell Biol. 1986 Jun;6(6):2080–2088. doi: 10.1128/mcb.6.6.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. J., Johnson W. A., Hirsh J., Heberlein U., Tjian R. A cis-acting element and associated binding factor required for CNS expression of the Drosophila melanogaster dopa decarboxylase gene. EMBO J. 1988 Jan;7(1):177–188. doi: 10.1002/j.1460-2075.1988.tb02798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn T. C., Vigoreaux J. O., Tobin S. L. Alternative 5C actin transcripts are localized in different patterns during Drosophila embryogenesis. Dev Biol. 1989 Feb;131(2):345–355. doi: 10.1016/s0012-1606(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Chung Y. T., Keller E. B. Regulatory elements mediating transcription from the Drosophila melanogaster actin 5C proximal promoter. Mol Cell Biol. 1990 Jan;10(1):206–216. doi: 10.1128/mcb.10.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearolf C. R., Topol J., Parker C. S. The caudal gene product is a direct activator of fushi tarazu transcription during Drosophila embryogenesis. Nature. 1989 Sep 28;341(6240):340–343. doi: 10.1038/341340a0. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell F. X., Sax C. M., Zehner Z. E. A negative element involved in vimentin gene expression. Mol Cell Biol. 1990 May;10(5):2349–2358. doi: 10.1128/mcb.10.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hertzberg R. P., Dervan P. B. Cleavage of DNA with methidiumpropyl-EDTA-iron(II): reaction conditions and product analyses. Biochemistry. 1984 Aug 14;23(17):3934–3945. doi: 10.1021/bi00312a022. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Hung M. C., Wensink P. C. The sequence of the Drosophila melanogaster gene for yolk protein 1. Nucleic Acids Res. 1981 Dec 11;9(23):6407–6419. doi: 10.1093/nar/9.23.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mohun T., Garrett N., Treisman R. Xenopus cytoskeletal actin and human c-fos gene promoters share a conserved protein-binding site. EMBO J. 1987 Mar;6(3):667–673. doi: 10.1002/j.1460-2075.1987.tb04806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988 Dec 20;7(13):4265–4273. doi: 10.1002/j.1460-2075.1988.tb03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. H., Lis J. T., Wu R. The positive and negative transcriptional regulation of the Drosophila Gapdh-2 gene. Genes Dev. 1988 Jun;2(6):743–753. doi: 10.1101/gad.2.6.743. [DOI] [PubMed] [Google Scholar]
- Vigoreaux J. O., Tobin S. L. Stage-specific selection of alternative transcriptional initiation sites from the 5C actin gene of Drosophila melanogaster. Genes Dev. 1987 Dec;1(10):1161–1171. doi: 10.1101/gad.1.10.1161. [DOI] [PubMed] [Google Scholar]



