Abstract
Sex is an important factor in the response to ischemic insults in both the laboratory and the clinic. Inflammation and cell death are points where sex-specific pathways diverge in stroke, and serum estrogen levels status affect the response to inflammation. The cytokine macrophage migration inhibitory factor (MIF) is detrimental in experimental stroke models in male animals. However MIF is known to have sex-specific actions on inflammation and wound healing. The role of MIF in the ischemic female brain has not been evaluated. A transient middle cerebral artery occlusion (MCAO/90 minutes) model was used to induce stroke in male, intact female, and ovariectomized female wildtype (WT) and MIF knockout (KO) mice. Infarct size was quantified 72 hours after stroke. Protein and cytokine levels were assessed post stroke. Female MIF KO mice had significantly larger strokes compared to WT females (mean hemispheric infarct±SEM: 63%±2% versus 29%±3%; n=8; p<0.05). Ovariectomized female MIF KO mice also had larger infarcts than ovariectomized WT littermates (70%±3% versus 47%±4%; n=11; p<0.05). In males, however, infarct size was equivalent between MIF KO and WT mice (63%±2% versus 67%±3%; n=9; p=0.25). There were no significant differences in cytokine levels at 6 hours post-infarct between mice of either genotype in brain. MIF KO females displayed more microglial activation (ionized calcium binding adaptor molecule 1 (Iba1) immunofluoresence) after stroke than did WT mice or MIF KO males. The larger infarcts in MIF KO females were associated with an early increase in mitochondrial localization of Jun activation domain-binding protein 1 (JAB1). Loss of MIF exacerbated injury in the female brain after experimental stroke, which was independent of changes in pro-inflammatory cytokine levels. This response is sex-specific, and is in part independent of physiological serum levels of estrogen.
Keywords: Focal cerebral ischemia, stroke, middle cerebral artery occlusion, macrophage migration inhibitory factor, MIF, JAB1, sex differences, estrogen, inflammation, mouse
Introduction
Each year acute stroke affects approximately 800,000 people in the U.S., with 12% of those patients dying within 1 month of stroke and a third of stroke survivors experiencing permanent disability (Roger et al., 2012). While the incidence of stroke is higher in men than in women throughout most of the lifespan, at older ages more women than men experience strokes (Roger et al., 2012). Estrogen (E2) is a potent neuroprotective agent in vitro and in vivo (Garcia-Segura et al., 2001; McCullough and Hurn, 2003; Stein, 2001). However, randomized clinical trials in postmenopausal women have not shown a benefit from chronic E2 treatment for stroke prevention (Viscoli et al., 2001; Wassertheil-Smoller et al., 2003), in part due to issues with study design (Turtzo and McCullough, 2008). Sex differences in stroke epidemiology and outcome cannot be explained on the basis of circulating levels of gonadal steroids alone (Du et al., 2004; Li et al., 2005; Zhang et al., 2003; Zhu et al., 2006). Laboratory and clinical data indicate that sex (e.g., male versus female) and gonadal steroid exposure both play important roles in the response to an ischemic insult (Turtzo and McCullough, 2008; Turtzo and McCullough, 2010). Sex differences in ischemic sensitivity may also become more apparent when the influence of gonadal steroids are removed by gonadectomy or with senescence.
Inflammation is a point where sex pathways diverge (Ritzel et al., 2012). A leading candidate for mediating the relationship between inflammation, E2, and sex is the cytokine macrophage migration inhibitory factor (MIF). MIF’s effects are mediated via interaction with the cell surface receptor CD74 (Savaskan et al., 2012) or through MIF’s endocytosis and binding to the intracellular protein JAB1 (also known as COP9 signalsome subunit 5 (CSN5)) (Rendon et al., 2009). MIF promotes inflammation in diseases including sepsis (Bernhagen et al., 1993) and atherosclerosis (Burger-Kentischer et al., 2002; Pan et al., 2004), and mediates the response to response to oxidative stress (Kudrin and Ray, 2007) and cardiac ischemia (Miller et al., 2008). A clear interaction between MIF and E2 exists in many divergent models including osteoporosis (Hsieh et al., 2007; Onodera et al., 2007a; Onodera et al., 2007b; Oshima et al., 2006) and wound healing (Ashcroft et al., 2003).
MIF plays a protective role in acute cardiac ischemic injury, as genetic deletion of MIF increased injury in males in a mouse model of cardiac ischemia (Miller et al., 2008), via inhibition of c-Jun N-terminal kinase (JNK)-mediated apoptosis and a reduction in oxidative cell stress during reperfusion (Luedike et al., 2012). MIF expression is rapidly upregulated in brain after experimental stroke (Wang et al., 2009), and an enriched environment results in the downregulation of MIF in male mice (Inacio et al., 2011c). In contrast to its role in cardiac injury MIF’s presence appears to be deleterious in male mice after transient cerebral ischemia, as shown by exacerbation of injury after middle cerebral artery occusion (MCAO) in male MIF KO mice (Inacio et al., 2011b). As MIF contributes to sex differences in other inflammatory diseases, we hypothesized that genetic deletion of MIF would have different consequences in male and female mice after stroke.
Materials and Methods
Animals
The MIF KO mice were from the original MIF genetic deletion (Bozza et al., 1999) backcrossed onto a C57BL6 background (Taylor et al., 2007; Taylor et al., 2006). Male and female C57BL6 littermate mice (ages 10 to 12 weeks) were utilized in this study. Genotype was determined by polymerase chain reaction with the following 5’ to 3’ primer sequences, specific for WT and mutant (the knockout allele contains a neomycin marker) alleles, respectively: ACGACATGAACGCTGCCAAC (forward) and ACCGTGGTCTCTTATAAACC (reverse); GAATGAACTGCAGGACGAGG (forward) and GCTCTTCGTCCAGATCATCC (reverse). This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research. All animal protocols were approved by the Center for Laboratory Animal Care of the University of Connecticut Health Center.
Ovariectomy (Ovx)
Ovaries were surgically removed from female mice 2 weeks prior to middle cerebral artery occlusion (MCAO) and a subcutaneous pellet containing either sesame oil or 17β-estradiol (180 µg/ml) was inserted, yielding physiological levels of E2 (Li et al., 2004; McCullough and Hurn, 2003). Serum E2 levels were measured by ELISA kit (IBL, Hamburg, Germany) and/or uterine weights at sacrifice were recorded to confirm successful ovariectomy.
Middle Cerebral Artery Occlusion (MCAO)
Male and female (intact and ovariectomized (Ovx) mice (weight 20 to 25 g) were subjected to MCAO as previously described (Sawada et al., 2000). In brief, mice were induced under 4% isoflurane, with maintenance at 1.5% isoflurane. Core body temperature was maintained at 37±0.5°C. A midline incision was made on the ventral neck, and the right common carotid artery and its bifurcation were exposed. A silicon-coated monofilament suture was introduced through the external carotid artery, passed into the internal carotid artery, and advanced to the root of right middle cerebral artery (MCA). The animal was allowed to awaken from anesthesia for documentation of neurological deficits to confirm successful occlusion. The animal was then reanesthetized and the suture removed after 90 minutes of MCAO. Survival (reperfusion) times varied depending upon the cohort examined. Sham surgery was identical to the MCAO protocol except that the monofilament was not inserted into the MCA. After sacrifice, brains were perfused and sectioned, or homogenized for protein extraction. All animals were randomly assigned to sham vs. MCAO cohorts.
Behavioral Testing
Neurologic deficit (ND) was scored 30 minutes after induction of ischemia and again at 6, 24, and/or 72 hours. The scoring system was as follows: 0, no deficit; 1, torso turning to the affected side and forelimb weakness when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, either barrel rolling or no spontaneous motor activity (Li et al., 2004).
Physiological Assessment
Physiological measurements were performed to compare all cohorts of mice. Monitoring of physiological variables and laser Doppler flowmetry (LDF) was performed in companion cohorts as previously described (n=4/gp in all genotypes)(McCullough et al., 2004; McCullough et al., 2005a; McCullough et al., 2005b; Sawada et al., 2000).
TTC Staining
Brain slices were incubated in 2,3,5-triphenyltetrazolium chloride (TTC) at 37°C and analyzed by an investigator blinded to mouse sex, hormonal status, and genotype as previously described (McCullough et al., 2004; McCullough et al., 2005a; McCullough et al., 2005b; Sawada et al., 2000).
Protein Lysis/Fractionation/Western Blot
At 6 hours after induction of stroke, brains were rapidly harvested, with removal of the frontal and occipital poles and cerebellum to enrich for territory supplied by the MCA. Brains were frozen at −80°C until preparation in lysis buffer, and fractionated into cytoplasmic, mitochondrial, and nuclear fractions prior to gel electrophoresis and Western blotting using primary antibodies to MIF (1:2000, Cell Sciences, Canton, MA), JAB1 (1:1000, Abcam, Cambridge, MA), β-actin (1:5000, Millipore, Billerica, MA), histone as a marker of nuclear samples (1:2000, US Biologicals, Swampscott, MA), and cytochrome c oxidase (COXIV) as a marker of mitochondrial samples (1:1000, Abcam, Cambridge, MA) as previously described (McCullough et al., 2005b). For fractionation, the infarcted (or sham equivalaent) hemispheres of two mice were pooled for each sample.
Co-immunoprecipitation
Co-immunoprecipitation was performed using Protein A-Sepharose beads(generously donated by Elizabeth Eipper, University of Connecticut Health Center). Mitochondrial lysate (100ug) was incubated with 1ug of the appropriate antibody (JAB, Abcam or MIF, Cell Sciences) at room temperature for 1hr to form the immune complex. Then 100ul of a 50% slurry of Protein A beads in PBS was added to the complex and incubated for 1 hour at room temperature. Beads were spun down, the unbound fraction removed and then the beads were extensively washed. To elute off the complex, the beads were incubated in sample buffer and boiled. Western blotting was performed to assess the complex as described above.
Cytokine assays
Cytokine expression from brain, and spleen at 6 hours post-stroke was assayed using the Ready-SET-Go! cytokine enzyme-linked immunosorbent assay (ELISA) kits for interleukin-1β (IL-1β), tumor necrosis factor- α (TNF-α), and interleukin-6 (IL-6) per manufacturer’s instructions (eBioscience, San Diego, CA).
Immunohistochemistry (IHC)
24 hours after MCAO induction, mice were perfused with ice-cold phosphate buffered saline (PBS) containing 1% heparin followed by 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde/PBS for 6 hours, then placed in sucrose/PBS. The cutting and staining protocol were as described (McCullough et al., 2005a; McCullough et al., 2005b; Rasband et al., 1999), using the following primary antibodies: MIF (Cell Sciences, Canton, MA); NeuN (Millipore, Billerica, MA), CD 74 (Abcam, Cambridge, MA), and Iba1 (Wako, Richmond, VA). To perform numerical quantificaation of Iba1-positive cells, four coronal brain slices/animal (n = 3 animals/group) were stained from standard coordinates (5.2 mm interaural, 1.4 mm bregma), (4.5 mm interaural, 0.7 mm bregma), (3.8 mm interaural, 0.0 mm bregma) and (3.1 mm interaural,- 0.7 mm bregma). Three 20× fields/section were analyzed in the penumbral area of the infarct from stroke brains and an anatomically similar area in sham animals. Iba1 positive cells were counted using MacBiophotonics ImageJ software with a DAPI counterstain by a blinded investigator (VV). The average total number of + cells from four sections for each animal with three fields of view/section was used for statistical analysis.
Statistical Analysis
All data were expressed as the mean ± standard error of the mean (SEM). Infarction volume data and Iba1 quantification were analyzed by one-way analysis of variance (ANOVA) with a post hoc Tukey test to correct for multiple comparisons. Estradiol levels were analyzed by Student’s t-test. Nonparametric behavioral scores were analyzed by the Mann-Whitney u-test. All infarct analysis, behavioral testing, and IHC was performed by an investigator blinded to genotype and sex. Physiological data were analyzed by two-way ANOVA with SPSS Statistics software (IBM, Armonk, NY) with each physiological paremeters as a dependent variable and time and genotype as fixed factors.
Results
To control for activational effects of sex steroids, males were compared to Ovx females in WT and MIF KO mice. Mice were subjected to a 90 minute MCAO followed by 72 hour post-stroke survival to include the peak stroke-induced inflammatory response (Jin et al., 2010; Schilling et al., 2003) (Figure 1). There was no difference in infarct size between male WT and KO mice (% infarct in hemisphere: WT= 66.7±2.7%; KO=63.0±1.5%; n=8 and 9, respectively; p>0.05). Surprisingly, intact female KO mice had a significant increase in infarct size compared to intact female WT mice (% infarct in hemisphere: intact female WT=29.4±3.0%; intact female KO=63.3±2.0%; p<0.05). After the loss of ovarian hormones by surgical ovariectomy, MIF KO females also had larger infarcts than WT females (% infarct in hemisphere: Ovx female WT=47.3±4.7%; Ovx female KO=70.7±3.0%; n=11 per group; p<0.05).
Figure 1. Genetic deletion of MIF increases infarct size in female mice, but has no effect in male mice at 72 hours after 90 minutes of transient MCAO.
Images A through D show representative TTC staining of infarcted brains at similar locations, with infarcted tissue appearing white and viable tissue appearing red. The graphs labeled E through G depict the quantification of infarct sizes based on TTC analysis. A) WT male (n=8); B) MIF KO male (n=9); C) WT intact female (n=6); D) MIF KO intact female (n=6). E) Male mice (n=8 for WT and 9 for MIF KO); F) Randomly hormonal cycling female mice with intact ovaries (*, **, *** = p<0.05) (n=6 per group); G) Ovariectomized female mice (*, **, *** = p<0.05) (n=9 per group).
Despite the differences in infarct sizes, neurobehavioral scores of MIF KO and WT male and female mice during stroke, or at 6, 24, or 72 hours after stroke were similar (Figure 2). During ischemia, cortical cerebral blood flow was reduced to a comparable degree by MCAO between WT and KO and was equivalently restored during early reperfusion (male: ischemia WT 14.9 ±1.3 vs. KO 14.3 ± 1.0%, reperfusion WT 81.8±7.9% vs. KO 77.2±3.0%; intact female: ischemia WT 13.0±1.3% vs. KO 15.3±1.2%, reperfusion WT 78.5 ±6.3% vs. KO 75.6±5.5%). No difference between physiological parameters such as blood glucose, CO2, O2, mean arterial blood pressure was observed between WT and KO mice in either male or intact females (Tables 1 and 2).
Figure 2. Neurological deficit scores in WT and MIF KO mice during cerebral ischemia, and at 6, 24, and 72 hours after 90 minutes of transient cerebral ischemia.
The horizontal midline of the box plots indicates the mean, with the top and bottom lines of the boxes mark the maximum and minimum values, respectively. A) Male mice; B) Female mice; (n = minimum of 6 per group).
Table 1.
There is no difference in physiological parameters between the WT and male KO mice
| Group | Condition | pH | pCO2 | pO2 | Glucose | MABP (mmHg) |
|---|---|---|---|---|---|---|
| WT | Pre- ischemic |
7.38 ± 0.02 | 39 ± 2.6 | 124 ± 5.4 | 147 ± 9.3 | 72 ± 2.9 |
| Intra- ischemic |
7.35 ± 0.02 | 38 ± 1.3 | 126 ± 8.5 | 145 ± 6.6 | 74 ± 5.1 | |
| KO | Pre- ischemic |
7.41 ± 0.01 | 34.8 ± 2.8 | 115 ± 5.8 | 143 ± 11 | 71 ± 4.5 |
| Intra- ischemic |
7.37 ± 0.02 | 37 ± 2.8 | 113 ± 4.8 | 153 ± 18 | 76 ± 1.8 |
Blood samples were collected from the femoral artery at the onset of MCAO or 60 min from the onset of MCAO. Data are expressed as Mean±SEM. n = 4 per group. Student’s t-test was used to compare the difference of each parameter between WT and male KO groups.
Table 2.
There is no difference in physiological parameters between the WT and intact female KO mice
| Group | Condition | pH | pCO2 | pO2 | Glucose | MABP (mmHg) |
|---|---|---|---|---|---|---|
| WT | Pre- ischemic |
7.40 ± 0.01 | 36 ± 4.0 | 111 ± 7.9 | 144 ± 16 | 73 ± 2.5 |
| Intra- ischemic |
7.35 ± 0.02 | 37 ± 4.2 | 108 ± 9.7 | 159 ± 3.4 | 76 ± 2.5 | |
| KO | Pre- ischemic |
7.39 ± 0.02 | 32 ± 3.9 | 114 ± 10 | 155 ± 7.8 | 72 ± 3.0 |
| Intra- ischemic |
7.35 ± 0.02 | 36 ± 2.0 | 119 ± 6.9 | 158 ± 15 | 74 ± 2.8 |
Blood samples were collected from the femoral artery at the onset of MCAO or 60 min from the onset of MCAO. Data are expressed as Mean±SEM. n = 4 per group. Student’s t-test was used to compare the difference of each parameter between WT and intact female KO groups.
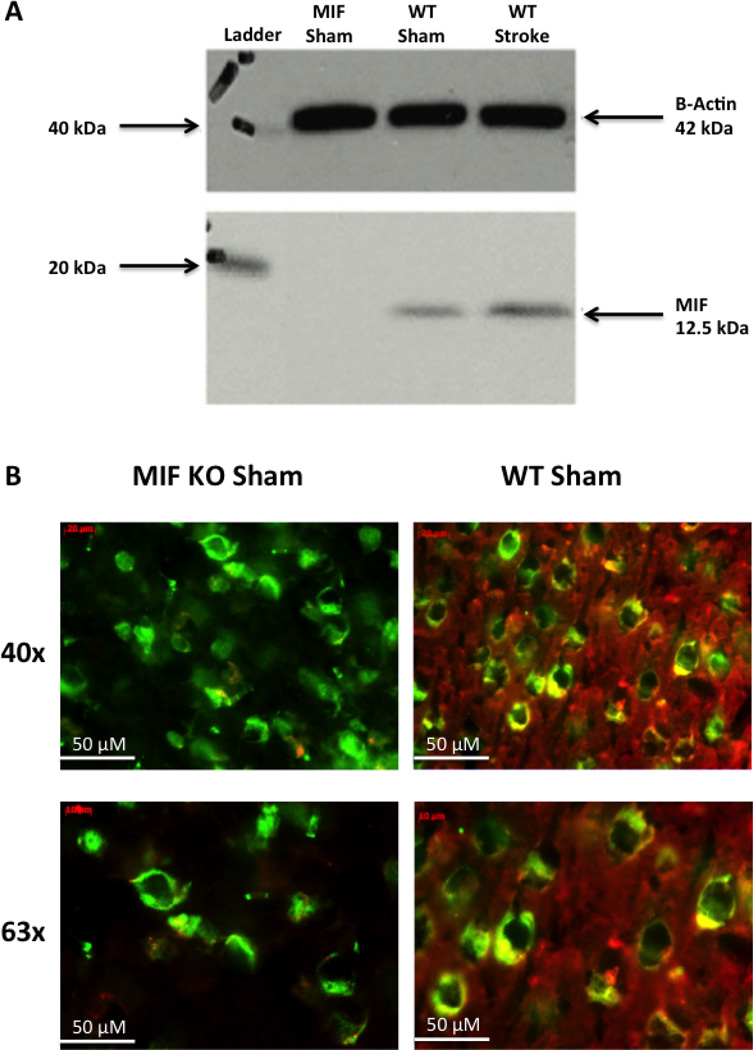
No MIF protein was detectable in MIF KO mice by Western blot or by immunohistochemistry, confirming the KO genotype (Figure 3).
Figure 3. MIF KO mice do not express MIF protein by Western blot (A) or immunofluorescent staining (B).
A) Western blot of total brain protein from MIF KO and WT intact females at 6 hours post-stroke. B) Immunofluorescence of mouse cerebral cortex for MIF (red) and NeuN (green) (n=3 per group). MIF is present in the cytoplasm of neurons in WT mice, while NeuN is located in neuronal nuclei of both WT and MIF KO mice.
As microglia are integral to the brain’s inflammatory response to stroke, and activation status can be influences by estrogen (Ritzel et al., 2012), the effects of MIF in microglial activation were examined (Figure 4). We first assessed Iba1 expression in sham brains of both male and female mice at 24 hours post surgery. No significant differences in the average number of Iba1 positive cells per field of vision was observed (WT female sham 19.2+/−0.8; KO female sham 18.5+/−0.9; p>0.05), (WT male sham 18.5+/−2; KO male sham 18.3+/−0.6; p>0.05). At 24 hours after MCAO surgery the number of Iba1 positive cells in the MIF KO female mice (38.7+/−2.1) was significantly highert (p<0.05) compared to to WT mice of either sex (WT male 29.4+/−2.3; WT female 26.3+/−1.5) and MIF KO male mice (30.2+/−1.9).
Figure 4. Microglial activation in cerebral cortex is higher in MIF KO female mice at 24 hours after stroke than in WT females, WT males, or MIF KO males, as assessed by Iba-1 staining of cerebral cortex.
A) WT female sham; B) WT female stroke; C) MIF KO female sham; D) MIF KO female stroke; E) WT male sham; F) WT male stroke; G) MIF KO male sham; H) MIF KO male stroke; Iba1 (red), NeuN (green), and DAPI (blue) (n= 3 per group). The yellow arrows point to examples of activated microglia in post-stroke cortex. Photographs were taken with a 20× objective at approximately 0.3 mm from Bregma within the infarcted cortex or an anatomically similar area in sham animals. I) Quantification of average number of Iba1-positive cell per field of vision (n=3 per group). The * symbol indicates a statistically different difference (p<0.05) from other groups by one-way ANOVA.
At 6 hours after stroke induction, the level of cytokine IL-1β was significantly lower in the spleens of ovariectomized MIF KO females than in ovariectomized WT females (8.5±0.4 pg/mL vs. 17.9±2.1 pg/mL, p=0.01) (Figure 5). Measured cytokine levels otherwise remained constant between WT and MIF KO mice in the spleen samples. No significant differences in cytokine levels were seen between male and female mice of either genotype. Brain samples at 6 hours post stroke showed no significant differences in IL-1β, IL-6, and TNF-α levels in WT versus MIF KO brains (56.4±12.5 vs. 28.3±1.8 pg/ml, p=0.09 n=3; 20.5±2.6 vs.16.2±1.9 pg/ml, p=0.19 n=3;and 8.8±0.2 vs. 6.6±0.8 pg/ml, p=0.055 n=3, respectively).
Figure 5. Splenic levels of IL-1β are decreased in ovariectomized female MIF KO mice versus WT.
A) IL-1β; B) IL-6; C) TNF-α (n = 3 per group). * = p<0.05.
To investigate the role of MIF’s downstream effectors in cerebral ischemia, CD74 and JAB1 levels were examined in fractionated samples from WT and MIF KO mice. No differences in CD74 levels were seen between male and female sham and stroke mice (data not shown) in any fraction. Mitochondrial JAB1 levels in MIF KO female mice increased at 6 hours after stroke in comparison to sham MIF KO and WT mice (p<0.05) (Figure 6A). WT and MIF KO female mice had similar levels of mitochondrial JAB1 by 24 hours after stroke (Figure 6B). No differences in mitochondrial JAB1 levels were observed between WT and MIF KO male mice at 6 hours and 24 hours post-stroke (data not shown). JAB1 levels in cytosol did not differ between WT and MIF KO mice of either sex (data not shown). MIF was detected in mitochondrial lysates from WT mice of both sexes by Western blotting (Figure 6D). Importantly, WT female mice do not upregulate JAB at 6 hrs (unlike the MIF KO females), suggesting that MIF has a supressive effect on JAB. This occurs selectively in the ischemic brain as WT and MIF KO Sham (C) mice had equivalent JAB levels (Figure 6A). This suppressive effect of MIF occurs via a direct interaction between MIF and JAB. Co-immunoprecipitation studies with anti-JAB and anti-MIF antibodies (Figure 6E and F) in the mitochondrial lysates of WT mice of both sexes, showed a clear interaction between MIF and JAB that was greates in intact females, whereas no changes in the amount of interaction was seen in males (Figures 6E and F).
Figure 6. JAB1 levels are upregulated in the mitochondria of MIF KO versus WT intact female mice at 6 hours post-stroke.
A) Fractionated protein from mitochondrial and nuclear fractions from intact females at 6 hours post-stroke (n=6 mice per genotype). The JAB1 protein detected is 38 kDa in size, while mitochondrial marker COXIV is 16 kDa; b) Fractionated protein from mitochondrial fractions from intact females at 24 hours post-stroke (n=6 mice per genotype); C) Quantification by densitometry of changes in the ratio of JAB1 to COXIV protein levels relative to sham in intact females at 6 hours and 24 hours post-stroke; D) Detection of MIF protein in mitochondrial lysates from WT mice by Western blotting with representative densitometry; E) Co-immunoprecipitation of JAB and MIF from WT mitochondrial lysates; F) Densitometry of co-immunoprecipitation shown in E expressed as ratio of stroke to sham. The * symbol indicates statistically significant differences (p<0.05) by one-way ANOVA with post-hoc Tukey analysis. Cyto = cytoplasmic fraction, L = ladder, Mito = mitochondrial fraction, C = surgical sham; St = stroke, F = female; Ovx F = ovariectomized female; M = male; Co-IP = co-immunoprecipitation.
Discussion
MIF’s role in experimental stroke manifests sex differences. Whereas genetic deletion of MIF has minimal effects on stroke size in male mice, loss of MIF in females is detrimental (Figure 1). MIF’s absence results in a significant increase in infarct size in both female mice with intact ovaries, as well as in Ovx mice. The effect of MIF in females after stroke contrasts with the results in male mice, where there is either no effect (as in the data presented here after 90 minutes of transient MCAO) or mild improvement in infarct size in the absence of MIF (as seen in (Inacio et al., 2011b) after 45 minutes of transient MCAO).
The exacerbation of ischemic damage seen in MIF KO Ovx females versus WT Ovx females (Figure 1) suggests that the absence of both MIF and/or E2 is detrimental in females. Since MIF KO females have larger strokes than WT females as well as enhanced microglial activation as assessed by Iba1 staining (Figure 4), this suggests that MIF’s normal role in the female brain is to inhibit excessive microglial activation after injury. Increased microglial activation could also be independent of a direct effect of MIF and simply be correlated with infarct size. MIF is an upstream regulator of inflammatory-immune processes (Renner et al., 2005). In particular, MIF has a pro-inflammatory action in local and systemic inflammatory and immune responses outside the brain, which can be highly detrimental, for example, in atherosclerosis, rheumatoid arthritis (Morand and Leech, 2005; Morand et al., 2006; Renner et al., 2005) and sepsis (Bernhagen et al., 1994; Bernhagen et al., 1993).
The data presented here are in contrast to those seen in female MIF KO mice subjected to spinal cord compression injury, where the absence of MIF was protective (Nishio et al., 2009). In the spinal cord injury study, the MIF KO mice were established on a Balb/c background (Honma et al., 2000), while mice in the present study were from a C57BL6 background (Bozza et al., 1999; Taylor et al., 2007; Taylor et al., 2006). The contrasting effects of MIF’s absence after spinal cord injury versus stroke likely result from differences in the mechanism of injury (mechanical vs. ischemic), the models utilized, and the genetic backgrounds of the mice used.
The finding that MIF deletion had no significant effect in males was somewhat surprising, as Inacio et al., found that the absence of MIF was protective in males (Inacio et al., 2011b). In that study male MIF KO mice had smaller infarcts and improved behavioral outcomes at 48 h and 7 days after transient MCAO compared to WT (Inacio et al., 2011b) suggesting that MIF promotes cell death. Of note in that study, the ischemic insult was much milder than that used in this study, with 45 minutes of occlusion resulting in much smaller infarcts (with infarct volumes of approximately 20 mm3 by NeuN staining) at 7 days post injury for both WT and MIF KO. The absolute differences in infarct volumes between WT and KO males were also very modest. In addition, 5% glucose was infused subcutaneously 30 minutes after reperfusion. In our study a more severe ischemic injury of 90 minutes of occlusion was performed (resulting in infarct volumes of 80 mm3 as measured on TTC analysis), and no post-ischemic glucose wass administered. These differences in stroke surgery protocol may explain the lack of neuroprotection in the MIF KO males in our study.
As in the current study where no differences were found in brain or serum cytokine levels after stroke in male or female mice regardless of MIF genotype, Inacio et al. also reported no differences were observed in the inflammatory response between MIF KO and WT male mice within the first week after 45 minutes of transient cerebral ischemia (Inacio et al., 2011a). The current work did show a significant decrease in the levels of cytokine IL-1β in the spleens of ovariectomized MIF KO versus WT females at 6 hours post-injury. Whether MIF’s sex-specific effects after stroke are mediated solely within the central nervous system, or in part through a systemic effect on inflammation, is unclear from the present evidence.
Use of the MIF KO model has several limitations. These mice have complete constitutive knockout of MIF. The complete absence of MIF in all cells at all stages of development may lead to compensation by other genes. In addition, although understanding of the differences in the immune system between male and female mice is still incomplete, estrogen has a major effect on inflammatory signaling (Ritzel et al., 2012). Differences may also exist between the innate inflammatory response to stroke in the central nervous system versus that seen in the periphery. The increasing recognition of the importance of the peripheral immune response to stroke outcome makes the development of cell type specific and inducible MIF KO mice an important future goal to address the importance of this cytokine to both stroke and the sexually dimorphic response to ischemia.
MIF’s actions are mediated by either its interaction with the cell surface receptor CD74, or via MIF’s endocytosis and subsequent interaction with Jun activation domain-binding protein 1 (JAB1)(Morand et al., 2006; Renner et al., 2005). After binding to CD74 on the cell surface, MIF triggers a cell signaling cascade affecting the toll-like receptor-4 (TLR4)(Roger et al., 2001) and nuclear factor kappa B (NF-κB) pathways(Daun and Cannon, 2000), which are key mediators of cytokine production, inflammation, and the response to stroke. MIF’s binding keeps JAB1 in an inactive form in the cytosol, preventing JAB1 from entering the nucleus to interact with transcription factors (Kleemann et al., 2000). Downstream targets of JAB1 include c-Jun (Kleemann et al., 2000), hypoxia-inducible factor-1α (HIF-1α)(Bae et al., 2002), and p53 (Oda et al., 2008), which also play roles after ischemic injury and in apoptosis. JAB1 also directly interacts with the progesterone receptor and steroid receptor coactivator-1 (SRC1), stabilizing their complex formation and subsequently potentiating their ability to enhance transcription (Chauchereau et al., 2000). JAB1 has also been implicated in mitochondrial apoptosis, possibly via interaction with the proapoptotic protein Bcl-Gs (Liu et al., 2008).
Data presented in this paper suggest a mechanism for the ischemic sensitivity seen in MIF KO females. Our observation of increased levels of JAB1 in the mitochondria of MIF KO females suggests the intriguing possibility that mitochondrial JAB1 may help mediate MIF’s effects in the female brain. Our data demonstrate that MIF is neuroprotective in the female brain, as its genetic knockout leads to larger strokes. These effects do not appear to be mediated by MIF’s cell surface receptor CD74, as CD74 levels are unchanged in the ischemic brain by western (data not shown) or by IHC (Inacio et al., 2011c). MIF’s neuroprotective effects instead may be mediated by MIF’s interaction with the intracellular protein JAB1, as suggested by the novel finding of an increase in mitochondrial localization of JAB1 seen 6 hours after stroke in female MIF KO mice. In WT animals, MIF levels are slightly higher in intact females compared to males, but this was not significant. Interestingly stroke induced a decrease in MIF protein in ovx females, but not in males or females. We then performed co-IP studies as the interaction of JAB with MIF was likely more relevant to ischemic outcome than absolute protein levels. Co-IP studies demonstrated that there was a more robust JAB/MIF interaction after stroke in intact females, whereas less interaction was seen in ovx females after stroke, and no differences were seen in males. This suggests that the early JAB/MIF interaction may restrain deleterious signaling in the female brain during stroke, and is consistent with results showing that the deletion of MIF is detrimental in females but has no effect in males. The downstream effects or targets of this increased MIF/JAB interaction in the female brain are not yet known. We hypothesize that in the absence of MIF, more activated JAB1 enters the mitochondria, enhancing ischemic cell death, possibly by impeding mitochrondrial autophagy (mitophagy) (see Figure 7 for a potential model). This could result in increased release of proapoptotic factors such as cytochrome c, which plays a more critical role in the female cell death pathway than in the male (Lang and McCullough, 2008).
Figure 7. JAB1 plays a critical role in the proposed model of MIF’s sex-specific effects in ischemia.
In WT mice, MIF’s binding to JAB1 keeps JAB1 sequestered in an inactive form in the cytosol (A). In MIF KO mice, the absence of MIF allows JAB1 to be active. The previously known role of active JAB1 is to affect transcription by binding to other factors such as SRC-1. In the absence of MIF, after stroke in female MIF KO mic, JAB1 enters the mitochondria (B) as demonstrated in the current study. There JAB1 is proposed to interact with Bcl-Gs and/or other factors to decrease mitophagy, resulting in increased release of cytochome C from damaged mitochondria. The increased levels of cytosolic cytochrome c trigger more apoptosis and larger infarcts via the cell death pathway preferred in females.
As little is known of JAB1’s function in the adult brain, and JAB1’s role in mitochrondrial apoptosis has only been reported in a single paper with a yeast two-hybrid screen (Liu et al., 2008), our finding that JAB1 is regulated by MIF, at least in the female brain, is very novel. Given the suggested links among JAB1, Bcl-Gs, MIF, and autophagy (Chuang et al., 2012; Bounkari and Bernhagen, 2012; Wu et al., 2012), the members of the Bcl-2 pathway and JAB1’s possible role in autophagy will be closely examined in future experiments.
Increases in MIF in serum after successful resuscitation post-cardiac arrest (Stoppe et al., 2012a) and in the plasma of patients with acute coronary syndrome (Muller et al., 2012) or stroke (Wang et al., 2009) have recently been reported. In patients after cardiac surgery, increased postoperative levels of MIF were inversely correlated with organ dysfunction (Stoppe et al., 2012b), suggesting a protective role for MIF after cardiac ischemia-reperfusion injury as seen in preclinical studies (Gao et al., 2011; Koga et al., 2011; Qi et al., 2009). The rise in MIF levels after stroke correlated to the severity of neurological deficits (Wang et al., 2009). Whether this is a beneficial or detrimental response to cerebral ischemic injury remains to be determined, and sex specific levels were not examined.
MIF inhibitors are currently in development for the treatment of a variety of diseases. While preclinical evidence is promising that MIF antagonism may be of clinical utility in chronic inflammatory diseases (Morand and Leech, 2005; Morand et al., 2006; Renner et al., 2005), in acute ischemia MIF may play a protective role as seen in the present study and in cardiac ischemia (Gao et al., 2011; Koga et al., 2011; Qi et al., 2009). The sex differences observed in the brain’s response to MIF’s absence should be taken as a cautionary note. Trials of MIF antagonists need to be tested in both sexes, and attention paid to potential sex specific side effects.
Conclusions
Loss of MIF is detrimental to female mice after stroke. The absence of MIF in females is associated with enhanced microglial activation and increased localization of JAB1 to the mitochondria in the infarcted brain. These results imply that MIF plays a critical role in mediating inflammation and cell death after cerebral ischemia in females, and that this may occur via a novel role of MIF’s intracellular binding partner JAB1.
Highlights.
Macrophage migration inhibitory factor (MIF) has sex-specific effects in stroke.
Genetic absence of MIF results in larger strokes in female mice but not in males.
MIF knockout females have increased early mitochondrial localization of JAB1.
Acknowledgements
We would like to thank the National Institutes of Health for funding support (LDM: 1RO1-NS055215, R21 NS066406 and 5F31-NS062608). We would also like to thank Chad Siegel for technical assistance in the blinding of images for the infarct size analysis, and Dr. George Kuchel for helpful comments, discussion, and providing MIF breeding pairs.
List of Abbreviations
- ANOVA
analysis of variance
- COXIV
cytochrome c oxidase
- CSN5
COP9 signalsome subunit 5; alternative name for JAB1
- E2
estrogen
- ELISA
enzyme-linked immunosorbent assay
- HIF-1α
hypoxia-inducible factor-1α
- Iba1
ionized calcium binding adaptor molecule 1
- IHC
immunohistochemistry
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- JAB1
Jun activation domain-binding protein 1
- JNK
c-Jun N-terminal kinase
- KO
knockout
- LDF
laser Doppler flowmetry
- MCA
middle cerebral artery
- MCAO
middle cerebral artery occlusion
- MIF
macrophage migration inhibitory factor
- ND
neurological deficit
- NF-κB
nuclear factor kappa B
- Ovx
ovariectomy
- PBS
phosphate buffered saline
- SEM
standard error of the mean
- SRC-1
steroid receptor coactivator-1
- SRC-3
steroid receptor coactivator-3
- TLR4
toll-like receptor-4
- TNF-α
tumor necrosis factor- α
- TTC
2,3,5-triphenyltetrazolium chloride
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
R Bucala is a coinventor on patents describing MIF inhibitors. The authors have no other conflicts of interest to disclose.
Authors' contributions
LCT, and JL performed the ovariectomies, MCAO surgeries, and neurobehavioral studies. RB supplied KO mice and assisted in study design. LCT, RP, SB, GW, VV, and LC performed the molecular analyses and immunofluorescence. LCT performed the infarct size and statistical analyses. LDM conceived of the study. LCT and LDM participated in its design and coordination. LCT, JL, and LDM drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
L. Christine Turtzo, Email: turtzolc@mail.nih.gov.
Jun Li, Email: Junli@uchc.edu.
Rebecca Persky, Email: persky@uchc.edu.
Sharon Benashski, Email: dimauro@uchc.edu.
Gillian Weston, Email: gweston@student.uchc.edu.
Richard Bucala, Email: Richard.Bucala@yale.edu.
Venugopal Venna, Email: venna@uchc.edu.
Louise D. McCullough, Email: lmccullough@uchc.edu.
References
- Ashcroft GS, et al. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae MK, et al. Jab1 interacts directly with HIF-1alpha and regulates its stability. J Biol Chem. 2002;277:9–12. doi: 10.1074/jbc.C100442200. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, et al. Macrophage migration inhibitory factor is a neuroendocrine mediator of endotoxaemia. Trends Microbiol. 1994;2:198–201. doi: 10.1016/0966-842x(94)90111-h. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bounkari OE, Bernhagen J. MIF and autophagy: a novel link beyond "eating". Cell research. 2012;22:950–953. doi: 10.1038/cr.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza M, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger-Kentischer A, et al. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation. 2002;105:1561–1566. doi: 10.1161/01.cir.0000012942.49244.82. [DOI] [PubMed] [Google Scholar]
- Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nature reviews. Immunology. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauchereau A, et al. JAB1 interacts with both the progesterone receptor and SRC-1. The Journal of biological chemistry. 2000;275:8540–8548. doi: 10.1074/jbc.275.12.8540. [DOI] [PubMed] [Google Scholar]
- Chuang YC, et al. Macrophage migration inhibitory factor induces autophagy via reactive oxygen species generation. PloS one. 2012;7:e37613. doi: 10.1371/journal.pone.0037613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daun JM, Cannon JG. Macrophage migration inhibitory factor antagonizes hydrocortisone-induced increases in cytosolic IkappaBalpha. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1043–R1049. doi: 10.1152/ajpregu.2000.279.3.R1043. [DOI] [PubMed] [Google Scholar]
- Du L, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Gao XM, et al. Deletion of macrophage migration inhibitory factor protects the heart from severe ischemia-reperfusion injury: a predominant role of anti-inflammation. Journal of molecular and cellular cardiology. 2011;50:991–999. doi: 10.1016/j.yjmcc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, et al. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Honma N, et al. Deficiency of the macrophage migration inhibitory factor gene has no significant effect on endotoxaemia. Immunology. 2000;100:84–90. doi: 10.1046/j.1365-2567.2000.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YC, et al. Downregulation of migration inhibitory factor is critical for estrogen-mediated attenuation of lung tissue damage following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1227–L1232. doi: 10.1152/ajplung.00479.2006. [DOI] [PubMed] [Google Scholar]
- Inacio AR, et al. Lack of macrophage migration inhibitory factor in mice does not affect hallmarks of the inflammatory/immune response during the first week after stroke. Journal of neuroinflammation. 2011a;8:75. doi: 10.1186/1742-2094-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, et al. Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011b;31:1093–1106. doi: 10.1038/jcbfm.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio AR, et al. Enriched environment downregulates macrophage migration inhibitory factor and increases parvalbumin in the brain following experimental stroke. Neurobiology of disease. 2011c;41:270–278. doi: 10.1016/j.nbd.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Jin R, et al. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of leukocyte biology. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann R, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. 2000;408:211–216. doi: 10.1038/35041591. [DOI] [PubMed] [Google Scholar]
- Koga K, et al. Macrophage migration inhibitory factor provides cardioprotection during ischemia/reperfusion by reducing oxidative stress. Antioxidants & redox signaling. 2011;14:1191–1202. doi: 10.1089/ars.2010.3163. [DOI] [PubMed] [Google Scholar]
- Kudrin A, Ray D. Cunning factor: macrophage migration inhibitory factor as a redox-regulated target. Immunol Cell Biol. 2007 doi: 10.1038/sj.icb.7100133. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. JAB1 accelerates mitochondrial apoptosis by interaction with proapoptotic BclGs. Cellular signalling. 2008;20:230–240. doi: 10.1016/j.cellsig.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Luedike P, et al. Cardioprotection Through S-Nitros(yl)ation of Macrophage Migration Inhibitory Factor. Circulation. 2012;125:1880–1889. doi: 10.1161/CIRCULATIONAHA.111.069104. [DOI] [PubMed] [Google Scholar]
- McCullough L, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–268. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McCullough LD, et al. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005a;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- McCullough LD, et al. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005b;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Miller EJ, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- Morand EF, Leech M. Macrophage migration inhibitory factor in rheumatoid arthritis. Front Biosci. 2005;10:12–22. doi: 10.2741/1501. [DOI] [PubMed] [Google Scholar]
- Morand EF, et al. MIF: a new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov. 2006;5:399–410. doi: 10.1038/nrd2029. [DOI] [PubMed] [Google Scholar]
- Muller II, et al. Macrophage migration inhibitory factor is enhanced in acute coronary syndromes and is associated with the inflammatory response. PloS one. 2012;7:e38376. doi: 10.1371/journal.pone.0038376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y, et al. Deletion of macrophage migration inhibitory factor attenuates neuronal death and promotes functional recovery after compression-induced spinal cord injury in mice. Acta Neuropathol. 2009;117:321–328. doi: 10.1007/s00401-008-0476-x. [DOI] [PubMed] [Google Scholar]
- Oda S, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS ONE. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera S, et al. A novel DNA vaccine targeting macrophage migration inhibitory factor protects joints from inflammation and destruction in murine models of arthritis. Arthritis Rheum. 2007a;56:521–530. doi: 10.1002/art.22407. [DOI] [PubMed] [Google Scholar]
- Onodera S, et al. Active immunization against macrophage migration inhibitory factor using a novel DNA vaccine prevents ovariectomy-induced bone loss in mice. Vaccine. 2007b doi: 10.1016/j.vaccine.2007.11.066. [DOI] [PubMed] [Google Scholar]
- Oshima S, et al. Macrophage migration inhibitory factor-deficient mice are resistant to ovariectomy-induced bone loss. FEBS Lett. 2006;580:1251–1256. doi: 10.1016/j.febslet.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Pan JH, et al. Macrophage migration inhibitory factor deficiency impairs atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:3149–3153. doi: 10.1161/01.CIR.0000134704.84454.D2. [DOI] [PubMed] [Google Scholar]
- Qi D, et al. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. The Journal of clinical investigation. 2009;119:3807–3816. doi: 10.1172/JCI39738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, et al. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon BE, et al. Mechanisms of macrophage migration inhibitory factor (MIF)- dependent tumor microenvironmental adaptation. Experimental and molecular pathology. 2009;86:180–185. doi: 10.1016/j.yexmp.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner P, et al. Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis. 2005;41(Suppl 7):S513–S519. doi: 10.1086/432009. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, et al. Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke. Hormones and Behavior. 2012 doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, et al. MIF regulates innate immune responses through modulation of Tolllike receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- Roger VL, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan NE, et al. Brain miffed by macrophage migration inhibitory factor. International journal of cell biology. 2012;2012:139573. doi: 10.1155/2012/139573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, et al. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Schilling M, et al. Microglial activation precedes and predominates over macrophage infiltration in transient focal cerebral ischemia: a study in green fluorescent protein transgenic bone marrow chimeric mice. Experimental neurology. 2003;183:25–33. doi: 10.1016/s0014-4886(03)00082-7. [DOI] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Stoppe C, et al. Blood levels of macrophage migration inhibitory factor after successful resuscitation from cardiac arrest. PloS one. 2012a;7:e33512. doi: 10.1371/journal.pone.0033512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppe C, et al. High postoperative blood levels of macrophage migration inhibitory factor are associated with less organ dysfunction in patients after cardiac surgery. Molecular medicine. 2012b doi: 10.2119/molmed.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, 3rd, et al. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer. 2007;7:135. doi: 10.1186/1471-2407-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, et al. Null mutation in macrophage migration inhibitory factor prevents muscle cell loss and fibrosis in partial bladder outlet obstruction. Am J Physiol Renal Physiol. 2006;291:F1343–F1353. doi: 10.1152/ajprenal.00144.2006. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, McCullough LD. Sex Differences in Stroke. Cerebrovasc Dis. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future Neurol. 2010;5:47–59. doi: 10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli CM, et al. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Upregulation of Macrophage Migration Inhibitory Factor Gene Expression in Stroke. Stroke. 2009 doi: 10.1161/STROKEAHA.108.530535. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. Jama. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wu MY, et al. Steroid receptor coactivator 3 regulates autophagy in breast cancer cells through macrophage migration inhibitory factor. Cell research. 2012;22:1003–1021. doi: 10.1038/cr.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, et al. Sex-related differences in neuronal cell survival and signaling in rats. Neurosci Lett. 2003;337:65–68. doi: 10.1016/s0304-3940(02)01179-5. [DOI] [PubMed] [Google Scholar]
- Zhu C, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]