Abstract
A Val66Met single nucleotide polymorphism (SNP) in the brain-derived neurotrophic factor (BDNF) gene impairs activity-dependent BDNF release in cultured hippocampal neurons and predicts impaired memory and exaggerated basal hippocampal activity in healthy humans. Several clinical genetic association studies, along with multi-modal evidence for hippocampal dysfunction in schizophrenia indirectly suggest a relationship between schizophrenia and genetically-determined BDNF function in the hippocampus. To directly test this hypothesized relationship, we studied 47 medication-free patients with schizophrenia or schizoaffective disorder and 74 healthy comparison individuals with genotyping for the Val66Met SNP and [15O]H2O positron emission tomography (PET) to measure resting and working memory-related hippocampal regional cerebral blood flow (rCBF). In patients, harboring a Met allele was associated with significantly less hippocampal rCBF. This finding was opposite to the genotype effect seen in healthy participants, resulting in a significant diagnosis-by-genotype interaction. Exploratory analyses of interregional resting rCBF covariation revealed a specific and significant diagnosis-by-genotype interaction effect on hippocampal-prefrontal coupling. A diagnosis-by-genotype interaction was also found for working-memory related hippocampal rCBF change, which was uniquely attenuated in Met allele-carrying patients. Thus, both task-independent and task-dependent hippocampal neurophysiology accommodates a Met allelic background differently in patients with schizophrenia than in control subjects. Potentially consistent with the hypothesis that cellular sequelae of the BDNF Val66Met SNP interface with aspects of schizophrenic hippocampal and frontotemporal dysfunction, these results warrant future investigation to understand the contributions of unique patient trait or state variables to these robust interactions.
Keywords: Schizophrenia, BDNF, hippocampus, PET, Val66Met, working memory
Introduction
Brain-derived neurotrophic factor (BDNF) is an important determinant of hippocampal function throughout the lifespan, facilitating neuronal survival and differentiation, synaptic structure and plasticity, long-term potentiation, and learning and memory1-8. Because schizophrenia is postulated to have a neurodevelopmental etiology involving disrupted hippocampal function9 and has been associated with abnormalities in hippocampal-dependent cognitive functions10, hippocampal basal neural activity11-14, and hippocampal-prefrontal functional relationships15,16, an important role for BDNF in schizophrenic pathophysiology has been repeatedly proposed17-19.
In keeping with this hypothesis, post-mortem studies have identified reduced BDNF expression in hippocampal20-22 and prefrontal21-24 (though see Durany et al20) tissues in schizophrenia. Consistent with these reports, BDNF blood levels are reduced in schizophrenia patients – a finding replicated in medication-naïve, first-episode patients25 and associated with cognitive or clinical measures in some experiments26-28, though definitive work linking peripheral and central BDNF measures is lacking. Finally, although negative studies have been published, in some cohorts there exists an association between variation in the BDNF gene and schizophrenia risk29,30 or treatment response31.
This variation includes a single nucleotide polymorphism (SNP), rs6265, in the conserved, 5′-pro-protein-coding region, which entails a valine-to-methionine substitution (Val66Met) causing inefficient BDNF trafficking and reduced activity-dependent BDNF secretion32. Healthy Met carriers demonstrate diminished episodic memory performance, reduced hippocampal neuronal integrity measured with magnetic resonance spectroscopy, and attenuated hippocampal deactivation during working memory32.
Despite evidence in schizophrenia for disturbances in the same hippocampal functions that are modulated by BDNF, abnormal post-mortem brain and in vivo peripheral BDNF measurements, and select genetic association data, it remains unclear whether there is any meaningful relationship between observed hippocampal pathophysiological changes and genetically determined BDNF signaling. Consequently, we took advantage of [15O]H2O PET, a gold-standard method, to ascertain direct measurements of task-independent (“resting”) and task-related rCBF in patients with schizophrenia and healthy comparators, all genotyped for the Val66Met polymorphism. We hypothesized that if such an interaction existed, not only might patients show abnormal hippocampal physiology at baseline11-13 and during cognitive challenge16, they might also show differential effects of the Val66Met polymorphism on hippocampal function compared to healthy individuals. Critically, given prior findings suggesting antipsychotic-induced changes in hippocampal function33 and hippocampal BDNF concentrations9, we studied patients in a medication-free state.
Subjects and Methods
Subjects
Forty-seven individuals (mean age 28+6, 34 male, 37 Caucasian, 40 right-handed) meeting DSM-IV criteria for schizophrenia or schizoaffective disorder (6) by clinician-administered structured interview34 and confirmatory longitudinal inpatient psychiatric evaluation participated in a double-blind, placebo-controlled pharmacotherapy withdrawal protocol. After stabilization on a single, standard antipsychotic medication (and no other psychotropic agents), patients were switched to placebo treatment and four weeks later, still medication-free, underwent PET scanning and clinician-administered Positive and Negative Symptom Scale (PANSS) ratings35. Duration of illness was estimated retrospectively by history obtained from the patient, family, and past clinical documentation28.
Comparison data were acquired from 74 healthy individuals (mean age 30+7, 50 male, 62 Caucasian, 69 right-handed), without Axis I disorder34. For all participants, absence of pregnancy, confounding medical condition, and recent psychoactive substance use were confirmed by clinical history, physical examination, and laboratory studies. A subset of resting rCBF and BDNF genotype data from healthy individuals was also used in prior work36. All participants provided written informed consent, and all procedures were approved by the NIH Institutional Review Board and Radiation Safety Committee.
Genotyping
DNA was extracted from blood samples via standard methods and genotyped for the BDNF Val66Met SNP via TaqMan 5′exonuclease assay (Applied Biosystems, Foster City, CA). Given previous work implicating the COMT Val158Met SNP in cognition and medial temporal lobe physiology37 and potential relevance of this SNP to schizophrenia, genotypes for this variant were similarly obtained to rule out confounding COMT stratification effects.
Scanning Procedures
Participants abstained from caffeine and nicotine for at least four hours prior to a single brain PET session using a GE Advance PET scanner operating in 3-dimensional mode. An eight-minute transmission scan for attenuation correction and sixteen 60-second emission scans for rCBF measurements, six minutes apart, each following intravenous injection of [15O]H2O (10 mCi/injection), were acquired. During emission scans, participants performed the n-back task (seven 0-back sensorimotor control scans alternating with seven 2-back working memory scans) as previously described16, or quietly rested with eyes closed (two scans). Unlike fMRI, which depends on detecting changes in signal between different task conditions performed in the same imaging run (typically a task of interest alternating with rest), this PET method maps rCBF during each condition separately with entirely independent scans. For n-back conditions, a continuous series of single digits (“1”-“4”) shown at the points of a diamond were presented in random order. Subjects responded with a right-handed button-press corresponding to either the present stimulus (for 0-back) or that of the stimulus two presentations prior (for 2-back).
Statistical Procedures
After attenuation-correction and reconstruction, scans were background-activity corrected, registered, spatially normalized (Montreal Neurological Institute space), smoothed (10 mm3 full-width/half-maximum Gaussian kernel), and proportionally scaled. To allow random-effects analyses, images were averaged by condition (resting, 0-back, or 2-back), and n-back scans were contrasted by condition (2-back scans minus 0-back scans) to create three single condition-specific rCBF maps (for resting, 0-back, and 2-back separately) and a single working memory activation map (2-back minus 0-back) for each individual, respectively. Mean hippocampal rCBF for all conditions and activation values were measured using a bilateral hippocampal anatomical region of interest (ROI) derived from WFU PickAtlas software. ROI, demographic and clinical data analyses employed SPSS (SPSS Inc., Chicago, IL). Hardy-Weinberg exact tests were performed in R (http://cran.r-project.org)38. In addition, voxel-wise, general linear analyses of hippocampal rCBF and activation were performed with SPM5 (Wellcome Department of Cognitive Neurology, London). Planned contrasts compared resting hippocampal rCBF or activation between diagnostic (patient versus control) and genotype groups (Val homozygote versus Met carrier), and tested for diagnosis-by-genotype interactions.
To understand how hippocampal regions most relevant to schizophrenia might differentially predict activity in other brain regions, particularly the prefrontal cortex39, based on diagnosis and BDNF genotype, we conducted post-hoc explorations of hippocampal functional coupling (“connectivity”) across the whole brain. ‘Functional coupling’ in this context refers to interindividual regional covariation ascertained by voxel-wise regression of resting rCBF or activation across the entire brain on mean-centered hippocampal seed resting rCBF or activation, respectively. Each seed (one for resting rCBF and one for activation) was defined as a 6mm radius sphere centered on the most significant peak voxel result from the hippocampal diagnostic group comparisons. Between-group differences in functional connectivity were ascertained by contrast tests of beta-weights associated with each group’s hippocampal seed regressor.
For all whole-brain analyses, reported results met a cluster-level threshold of p<0.05, corrected for multiple comparisons, as determined by 10,000 Monte Carlo simulations implemented with AFNI AlphaSim software (NIMH, Bethesda, MD) using a voxel-level uncorrected threshold of p<0.00540. For all hippocampal rCBF or activation voxel-wise comparisons, the search volume was restricted to the hippocampal ROI, and a voxel-wise false discovery rate (FDR) correction for multiple comparisons was applied.
Results
Demographics, Genotypes and Clinical Ratings
In the schizophrenia group, there were 32 BDNF Val homozygotes (mean age 27+5, 22 male, 26 Caucasian, 27 right-handers, 21 COMT Val carriers, mean age at onset 21+4, mean illness duration 6+4) and 15 BDNF Met carriers (mean age 29+9, 12 male, 11 Caucasian, 13 right-handers, 10 COMT Val carriers, mean age at onset 19+3, mean illness duration 10+8). In the control group there were 49 BDNF Val homozygotes (mean age 30+7, 34 male, 43 Caucasian, 45 right-handers, 32 COMT Val carriers) and 25 BDNF Met carriers (mean age 29+7, 16 male, 19 Caucasian, 23 right-handers, 17 COMT Val carriers). Across these four groups, there were no significant differences in age (F(3,117)=1.223, p=0.304) or in sex, race, handedness, or COMT genotype distributions (all Fisher’s exact test statistics ≤1.612, p≥0.711). Healthy and patient groups did not differ in BDNF genotype distribution (χ2(1,N=121)=0.045, p=0.831). No deviations from Hardy-Weinberg equilibrium existed for either gene (all p’s≥0.133). Within the patient group, there were no significant differences in illness duration by genotype (t(45)=1.674, p=0.112).
Inpatient PANSS ratings were available for all but five individuals whose data were lost due to transcription error. Average ratings for total PANSS (maximum 210), and Negative (maximum 49), Positive (maximum 49), and General Psychopathology (maximum 112) subscales were 72+22, 20+7, 17+7, and 34+11, respectively, and did not significantly vary between genotypes (all t’s(40)≤1.237; p≥0.223).
Resting rCBF data were available for all participants. Working memory rCBF data for nine patients were excluded due to data acquisition problems (three) or 2-back performance at or below chance (six). The remaining patient group contained 25 Val homozygotes (mean age 26+5) and 13 Met carriers (mean age 27+8). Mean age differences across groups did not reach statistical significance (F(3,108)=1.904, p=0.133). Post-hoc pair-wise age comparisons (Tukey’s HSD test) of all groups were performed and indicated that control Val homozygotes (mean age 30+7) non-significantly tended to be older than patient Val homozygotes (p=0.128; all other p’s>0.483). Demographic comparisons across these subgroups remained nonsignificant for sex, race, handedness, and COMT genotype distributions (Fisher’s exact test statistics (N=112)≤2.298, p≥0.517). Again, healthy and patient groups did not differ in BDNF genotype distributions (χ2(1,N=112)=0.002, p=0.964).
Inpatient PANSS ratings were available for all but two individuals in the working memory patient group. Average ratings on the total PANSS, and Negative, Positive, and General Psychopathology subscales were 66+16, 19+6, 16+5, and 32+9, respectively. These did not significantly vary between genotypes (t’s(34)≤0.501, p≥0.620).
Working Memory Task Performance
As expected, patients showed worse accuracy on the 2-back task than healthy volunteers (patients mean=70%+0.2, controls mean=83%+0.16; t(108)=3.912, p<0.001), but all performed above chance (25%) level. A trend for worse accuracy among Met carriers was not significant (Met carriers mean=76%+0.19, Val homozygotes mean=81%+0.17; t(108)=1.744, p=0.084), and there was no significant diagnosis-by-genotype interaction (t(108)=0.355, p=0.186).
Resting Hippocampal rCBF: ROI Comparisons
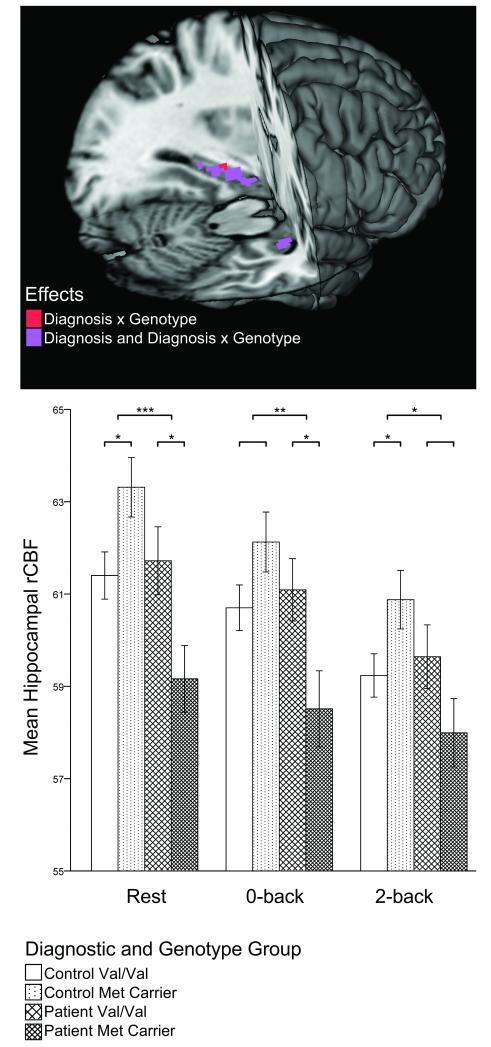
Planned, two-tailed contrast tests revealed a main effect of diagnostic group on mean hippocampal resting rCBF (t(117)=3.042; p=0.003) indicating lower basal hippocampal rCBF in patients. No main effect of genotype existed (t(117)=0.511, p=0.611), but a highly robust diagnosis-by-genotype interaction (t(117)=3.258, p=0.001) did: whereas control Met carriers had greater resting hippocampal rCBF than Val homozygotes (t(117)=2.224, p=0.028), patients showed the opposite pattern (t(117)=2.396, p=0.018), with abnormally low rCBF in patient Met carriers. Notably, this same interaction pattern was observed within the 0-back (t(108)=2.841, p=0.005) and 2-back (t(108)=2.414, p=0.017) conditions alone (Figure 1). PANSS ratings (total and subscales) did not correlate with resting hippocampal rCBF in patients (all r’s≤|0.075|, p’s≥0.635).
Figure 1.
Scaled Mean Hippocampal Regional Cerebral Blood Flow (rCBF) by Diagnosis and BDNF Val66Met Genotype During Rest, Sensorimotor (0-back), and Working Memory (2-back) Conditions.
In the lower panel, error bars represent standard errors of the mean. Significant within diagnostic group genotype comparisons (lower brackets) and significant diagnosis-by-genotype interactions (upper brackets) are indicated with asterisks (* p≤0.05; * p≤0.005; *** p≤0.001). The upper panel reveals localization of results for voxel-wise resting rCBF comparisons. The search volume was restricted to the hippocampal ROI, and a voxel-wise FDR corrected threshold of p<0.05 was used.
Resting Hippocampal rCBF: Voxel-wise Comparisons
Confirmatory voxel-wise hippocampal rCBF comparisons identified bilateral diagnosis effects (controls>patients), with the strongest difference in the left anterior hippocampus; Figure 1). As with the ROI analyses above, no main effects of genotype on hippocampal rCBF were found. The robust diagnosis-by-genotype interaction observed in the ROI analyses also localized bilaterally, with the strongest effect in the left mid-posterior hippocampus (Figure 1; Supplementary Table 1).
Resting Hippocampal Functional Coupling: Voxel-wise Comparisons
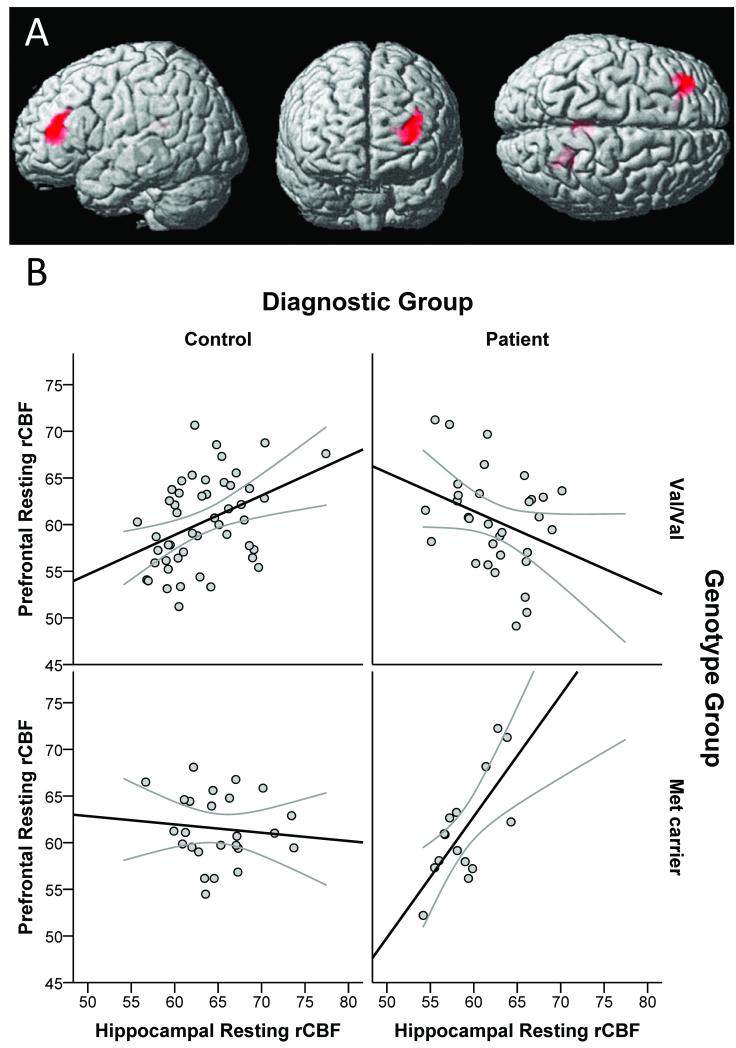
When whole-brain, voxel-wise patterns of resting hippocampal functional coupling were explored, there was no significant main effect of diagnosis. A genotype main effect arose in several midline regions, including the medial frontal gyrus and several cingulate cortex clusters as well as in the amygdala and left inferior temporal and postcentral gyri, where strong positive relationships with hippocampal rCBF occurred in Met carriers but not Val homozygotes. The opposite effect (Val homozygotes showing more positive relationships with hippocampal rCBF than Met carriers) existed in the middle occipital and superior temporal gyri. Importantly, a highly significant diagnosis-by-genotype interaction localized to a large lateral prefrontal cortex (PFC) region (Table 1): whereas healthy Val homozygotes showed a significant positive relationship between resting hippocampal and prefrontal rCBF, healthy Met carriers had a trend for an inverse relationship between these regions; in contrast, patients evidenced the opposite pattern, with Met carriers, but not Val homozygotes, demonstrating a strong positive hippocampal-PFC relationship (Figure 2). Less significant diagnosis-by-genotype interactions occurred in select posterior regions: lingual and fusiform gyri and posterior cingulate (Table 1).
Table 1.
Results for Voxel-Wise Analyses of Hippocampal Resting rCBF (Upper) and Activation (2-Back Greater Than 0-Back; Lower) Functional Coupling by Diagnosis and BDNF Val66Met Genotype Groups.
| Contrast | Laterality and Region |
Peak Voxel Coordinate |
Peak Voxel Uncorrected p Value |
Peak T Value | Cluster Size |
|---|---|---|---|---|---|
| Diagnosis Main Effects | |||||
|
| |||||
| Control > Patient | - | - | - | - | - |
| Patient > Control | |||||
| Left Insula* Left Globus |
(−42 4 −2) | <0.001 | 3.44 | 330 | |
| Pallidus/ Thalamus |
(−18 0 0) | <0.001 | 3.17 | 327 | |
| Genotype Main Effects | |||||
|
| |||||
| BDNF Val > Met Carrier | |||||
| Right Middle Occipital Gyrus |
(24 −88 −2) | <0.001 | 4.15 | 1066 | |
| Right Superior Temporal Gyrus* |
(52 −52 8) | <0.001 | 3.17 | 300 | |
| BDNF Met Carrier > Val | |||||
| Left Inferior Temporal Gyrus |
(−52 −8 −42) | <0.001 | 4.41 | 535 | |
| Left Postcentral Gyrus |
(−48 −32 44) | <0.001 | 4.35 | 2320 | |
| Left Amygdala | (−22 −4 −22) | <0.001 | 4.12 | 341 | |
| Left Medial Frontal Gyrus |
(−4 −16 76) | <0.001 | 3.99 | 836 | |
| Right Anterior Cingulate Cortex |
(4 40 16) | <0.001 | 3.93 | 1001 | |
| Right Posterior Cingulate Cortex |
(6 -36 24) | <0.001 | 3.87 | 2015 | |
| Right Middle Cingulate Cortex |
(6 4 34) | <0.001 | 3.62 | 587 | |
| Diagnosis x Genotype Interactions | |||||
|
| |||||
| Patient Val/Val and Control Met Carrier > Patient Met Carrier and Control Val/Val |
|||||
| Left Lingual Gyrus* |
(−4 −94 −10) | <0.001 | 3.59 | 456 | |
| Right Fusiform Gyrus |
(44 −54 −14) | 0.001 | 3.21 | 457 | |
| Patient Met Carrier and Control Val/Val > Patient Val/Val and Control Met Carrier |
|||||
| Left Middle Frontal Gyrus |
(−30 40 12) | <0.001 | 4.53 | 391 | |
| Right Callosal Forceps Major/ Posterior Cingulate Cortex |
(22 -50 20) | <0.001 | 3.57 | 351 | |
| Posterior Cingulate# |
(4 −44 18) | 0.001 | 3.14 | 289 | |
| Hippocampal Activation (2-back greater than 0-back) Coupling Voxel-Wise Comparisons | |||||
| Contrast |
Laterality and
Region |
Peak Voxel
Coordinate |
Peak Voxel
Uncorrected p Value |
Peak T Value | Cluster Size |
|
| |||||
| Diagnosis Main Effects | |||||
|
| |||||
| Control > Patient | |||||
| Precuneus Left Inferior |
(0 −76 52) (−52 −46 38) |
<0.001 <0.001 |
4.09 4.00 |
517 310 |
|
| Parietal Lobule Right Inferior Frontal Gyrus |
(52 18 24) | <0.001 | 3.70 | 283 | |
| Right Middle Frontal Gyrus |
(40 4 56) | <0.001 | 3.66 | 379 | |
| Right Middle Occipital Gyrus |
(54 −72 4) | <0.001 | 3.60 | 535 | |
| Left Postcentral Gyrus |
(−48 −18 48) | <0.001 | 3.45 | 415 | |
| Patient > Control | |||||
| Right Anterior Cingulate Cortex |
(2 30 0) | <0.001 | 4.38 | 525 | |
| Left Posterior Cingulate Cortex |
(−10 −44 −6) | <0.001 | 3.87 | 318 | |
| Genotype Main Effects | |||||
|
| |||||
| BDNF Val > Met Carrier | - | - | - | - | - |
| BDNF Met Carrier > Val | |||||
| Left Inferior Frontal Gyrus |
(−34 36 −4) | <0.001 | 5.01 | 628 | |
| Diagnosis x Genotype Interactions | |||||
|
| |||||
| Patient Val/Val and Control Met Carrier > Patient Met Carrier and Control Val/Val |
- | - | - | - | - |
| Patient Met Carrier and Control Val/Val > Patient Val/Val and Control Met Carrier |
|||||
| Left Inferior Parietal Lobule# |
(−34 −78 42) | <0.001 | 3.77 | 344 | |
The search volume was the whole brain, and results meet a cluster-level threshold of p<0.05, corrected for multiple comparisons. All findings except those asterisked continued to meet corrected significance after including age, sex, or COMT genotype in the statistical model. Findings with a pound sign no longer met corrected significance after accounting for individuals who were confirmed to have smoked on the day of the scan (but at least 4 hours prior to the scan session) in the statistical model. Additionally, all activation findings remained significant after controlling for working memory performance. Kolmogorov-Smirnov tests performed on the hippocampal seed regressor values (resting and activation) for each of the four diagnosis-genotype groups confirmed no significant pair-wise group differences (for all, p≥0.370), nor any differences from the normal distribution (for all, p>0.488).
Figure 2.
Hippocampal-Prefrontal Functional Coupling by Diagnosis and BDNF Val66Met Genotype During Rest.
The upper panel (A) shows areas of significant diagnosis by genotype interactions for resting condition hippocampal coupling (cluster-level p<0.05, corrected for multiple comparisons). This interaction localized to the prefrontal cortex and is shown in the lower panels (B) which shows plots of each individual’s mean prefrontal rCBF measured from a 6 mm radius sphere centered at (−30, 40, 14) versus mean hippocampal rCBF measured from a 6 mm radius sphere centered at (−26, −14, −22). Linear fits are also displayed along with 95% confidence intervals. Graphs are divided by diagnostic and genotype group combinations (healthy control subjects in the left column, patients with schizophrenia or schizoaffective disorder in the right column, BDNF Val/Val subjects in the upper row, BDNF Met carriers in the lower row).
Working Memory-Related Hippocampal Activation: ROI Comparisons
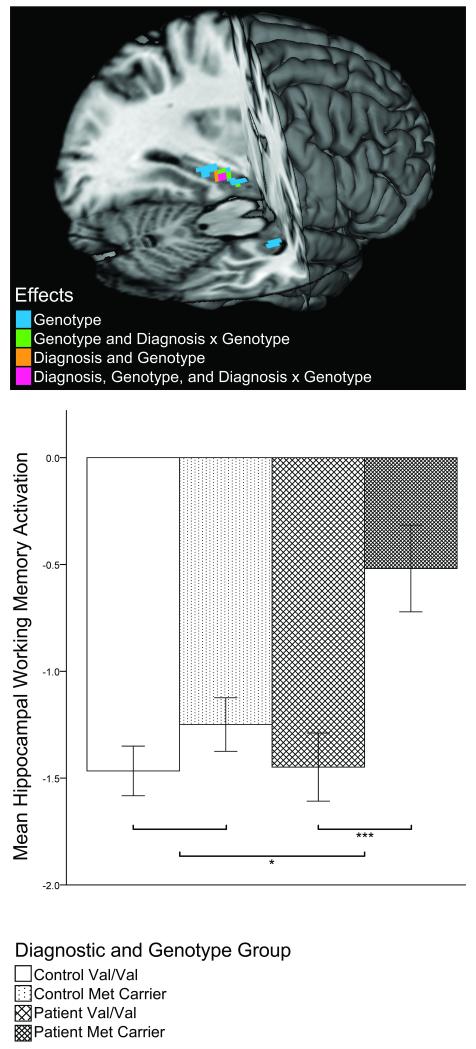
As expected from previous reports32,39, hippocampal rCBF was generally lower during working memory relative to during the sensorimotor 0-back condition (i.e., ‘deactivation’; see Figure 1). However, planned two-tailed contrast tests revealed a significant diagnosis main effect on mean hippocampal working memory activation (t(108)=2.330; p=0.022) indicating that patients showed diminished hippocampal deactivation. Also, whereas healthy volunteers showed no significant genotype-related hippocampal activation differences (t(108)=1.158, p=0.249), patient Met carriers had significantly less hippocampal deactivation than Val homozygote patients (t(108)=3.564, p=0.001), resulting in an overall main effect of genotype (t(108)=3.570; p=0.001) and diagnosis-by-genotype interaction (t(108)=2.218, p=0.029; Figure 3) Working memory performance did not correlate with hippocampal activation (r=−0.05, p=0.603), and diagnosis, genotype and diagnosis-by-genotype interactions remained significant even when accounting for performance or age in the model (all p’s≤0.024). In patients, correlations between PANSS ratings (total and subscales) and hippocampal activation did not reach significance (all r’s<0.274, p≥0.106).
Figure 3.
Scaled Mean Hippocampal Working Memory Activation (2-back – 0-back) by Diagnosis and BDNF Val66Met Genotype.
In the lower panel, error bars represent standard errors of the mean. Significant within-diagnostic group genotype comparisons (upper brackets) and significant diagnosis-by-genotype interaction (lower bracket) are indicated with asterisks (* p≤0.05; *** p≤0.001). The upper panel reveals localization of results for voxel-wise resting rCBF comparisons. The search volume was restricted to the hippocampal ROI, and a voxel-wise FDR corrected threshold of p<0.05 was used.
Working Memory-Related Hippocampal Activation: Voxel-wise Comparisons
Confirmatory voxel-wise comparisons of hippocampal working memory activation identified greatest effects of diagnosis (less deactivation in patients), genotype (less deactivation in Met carriers), and diagnosis-by-genotype interaction (greater genotype effect in patients) in the left anterior hippocampus (Figure 3; Supplementary Table 1). These results remained significant even when accounting for performance or age in the model.
Working Memory-Related Hippocampal Activation Functional Coupling: Voxel-wise Comparisons
When voxel-wise patterns of hippocampal covariation across the brain were explored, diagnosis predicted differences in functional coupling within critical working memory and ‘default’ network nodes, including the lateral PFC and cingulate cortices respectively (Table 1). In patients but not controls, greater hippocampal deactivation predicted stronger activation in the lateral PFC and stronger deactivation in the anterior and posterior cingulate cortices. Additionally, there was a regionally specific main effect of genotype in the left inferior frontal gyrus, where greater hippocampal deactivation was more predictive of less activation among Met carriers relative to Val homozygotes. A significant diagnosis-by-genotype interaction existed in the left inferior parietal lobule, where greater activation was predicted by less hippocampal deactivation only in patient Met carriers and control Val homozygotes. No interactions existed in the PFC.
Discussion
Resting rCBF Studies
In the hippocampus, a BDNF-rich structure consistently implicated in schizophrenic pathophysiology, functional genetic variation in BDNF predicted basal blood flow in a markedly divergent manner depending on diagnosis. BDNF enhances glutamatergic signaling and long-term potentiation in the hippocampus, promoting neuronal excitability in animal and tissue preparation models41-45. Knock-out mice and chronic BDNF infusion studies have supported positive effects of BDNF on both activity-dependent and basal neurotransmission in the hippocampus2,46. However, in healthy Met carriers, selective and moderate reductions of activity-dependent BDNF signaling32 are hypothesized to result in compensatory increases in resting paralimbic neural activity36. Such an increase was not observed in Met carriers with schizophrenia, who, in contrast, showed marked reduction in hippocampal activity, yielding the observed gene-by-diagnosis interaction. Assuming equivalent cellular effects of the Val66Met polymorphism on BDNF trafficking and secretion in patients and healthy individuals, the present data suggest that genetically-determined inefficiency in BDNF signaling32 differentially biases task-independent hippocampal neurophysiology in medication-free patients with schizophrenia.
We found lower basal hippocampal rCBF in patients than controls, consistent with several11-13 but not all47,48 PET reports of resting hippocampal activity in medication-free individuals with schizophrenia. This is in contrast to resting state studies of medicated individuals with schizophrenia, which have more consistently suggested elevated resting hippocampal activity14,49-52. Because of the heterogeneity existing even within studies of patients in medication-free states (e.g., see Medoff et al47, showing hippocampal hyperperfusion and Erkwoh et al48, showing lateral but not medial temporal hypoperfusion), important variation in past findings has remained unaccounted for. Given that patient and control Val homozygotes showed equivalent mean hippocampal rCBF, the diagnostic main effect found here was largely driven by the Met carriers, indicating that some aspects of observed resting state neuropathophysiology in schizophrenia, and, by extension, heterogeneity across the literature, may be attributable to differential effects of common polymorphisms within patients, such as those reported here, and not simply to clinical illness or its correlates alone.
Importantly, despite compelling recent results obtained from basal, resting fMRI53 and rCBF37,54 measurements in schizophrenia (as used here), variation in thought processes and content during the resting state are necessarily unrestricted, and genetic and illness-related predilections toward certain types of unguided intrapsychic behaviors, rather than, or in conjunction with, differential neural physiology independent of cognitive condition, could potentially contribute to resting state findings. It is particularly striking, therefore, to note the same diagnosis-by-genotype interaction pattern of absolute, mean hippocampal rCBF was preserved under diverse cognitive conditions (i.e., resting, sensorimotor, and working memory tasks), which suggests that this particular effect may be robust to substantial state variations (Figure 1).
Aberrant hippocampal-prefrontal connectivity has been increasingly identified as an important schizophrenia neuroimaging phenotype16, in line with preclinical neurodevelopmental models9. Because BDNF genotype may impact not only the degree of local hippocampal activity, but also the structure and function of excitatory synapses in both prefrontal and hippocampal cortices55,56, it is tempting to posit a modulatory role for BDNF in aspects of frontotemporal functional relationships that might be relevant to schizophrenia. The reversed genotype-determined hippocampal-prefrontal basal rCBF relationships in patients versus controls observed here is therefore particularly intriguing. Within the control group, greater positive associations existed between hippocampal and prefrontal cortical resting neural activity in Val homozygotes relative to Met carriers, consistent with previous work in healthy individuals57. The opposite result in our medication-free patients suggests that biases in resting state interregional coactivity set by trait BDNF function are markedly impacted by aspects of illness. However, the lack of a diagnosis main effect on this measure – due to the dramatic reversal of genotype effects in patients – indicates that interindividual trends in hippocampal-prefrontal resting rCBF covariation alone do not consistently characterize schizophrenia.
Working Memory Activation Studies
During the 2-back relative to 0-back condition, hippocampal activity is expected to be substantially reduced. As previously observed39, we demonstrated attenuated suppression of hippocampal activity to working memory challenge in patients with schizophrenia relative to healthy individuals, but, analogous to the resting state hippocampal findings, this diagnostic effect represents a dominant contribution from patient Met carriers (Figure 3). The resultant diagnosis-by-genotype interaction implies that in schizophrenia, differential genotype effects on hippocampal physiology are not limited to task-independent activity but also apply to dynamic responses to cognitive challenge. One persistent difficulty in understanding systems-level findings in complex neuropsychiatric disease, particularly schizophrenia, is the substantial variability frequently observed in patient neurophysiological data. This convergent evidence that among patient participants, carrying BDNF Met alleles predicts the hippocampal neurophysiological characteristics previously associated with schizophrenia itself, serves as a potent example of functional genetic variation underlying previously unexplained phenotypic heterogeneity.
The present data do not confirm a strong BDNF genotype effect on hippocampal activation within healthy volunteers, unlike past work using an fMRI paradigm32. Whether this is attributable to differences in experiment design, such as imaging modality58, remains unclear, but it is unlikely to imply a sweeping insensitivity of our methodology to BDNF genotype effects, given the ability of this assay to detect distinctive hippocampal activation patterns in the patient Met carriers.
As presaged by prior observations16,39, we found aberrant frontotemporal coupling during the working memory task in the patient group. Patients with more hippocampal deactivation generated greater prefrontal activation and greater cingulate deactivation, whereas this relationship was absent in healthy individuals. The fact that hippocampal deactivation selectively operated as a predictor of wide-spread working memory network-response in patients, conforms to notions of this region being a critical determinant of cognitive systems dysfunction in schizophrenia9 and, conversely, suggests that in health, the degree of hippocampal deactivation achieved is far less emblematic of broader executive neural network responsiveness. This is in keeping with findings of abnormally persistent hippocampal-prefrontal coupling during working memory in individuals with schizophrenia39. Likewise, stronger relationships between brain areas whose activity is normally reduced during working memory challenge, such as medial temporal and midline cortical structures, have been previously observed in schizophrenia patients53,59, and here we demonstrate this to be true of interindividual hippocampal-cingulate coupling. Importantly, we show these effects to be independent of BDNF genotype. The rs6265 polymorphism did predict associations between hippocampal deactivation and inferior frontal gyrus activation, but there were no diagnosis-by-genotype interactions in the frontal lobes to support the hypothesis that this polymorphism modulates working-memory related frontotemporal relationship abnormalities in patients. This suggests the possibility that if there is a significant connection between BDNF biology and dysfunctional frontal-hippocampal cooperativity in schizophrenia, as supported by the resting state data, it may be prominent only during certain cognitive states. This underscores the value of obtaining both basal and cognitive-challenge neurophysiological measurements when investigating complex gene-disease interactions on circuits characterized by long-range, multisynaptic connections60.
Conclusions
Overall, these results suggest an important intersection between BDNF biology, in particular, cellular changes conferred by the Val66Met polymorphism, and hippocampal dysfunction observed in schizophrenia. The data’s relational nature precludes definitive mechanistic explanation. However, several illness-related factors could potentially conspire with this polymorphism to generate the patient Met carriers’ aberrant hippocampal physiology. For instance, specific molecular abnormalities downstream of BDNF might modulate the impact of Val66Met genotype, a possibility supported by other candidate pathogenic mechanisms in BDNF-related pathways (e.g., AKT dysregulation61, NMDA hypofunction55). Alternatively, given BDNF’s importance in neural maturation, hippocampal developmental insults in schizophrenia may also interact with BDNF genotype to generate these results62.
We cannot exclude effects of past medication treatment or other illness-associated epiphenomena, though our collection of patient data after four weeks of medication withdrawal in a rigorously controlled inpatient setting is a particular strength of this experiment. The present results are unlikely explained by age, sex, race, or COMT given the well-matched groups; however, stratification from unmeasured genetic trait or clinical state variables remains possible. Furthermore, though we restricted nicotine prior to scanning, limiting the influence of acute smoking effects, we cannot exclude the possibility that subacute or otherwise occult effects of tobacco exposure may affect our blood flow measurements. Additional studies of other major neuropsychiatric conditions will help determine the specificity of the current findings. Finally, we did not observe a diagnosis-by-genotype interaction in working-memory accuracy, which, along with the lack of correlation between accuracy and hippocampal activation, suggests that rCBF interaction effects are not simply proxies for gross performance differences. Nonetheless, n-back accuracy, especially when obtained in-scanner, is expected to be a less sensitive measure of neural function than rCBF measurements, and the lack of behavioral gene-by-diagnosis interaction effects could be due to insufficient power.
In conclusion, these data demonstrate statistically robust interactions between BDNF genotype and schizophrenia diagnosis on basal hippocampal rCBF and hippocampal-prefrontal coupling as well as on hippocampal activation during cognitive challenge, suggesting the possibility that variation in BDNF function conferred by the Val66Met polymorphism might differentially impact fundamental aspects of frontotemporal neurophysiology when occurring in the context of schizophrenia. Future work investigating possible contributions of illness-associated state and trait variables to this finding will help to refine the importance of these data for understanding schizophrenic hippocampal pathophysiology.
Supplementary Material
Supplementary Figure 1. Hippocampal Region of Interest.
The hippocampal region of interest is shown in red and overlaid on the mean resting rCBF map (green (low rCBF) – orange (high rCBF)) and a grayscale, standard T1-weighted MNI template.
Acknowledgments
This research was supported by the Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, 20892.
We would like to thank the staff of the NIH PET Center and the NIMH GCAP Inpatient Program for their assistance in data acquisition.
Footnotes
Conflict of Interest
All authors report no financial conflict of interest with regard to this manuscript.
References
- 1.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Devel Neurobio. 2010;70(5):271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson SL, Abel T, Deuel TAS, Martin KC, Rose JC, Kandel ER. Recombinant BDNF Rescues Deficits in Basal Synaptic Transmission and Hippocampal LTP in BDNF Knockout Mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 3.Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- 4.Lu B. BDNF and Activity-Dependent Synaptic Modulation. Learn Mem. 2003;10(2):86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein Synthesis-dependent and -independent Regulation of Hippocampal Synapses by Brain-derived Neurotrophic Factor. J Biol Chem. 2001;276(40):37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 6.Barnabe-Heider F, Miller FD. Endogenously Produced Neurotrophins Regulate Survival and Differentiation of Cortical Progenitors via Distinct Signaling Pathways. J Neurosci. 2003;23(12):5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang H, Schuman E. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267(5204):1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 8.Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-Derived Neurotrophic Factor and Antidepressant Drugs Have Different But Coordinated Effects on Neuronal Turnover, Proliferation, and Survival in the Adult Dentate Gyrus. J Neurosci. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14(1):135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- 10.Owens SF, Picchioni MM, Rijsdijk FV, Stahl D, Vassos E, Rodger AK, et al. Genetic overlap between episodic memory deficits and schizophrenia: results from The Maudsley Twin Study. Psychol Med. 2011;41(03):521–532. doi: 10.1017/S0033291710000942. [DOI] [PubMed] [Google Scholar]
- 11.Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic System Abnormalities Identified in Schizophrenia Using Positron Emission Tomography With Fluorodeoxyglucose and Neocortical Alterations With Deficit Syndrome. Arch Gen Psychiatry. 1992;49(7):522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 12.Nordahl TE, Kusubov N, Carter C, Salamat S, Cummings AM, O’Shora-Celaya L, et al. Temporal lobe metabolic differences in medication-free outpatients with schizophrenia via the PET-600. Neuropsychopharmacology. 1996;15(6):541–554. doi: 10.1016/S0893-133X(96)00098-X. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen NC, O’Leary DS, Flaum M, Nopoulos P, Watkins GL, Ponto LLB, et al. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. The Lancet. 1997;349(9067):1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- 14.Heckers S, Rauch S, Goff D, Savage C, Schacter D, Fischman A, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 15.Ragland JD, Gur RC, Valdez JN, Loughead J, Elliott M, Kohler C, et al. Levels-of-Processing Effect on Frontotemporal Function in Schizophrenia During Word Encoding and Recognition. Am J Psychiatry. 2005;162(10):1840–1848. doi: 10.1176/appi.ajp.162.10.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for Abnormal Cortical Functional Connectivity During Working Memory in Schizophrenia. Am J Psychiatry. 2001;158(11):1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 17.Buckley PF, Mahadik S, Pillai A, Terry A., Jr. Neurotrophins and schizophrenia. Schizophr Res. 2007;94(1-3):1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15(3):319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10(4):345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 20.Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52(1-2):79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5(3):293–300. doi: 10.1038/sj.mp.4000718. [DOI] [PubMed] [Google Scholar]
- 22.Wong J, Hyde TM, Cassano HL, Deep-Soboslay A, Kleinman JE, Weickert CS. Promoter specific alterations of brain-derived neurotrophic factor mRNA in schizophrenia. Neuroscience. 2010;169(3):1071–1084. doi: 10.1016/j.neuroscience.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, et al. Relationship of Brain-Derived Neurotrophic Factor and Its Receptor TrkB to Altered Inhibitory Prefrontal Circuitry in Schizophrenia. J Neurosci. 2005;25(2):372–383. doi: 10.1523/JNEUROSCI.4035-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- 25.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2010;16(9):960–972. doi: 10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 26.Chen D, Wang J, Wang B, Yang S, Zhang C, Zheng Y, et al. Decreased levels of serum brain-derived neurotrophic factor in drug-naive first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology. 2009;207(3):375–380. doi: 10.1007/s00213-009-1665-6. [DOI] [PubMed] [Google Scholar]
- 27.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is Serum Brain-Derived Neurotrophic Factor a Biomarker for Cognitive Enhancement in Schizophrenia? Biol Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chao HM, Kao HT, Porton B. BDNF Val66Met variant and age of onset in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(4):505–506. doi: 10.1002/ajmg.b.30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neves-Pereira M, Cheung JK, Pasdar A, Zhang F, Breen G, Yates P, et al. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol Psychiatry. 2005;10(2):208–212. doi: 10.1038/sj.mp.4001575. [DOI] [PubMed] [Google Scholar]
- 30.Muglia P, Vicente AM, Verga M, King N, Macciardi F, Kennedy JL. Association between the BDNF gene and schizophrenia. Mol Psychiatry. 2002;8(2):1478–1148. doi: 10.1038/sj.mp.4001221. [DOI] [PubMed] [Google Scholar]
- 31.Zai GCM, Zai CCH, Chowdhury NI, Tiwari AK, Souza RP, Lieberman JA, et al. The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2012 doi: 10.1016/j.pnpbp.2012.05.014. In press.: doi: 10.1016/j.pnpbp.2012.1005.1014. [DOI] [PubMed] [Google Scholar]
- 32.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 33.Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53(7):601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Miriam G, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders: SCID-I. Research Version. 2001 [Google Scholar]
- 35.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 36.Wei SM, Eisenberg DP, Kohn PD, Kippenhan JS, Kolachana BS, Weinberger DR, et al. Brain-Derived Neurotrophic Factor Val66Met Polymorphism Affects Resting Regional Cerebral Blood Flow and Functional Connectivity Differentially in Women Versus Men. J Neurosci. 2012;32(20):7074–7081. doi: 10.1523/JNEUROSCI.5375-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberg DP, Sarpal D, Kohn PD, Meyer-Lindenberg A, Wint D, Kolachana B, et al. Catechol-o-methyltransferase valine(158)methionine genotype and resting regional cerebral blood flow in medication-free patients with schizophrenia. Biol Psychiatry. 2010;67(3):287–290. doi: 10.1016/j.biopsych.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wigginton JE, Cutler DJ, Abecasis GaR. A Note on Exact Tests of Hardy-Weinberg Equilibrium. Am J Hum Genet. 2005;76(5):887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 40.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 41.Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295(5560):1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- 42.Lohof AM, Ip NY, Poo M-m. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 43.Binder DK, Croll SD, Gall CM, Scharfman HE. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24(1):47–53. doi: 10.1016/s0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- 44.Rex CS, Lin C-Y, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-Derived Neurotrophic Factor Promotes Long-Term Potentiation-Related Cytoskeletal Changes in Adult Hippocampus. J Neurosci. 2007;27(11):3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Messaoudi E, BÃ¥rdsen K, Srebro B, Bramham CR. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 1998;79(1):496–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- 46.Sherwood NT, Lo DC. Long-Term Enhancement of Central Synaptic Transmission by Chronic Brain-Derived Neurotrophic Factor Treatment. J Neurosci. 1999;19(16):7025–7036. doi: 10.1523/JNEUROSCI.19-16-07025.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: Contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- 48.Erkwoh R, Sabri O, Steinmeyer EM, Büll U, Saß H. Psychopathological and SPECT findings in never-treated schizophrenia. Acta Psychiatr Scand. 1997;96(1):51–57. doi: 10.1111/j.1600-0447.1997.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 49.Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, et al. Resting neural activity distinguishes subgroups of schizophrenia patients. Biol Psychiatry. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39(2):117–127. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 51.al-Mousawi AH, Evans N, Ebmeier KP, Roeda D, Chaloner F, Ashcroft GW. Limbic dysfunction in schizophrenia and mania. A study using 18F-labelled fluorodeoxyglucose and positron emission tomography. Br J Psychiatry. 1996;169(4):509–516. doi: 10.1192/bjp.169.4.509. [DOI] [PubMed] [Google Scholar]
- 52.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, Jr, McMahon R. Correlations Between rCBF and Symptoms in Two Independent Cohorts of Drug-Free Patients with Schizophrenia. Neuropsychopharmacology. 2005;31(1):221–230. doi: 10.1038/sj.npp.1300837. [DOI] [PubMed] [Google Scholar]
- 55.Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, et al. The BDNF Val66Met Polymorphism Impairs NMDA Receptor-Dependent Synaptic Plasticity in the Hippocampus. J Neurosci. 2010;30(26):8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu R-J, Lee FS, Li X-Y, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2012;71(11):996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomason ME, Yoo DJ, Glover GH, Gotlib IH. BDNF genotype modulates resting functional connectivity in children. Front Hum Neurosci. 2009;3:55. doi: 10.3389/neuro.09.055.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally Specific Sensitivity Differences in fMRI and PET: Where Do They Come From? NeuroImage. 2000;11(6):575–588. doi: 10.1006/nimg.2000.0581. [DOI] [PubMed] [Google Scholar]
- 59.Wolf RC, Vasic N, Sambataro F, Höse A, Frasch K, Schmid M, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 60.Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- 61.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt Signaling Pathways in Schizophrenia and Antipsychotic Drug Action. Am J Psychiatry. 2009;167(4):388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicodemus KK, Marenco S, Batten AJ, Vakkalanka R, Egan MF, Straub RE, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13(9):873–877. doi: 10.1038/sj.mp.4002153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Hippocampal Region of Interest.
The hippocampal region of interest is shown in red and overlaid on the mean resting rCBF map (green (low rCBF) – orange (high rCBF)) and a grayscale, standard T1-weighted MNI template.