Abstract
Dendritic cells (DCs) regulate immunity and immune tolerance in vivo. However, the mechanisms of DC-mediated tolerance have not been fully elucidated. Here we demonstrate that intravenous (i.v.) transfer of bone marrow-derived DCs pulsed with MOG peptide blocks the development of experimental autoimmune encephalomyelitis (EAE) in C57BL/6J mice. i.v. transfer of MOG-pulsed DCs leads to down-regulation of production of IL-17A and IFN-γ and up-regulation of IL-10 secretion. The development of regulatory T cells (Tregs) is facilitated via up-regulation of FoxP3 expression and production of IL-10. The number of suppressive CD4+ IL-10+ IFN-γ+ T cells is also improved. The expression of OX40, CD154 and CD28 is down-regulated, but the expression of CD152, CD80, PD-1, ICOS and BTLA is up-regulated on CD4+ T cells after i.v. transfer of immature DCs. The expression of CCR4, CCR5 and CCR7 on CD4+T cells is also improved. Our results suggest that immature DCs may induce tolerance via facilitating development of CD4+ FoxP3+ regulatory T cells and suppressive CD4+ IL-10+ IFN-γ+ T cells in vivo.
Keywords: Dendritic cell, EAE, Immune tolerance, Autoimmunity
Introduction
Dendritic cells (DCs) play an important role in both autoimmunity and immune tolerance. Although DC-based immunotherapy has been used to treat cancer and to target autoimmune diseases, the regulatory mechanisms controlling DC-mediated immune responses have not been fully elucidated [1, 2]. Understanding these regulatory mechanisms could facilitate the development of immunotherapy for use in autoimmune clinical trials.
DCs modulate the activity of T cells through three pathways. Signal 1: peptide/MHC complexes presented on DCs bind to T cell receptor (TCR) so that T cells can recognize target cells. Signal 2: multiple co-stimulatory molecules expressed on DCs bind to the ligands expressed on T cells and then initiate or inhibit T cell activity. For example, CD80 and CD86 expressed on DCs interact with CD28 or CD152 expressed on T cells to lead to autoimmunity or tolerance. B7H-1, B7-H-2 expressed on DCs bind to programmed death-1 (PD-1) or CD80 expressed on T cells to inhibit T cell activation. Signal 3: DCs secrete cytokines such as IL-12, IL-23 and IL-27 to modulate T cell activity [3].
Experimental autoimmune encephalomyelitis (EAE) is an animal model of multiple sclerosis (MS) [4, 5]. It has been reported that Th17 cells are major pathogenic cells in EAE/MS development [4]. DCs play an important role in induction of immune tolerance during EAE [6]. However, the molecular mechanisms of DC-mediated immune tolerance have not been fully elucidated. An animal model of immune tolerance in EAE mice induced by i.v. transfer of immature bone marrow-derived DCs has been established in this experiment. It will be investigated how DCs modulate the activity of CD4+ T cells in vivo.
The trafficking of regulatory CD4+ T cells from peripheral lymph organs into the central nervous system (CNS) is necessary for inhibition of EAE. C-C chemokine receptor 4 (CCR4), CCR5, CCR6 and CCR7 expressed on regulatory CD4+ T cells play an important role in regulatory CD4+ T cell trafficking to local environment and inhibition of peripheral inflammation [7–12]. In the present study, we will investigate whether MOG-pulsed DCs can modulate the protein expression of CCR4, CCR5, CCR6 and CCR7 on CD4+ T cells. This may affect regulatory CD4+ T cell trafficking to CNS and then lead to inhibition of EAE development.
Materials and Methods
Mice
C57BL/6J female mice (8–12 weeks) were ordered from The Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred in the Thomas Jefferson Animal Care facilities. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Immunogen and Peptide
Mouse MOG35–55 peptide(MEVGWYRSPFSRVVHLYRNGK) is part of myelin oligodendrocyte glycoprotein (MOG) and was purchased from Invitrogen (Invitrogen, Carlsbad, California, USA).
Generation of bone marrow-derived dendritic Cells
As described preciously [13, 14], femurs and tibiae of mice were isolated from muscle tissue by rubbing with Kleenex tissues. The intact bones were then put into 70% ethanol for 5 min for disinfection and washed with phosphate-buffered saline (PBS). Both ends of the bones were cut with scissors and the marrow was flushed with PBS by using a syringe with 0.45 mm diameter needle. Clusters within the marrow suspension were disintegrated by vigorous pipetting and then washed with PBS.
As described previously [13, 14], the leucocytes from bone marrow were fed in bacteriological 100 mm Petri dishes (Falcon, Becton Dickinson, Heidelberg, Germany) at 2×106 cells per dish. The cells were cultured in RPMI1640 complete medium (Gibco-BRL, Regenstein, Germany) including penicillin (100 U/ml, Sigma, St. Louis, MO, USA), streptomycin (100 U/ml, Sigma), L-glutamine (2 mM, Sigma), 2-mercaptoethanol (2-ME, 50 μM, Sigma), 10% heated inactivated and filtered (0.22 μm, Milipore, Inc., Bedford, MA, USA) Fetal Calf Serum (FCS, Sigma) and granulocyte-macrophage colony-stimulating factor (GM-CSF, PetroTech, Rocky Hill, NJ, USA) at 20 ng/ml at day 0 (10 ml medium per dish).
At day 3, 10 ml fresh medium with GM-CSF at 20 ng/ml was added to each dish, and at day 6, half of the medium (about 10 ml supernatant) was collected and centrifuged at 300 g for 5 min. Subsequently, the cells were resuspended in 10 ml fresh medium with GM-CSF (20 ng/ml) and were then re-fed in the original dish.
The DCs were collected by gentle pipetting and washed with PBS at 300 g for 5 min before conducting intravenous (i.v.) transfer at day 8.
EAE induction and DC treatment
C57BL/6J mice (female, 8–12 week) were immunized with MOG peptide/Complete Freund’s adjuvant (CFA, Sigma) at 200 μg/200 μl/per mouse (subcutaneous injection). Pertussis toxin (Sigma) was simultaneously injected at 200 ng/per mouse (intraperitoneal injection) and a second injection was administered after 48 hrs (post immunization p.i.). EAE disease was evaluated as following standard clinical score: 0.5: half of tail paralysis, 1: whole tail paralysis, 2: tail and one leg paralysis, 3: tail and two legs paralysis, 4: moribund, 5: death.
DCs (5 × 105 cells/per mouse/per time) were pulsed with MOG peptide (0.1 μM) for 6 hours and washed twice at 300 g ×5 min. DCs were i.v. injected into EAE mice on days 11, 14 and 17 p.i..
Generation of MOG-primed T lymphocytes
C57 BL/6J mice were immunized with MOG/CFA to induce EAE. Spleen cells were isolated from mice with EAE or tolerance at day 30 (p.i.). Splenocytes had been cultured in Click’s medium (Gibco) with mouse IL-2 (1ng/ml) and restimulated with MOG peptide (0.1μM) for5 days. Cells were then collected for flow cytometry and supernatant was harvested for ELISA.
Flow Cytometry
MOG-primed T lymphocytes were isolated from EAE mice and incubated with anti-mouse OX40, CD152 (CTLA-4), CD80, CD154 (CD40L), PD-1, CD28, inducible co-stimulator (ICOS), B and T lymphocyte attenuator (BTLA), IFN-γ, IL-10, FOXP3 and anti-CD4 antibodies (Biolegend, San Diego, CA, USA) at 4 °C for 1 hr. Cells were then washed twice with 5% FCS in PBS at 300 g for 5 min. and were fixed with 5% formalin in PBS. Fixed cells were run on a FACS Aria (BD Biosciences, San Jose, CA, USA) and data were analyzed with Flow Jo software (Treestar, Ashland, OR, USA).
For intracellular cytokine staining, MOG-primed T lymphocytes derived from spleen were treated with leukocyte activation cocktail with BD GolgiPlug™ (BD Pharmingen) including the phorbol ester, Phorbol 12-Myristate 13-Acetate (PMA), a calcium ionophore (Ionomycin) and protein transport inhibitor BD GolgiPlug™ (Brefeldin A) for 5 hrs. Cell surface staining described as above was firstly performed and cells were then fixed with 5% paraformaldehyde and permeabilized in PBS containing 0.1% saponin (Biolegend) for 20 min at room temperature. MOG-primed T lymphocytes were stained with anti-mouse FoxP3, IL-10 and IFN-γ antibodies (Biolegend) at 4 °C for 24 hrs before conducting flow cytometry.
ELISA
Anti-mouse ELISA kits including IFN-γ, IL-10 and IL-17A were purchased from R&D Systems (Minneapolis, MN, USA). Assays were conducted according to the manufacturer’s instructions. Plates were read out in Labsystems Multiskan MCC/340 (Fisher Scientific, Suwannee, GA, USA) and data were analyzed with DSJV ELISA software (Fisher Scientific).
Statistical Analysis
Experimental data were analysed using Prism software (GraphPad, La Jolla, CA, USA). A t test was conducted. Data represent the mean and SD. Results were regarded as showing a significant difference if the P value was less than 0.05.
Results
1. i.v. transfer of immature DCs pulsed with MOG peptide blocks EAE development in vivo
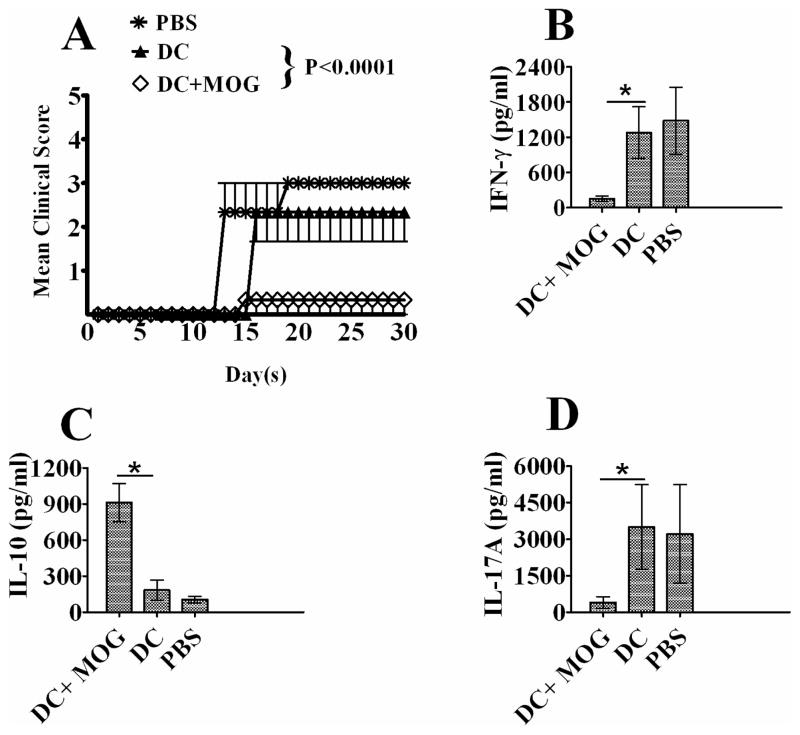
To investigate whether i.v. transfer of DCs can affect EAE development, PBS and DCs pulsed with MOG peptide or without loading MOG peptide were i.v. injected into C57 BL/6J mice immunized with MOG/CFA. Our results showed that i.v. transfer of MOG-pulsed DCs significantly suppressed EAE development (Fig. 1). The production of inflammatory cytokines including IL-17A and IFN-γ was down-regulated (Figs. 1B and C) while the production of anti-inflammatory cytokine IL-10 was elevated after i.v. transfer of MOG-pulsed DCs (Fig. 1D). By contrast, DCs without loading MOG peptide cannot affect EAE development and cytokine production by DCs. It can be concluded that i.v. transfer of bone marrow-derived immature dendritic cells pulsed with MOG peptide is able to induce tolerance in C57 BL/6J mice with EAE.
Figure 1.
Intravenous (i.v.) transfer of bone marrow-derived DCs inhibits EAE development. EAE was induced in mice via immunization with MOG/CFA. PBS and DC pulsed with MOG (0.1μM) (DC-MOG) or not(DC) were i.v. injected into EAE mice at days 11, 14 and 17 p.i (3×105 cells/ mouse/injection). EAE development with clinical score (A) from day 0 to day 30 p.i is shown. This experiment was repeated three times with similar results. Data represent the mean and SEM of EAE clinical score (n=3, t test, * P<0.05) in one experiment. Spleen cells were isolated from mice treated with DC-MOG, DC or PBS at day 30 and re-stimulated with MOG peptide (0.1 μM) and mouse IL-2 (1 ng/ml) for 5 days. The supernatant was collected to detect IFN-γ(B) and IL-10 (C) and IL-17A (D) by ELISA. The error bars shown in B, C and D represent mean and SD of the cytokine concentration in three independent experiments (n=3, t test, *P<0.05).
2. i.v. transfer of bone marrow-derived immature DCs facilitates the development of Tregs and suppressive CD4+ IFN-γ+ IL-10+ T cells in mice with EAE
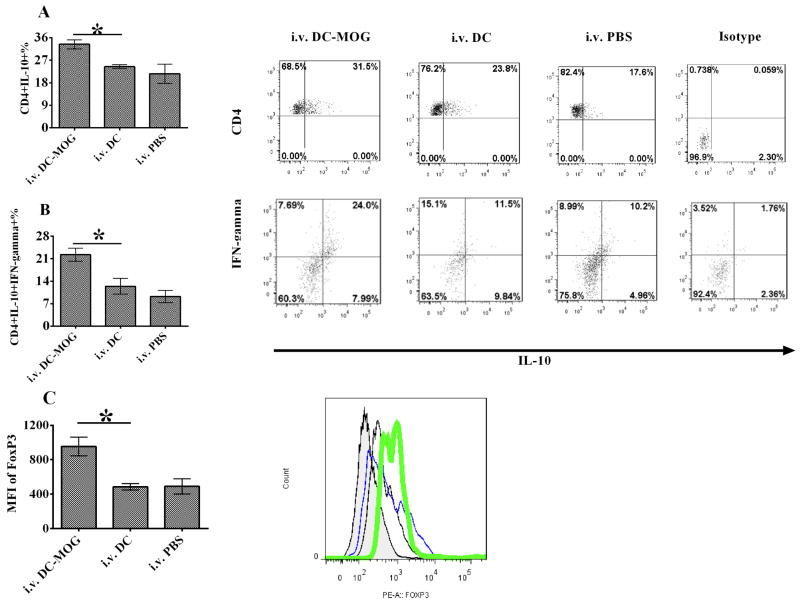
To test whether i.v. transfer of bone marrow-derived DCs can affect Treg development, the number of CD4+IL-10+ cells in mice with EAE or tolerance was detected using flow cytometry. Our results demonstrated that i.v. transfer of bone marrow-derived DCs pulsed with MOG peptide causes improvement of numbers of CD4+ IL10+ T cells and CD4+ IFN-γ+ IL-10+ T cells in mice with tolerance comparing with those in mice with EAE (Figs. 2A and B). Moreover, the expression of FoxP3 is up-regulated after i.v. transfer of DCs pulsed with MOG peptide (Fig. 2C). However, i.v. transfer of DCs without loading MOG peptide cannot affect development of CD4+ IL-10+ cells and CD4+ IFN-γ+ IL-10+ T cells in mice with EAE. The expression of FoxP3 also cannot be improved after i.v. transfer of DCs without loading MOG peptide (Fig. 2). These results imply that bone marrow-derived immature DCs pulsed with MOG peptide may induce tolerance through enhancement development of Treg and CD4+ IFN-γ+ IL-10+ suppressive T cells.
Figure 2.
Intravenous transfer of bone marrow-derived DCs up-regulates the numbers of IL-10 producing CD4+ T cells, suppressive CD4+ IL-10+ IFN-γ+ T cells and FoxP3+ expression in CD4+ T cells. EAE mice were treated with i.v. PBS and DCs pulsed with MOG peptide at 0.1 μM (DC-MOG) or not (DC). Splenocytes were isolated from mice and stained. CD4+ T cells were gated. The production of IL-10 in CD4+ T cells (A), CD4+ IL-10+ IFN-γ+ (B) and expression of FoxP3 (C) in CD4+ T cells were determined by flow cytometry. (C) Expression of FoxP3 in CD4+ T cells is improved after i.v. transfer of DC-pulsed with MOG peptide. Expression of FoxP3 was detected in CD4+ T cells which were isolated from mice with EAE treated with MOG-pulsed DCs (green), DCs without loading MOG peptide (DC) (blue) or PBS (black) as described in Fig. 1. Isotype control is also shown (shade). Error bars shown in this figure represent the mean and SD of triplicate determinations of the percentage of CD4+ T cells (A and B) or mean fluorescence of intensity (MFI) (C) in three independent experiments (n=3, t test, * P<0.05).
3. i.v. transfer of bone marrow-derived immature DCs pulsed with MOG peptide modulates protein expression of co-stimulatory molecule receptors on CD4+ T cells
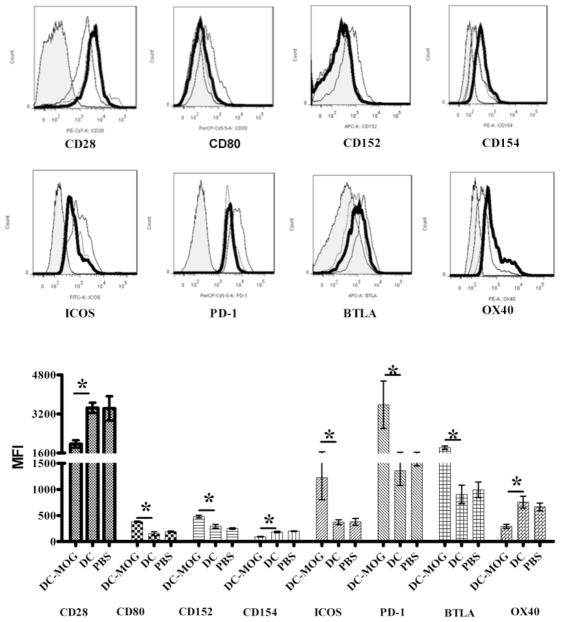
Since co-stimulatory molecule receptors expressed on CD4+ T cells play an important role in modulation of autoimmunity and tolerance in vivo, a systemic investigation was carried out to detect whether bone marrow-derived immature DCs can affect expression of co-stimulatory receptors on CD4+ T cells. Our results demonstrated that the expression of OX40, CD154 and CD28 is down-regulated. However, the expression of CD152, CD80, PD-1, BTLA and ICOS is up-regulated after i.v. transfer of bone marrow-derived DCs pulsed with MOG peptide (Fig. 3). By contrast, i.v. DCs without loading MOG peptide cannot affect expression of any co-stimulatory molecule ligands on CD4+ T cells (Fig. 3). These results showed that bone marrow-derived DCs pulsed with MOG peptide affect expression of co-stimulatory ligands with different effects.
Figure 3.
Intravenous transfer of bone-marrow-derived DCs modulates protein expression of ligands of co-stimulatory molecules on MOG-primed CD4+ T cells. Splenocytes were isolated from EAE mice treated with i.v. PBS, DCs pulsed with MOG peptide (0.1 μM) (DC-MOG) or not (DC).Cells were stained and CD4+ T cells were gated. Protein expression of OX40, CD152, CD80, CD154, PD-1, CD28, ICOS and BTLA on MOG-stimulated CD4+ T cells derived from mice treated with DC-MOG (thin line), DC without MOG peptide treatment (DC, dot line) and PBS (thick line) is shown. Isotype controls (shade) are also indicated. The error bars shown in this figure represent the mean and SD of triplicate determinations of mean fluorescence intensity (MFI) of co-stimulatory molecule ligands expressed on CD4+ T cells in three independent experiments (n=3, t test, * P<0.05).
4. i.v. transfer of bone marrow-derived immature DCs pulsed with MOG peptide regulates the expression of CCR4, CCR5 and CCR7 on CD4+ T cells
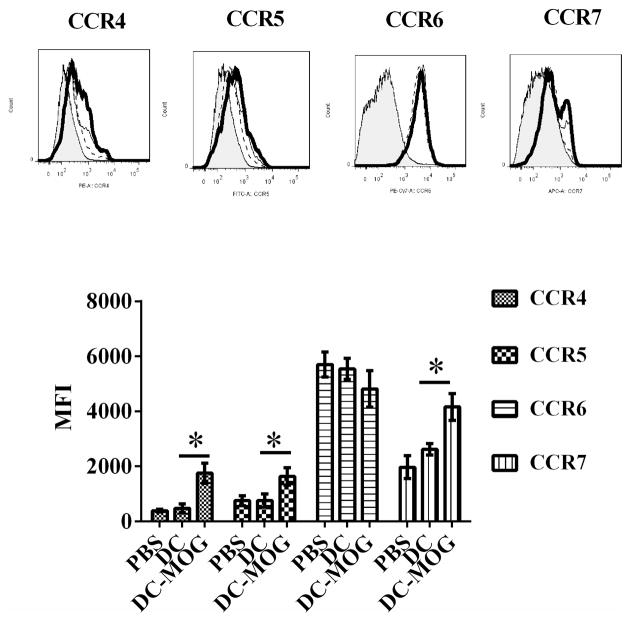
Since chemokine receptors expressed on CD4+ T cells can affect migration of effector CD4+ T cells to local environment such as CNS and affect EAE development, we tested whether i.v. transfer of bone marrow-derived immature DCs pulsed with MOG peptide or without loading MOG peptide can affect expression of CCR4, CCR5, CCR6 and CCR7 on CD4+ T cells or not. Our results indicated that the expression of CCR4, CCR5 and CCR7 on CD4+ T cells is increased after i.v. transfer of bone marrow-derived DCs pulsed with MOG peptide. However, MOG-pulsed DCs do not affect expression of CCR6 on CD4+ T cells. i.v. transfer of DCs without loading MOG peptide cannot affect expression of CCR4, CCR5, CCR6 and CCR7 on CD4+ T cells(Fig. 4). Our results suggest that bone-marrow-derived immature DCs pulsed with MOG peptide may affect migration of CD4+ T cells and EAE development through modulating expression of chemokine receptors such as CCR4, CCR5, and CCR7 on CD4+ T cells.
Figure 4.
Intravenous transfer of bone marrow-derived DCs pulsed with MOG peptide up-regulates protein expression of CCR4, CCR5 and CCR7 on CD4+ T cells. Splenocytes were isolated from mice treated with PBS (thin line), DC spulsed with MOG peptide (thick line) or DCs without loading MOG peptide (dash line). The isotype (shade) controls are also shown. CD4+ T cells were gated and their expression of CCR4, CCR5, CCR6 and CCR7 was determined by flow cytometry. Error bars shown in this figure represent mean and SD of mean of fluorescence intensity (MFI) of CCR4, CCR5, CCR6 and CCR7 expression on CD4+ T cells in three independent experiments ( n=3,t test, *P<0.05).
Discussion
DCs are professional antigen presenting cells (APCs) that play an important role in both autoimmunity and immune tolerance [15, 16]. However, it has not been fully elucidated how DCs modulate the balance between autoimmunity and tolerance in vivo. In general, immature DCs modulate tolerance and mature DCs stimulated by inflammatory signals facilitate the activity of T cells and lead to inflammation [15, 16]. Here an experimental model of tolerance induced by bone marrow-derived immature DCs was established. EAE induction is dependent on the activity of Th1 cells which are IFN-γ producing CD4+ T cells, and Th17 cells which are IL-17A producing CD4+ T cells [17]. Since the production of both IL-17A and IFN-γ was decreased after i.v. transfer of MOG peptide-pulsed immature DCs, our results suggest that MOG peptide–pulsed immature DCs may block the activity of Th17 and Th1 cells and then lead to immune tolerance in vivo.
Regulatory T cells are IL-10 producing CD4+ FOXP3+ T cells and can inhibit inflammation in vivo [18]. Since i.v. transfer of immature DCs leads to increase the production of IL-10 and the expression of FoxP3, it suggests that immature DCs may facilitate the development of regulatory T cells so that there is higher amount of anti-inflammatory cytokines such as IL-10 to be synthesized to inhibit inflammatory responses in EAE.
In addition, another important type of suppressive CD4+ T cells that can produce both IL-10 and IFN-γ was found in the 1990s [19]. CD4+ IL-10+ IFN-γ+ T cells are different from conventional Treg cells in that they are FoxP3− and T-bet+[20]. Even though CD4+ IL-10+ IFN-γ+ T cells are T-bet and produce IFN-γ, they are not Th1 cells, given that they inhibit inflammatory responses while Th1 cells, on the contrary, facilitate immune responses. It is unclear whether CD4+ IL-10+ IFN-γ+ suppressive T cells can inhibit EAE development. Our results demonstrate that the numbers of CD4+ IL-10+ IFN-γ+ are increased in mice with tolerance induced by i.v. transfer of MOG-pulsed immature DCs, suggesting that these DCs may block EAE development through up-regulation of numbers of CD4+ IL-10+ IFN-γ+ suppressive T cells.
DCs interact with T cells through peptide /MHC complexes binding to T cell receptor (TCR) on T cells (Signal 1) and co-stimulatory molecule-mediated signal transduction pathways (Signal 2) [3]. There are at least 8 ligands of co-stimulatory molecules expressed on T cells including OX40, CD154, CD80, CD28, CD152, PD-1, BTLA and ICOS [21]. Among them, OX40, CD154, and CD28 are ligands of positive co-stimulatory molecules which facilitate T-cell-mediated immunity in EAE [22–24]. In contrast, CD80, CD152, PD-1, BTLA and ICOS-mediated signal transduction pathways play an important role in induction of immune tolerance in EAE [25–29]. Our results indicate that i.v. transfer of immature DCs pulsed with MOG peptide down-regulates expression of CD154, CD28, and OX40, which are necessary for T cell activation on CD4+ T cells. However, expression of CD80, CD152, PD-1, BTLA and ICOS, which can inhibit T cell activity on CD4+ T cells, is up-regulated after i.v. transfer of DCs. These results suggest that immature DCs may inhibit the activity of auto-reactive CD4+ T cells by modulating expression of ligands of co-stimulatory molecules on auto-reactive CD4+ T cells and then block EAE development.
For EAE inhibition, it is necessary that regulatory T cells are recruited to the target tissue, the CNS. It is known that CCR4, CCR5, CCR6 and CCR7 expressed on regulatory CD4+ T cells play an important role in regulatory T cell trafficking to the peripheral environment [7–12]. However, it is not yet known whether immature DCs suppress EAE by regulating these molecules. Our study demonstrates that i.v. transfer of immature DCs pulsed with MOG peptide up--regulates the expression of CCR4, CCR5 and CCR7 on CD4+ T cells. This implies that immature DCs may affect trafficking of regulatory CD4+ T cells by up-regulating protein expression of CCR4, CCR5, and CCR7 in vivo, and then facilitate the migration of regulatory CD4+ T cells into the CNS to inhibit EAE.
It has been known that CCR7 is necessary for expression of FoxP3 in regulatory T cells [28]. i.v. transfer of MOG-pulsed immature DCs improves expression of FoxP3 and CCR7 in CD4+ T cells. This implies that immature DCs may facilitate expression of FoxP3 in regulatory T cells through enhance expression of CCR7 on CD4+ T cells.
In summary, our results show that immature DCs may induce tolerance and block EAE development via multiple pathways such as inducing CD4+ FoxP3+ IL-10+ Tregs, modulating expression of ligands of co-stimulatory molecules and chemokines on CD4+ T cells. Our research reveals a potential mechanism of immature DC-mediated immune tolerance and may be applied for immunotherapy to target MS and other auto immune diseases in clinical trials.
Acknowledgments
This study was supported by the NIH and the National Multiple Sclerosis Society.
We thank Katherine Regan for editorial assistance.
Abbreviations
- APC
Antigen presenting cell
- BTLA
B and T lymphocyte attenuator
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- DC
Dendritic cell
- EAE
Experimental autoimmune encephalomyelitis
- FCS
Fetal Calf Serum
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- ICOS
Inducible co-stimulator
- i.v
Intravenous
- MOG
myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- PD-1
Programmed death-1
- TCR
T cell receptor
- Tregs
Regulatory T cells
- 2-ME
2-mercaptoethanol
References
- 1.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–83. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Nikolic T, Welzen-Coppens JM, Leenen PJ, Drexhage HA, Versnel MA. Plasmacytoid dendritic cells in autoimmune diabetes - potential tools for immunotherapy. Immunobiology. 2009;214:791–9. doi: 10.1016/j.imbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–43. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 4.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11:625–30. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Farez M, Gilmore W. Vaccines for multiple sclerosis: progress to date. CNS Drugs. 2008;22:175–98. doi: 10.2165/00023210-200822030-00001. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. Journal of immunology. 2008;181:2483–93. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–44. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–87. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Xu W, Qiu S, Xiong S. Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clin Immunol. 2010;135:466–75. doi: 10.1016/j.clim.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Eller K, Weber T, Pruenster M, Wolf AM, Mayer G, Rosenkranz AR, Rot A. CCR7 deficiency exacerbates injury in acute nephritis due to aberrant localization of regulatory T cells. J Am Soc Nephrol. 2010;21:42–52. doi: 10.1681/ASN.2009020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueha S, Yoneyama H, Hontsu S, Kurachi M, Kitabatake M, Abe J, Yoshie O, Shibayama S, Sugiyama T, Matsushima K. CCR7 mediates the migration of Foxp3+ regulatory T cells to the paracortical areas of peripheral lymph nodes through high endothelial venules. J Leukoc Biol. 2007;82:1230–8. doi: 10.1189/jlb.0906574. [DOI] [PubMed] [Google Scholar]
- 12.Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735–45. doi: 10.1084/jem.20061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang GX, Kishi M, Xu H, Rostami A. Mature bone marrow-derived dendritic cells polarize Th2 response and suppress experimental autoimmune encephalomyelitis. Mult Scler. 2002;8:463–8. doi: 10.1191/1352458502ms857oa. [DOI] [PubMed] [Google Scholar]
- 15.Morel PA, Turner MS. Dendritic cells and the maintenance of self-tolerance. Immunol Res. 2011;50:124–9. doi: 10.1007/s12026-011-8217-y. [DOI] [PubMed] [Google Scholar]
- 16.Carreno LJ, Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Modulation of the dendritic cell-T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy. 2011;3:6–11. doi: 10.2217/imt.11.38. [DOI] [PubMed] [Google Scholar]
- 17.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;24:641–51. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–8. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- 19.Gerosa F, Nisii C, Righetti S, Micciolo R, Marchesini M, Cazzadori A, Trinchieri G. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol. 1999;92:224–34. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Liu XS. Development and function of IL-10 IFN-gamma-secreting CD4(+) T cells. J Leukoc Biol. 2009;86:1305–10. doi: 10.1189/jlb.0609406. [DOI] [PubMed] [Google Scholar]
- 21.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162:1818–26. [PubMed] [Google Scholar]
- 23.Girvin AM, Dal Canto MC, Miller SD. CD40/CD40L interaction is essential for the induction of EAE in the absence of CD28-mediated co-stimulation. J Autoimmun. 2002;18:83–94. doi: 10.1006/jaut.2001.0573. [DOI] [PubMed] [Google Scholar]
- 24.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–43. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 25.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. Journal of immunology. 2006;177:2948–58. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 26.Ratts RB, Arredondo LR, Bittner P, Perrin PJ, Lovett-Racke AE, Racke MK. The role of CTLA-4 in tolerance induction and ttigen administration cell differentiation in experimental autoimmune encephalomyelitis: i.v antigen administration. Int Immunol. 1999;11:1889–96. doi: 10.1093/intimm/11.12.1889. [DOI] [PubMed] [Google Scholar]
- 27.Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, Benoit S, Ireland G, Luxenberg D, Askew GR, Milarski KL, Groves C, Brown T, Carito BA, Percival K, Carreno BM, Collins M, Marusic S. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;182:124–34. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–9. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 29.Galicia G, Kasran A, Uyttenhove C, De Swert K, Van Snick J, Ceuppens JL. ICOS deficiency results in exacerbated IL-17 mediated experimental autoimmune encephalomyelitis. J Clin Immunol. 2009;29:426–33. doi: 10.1007/s10875-009-9287-7. [DOI] [PubMed] [Google Scholar]