Abstract
BACKGROUND
Lymphatic invasion (LI) identified by immunohistochemical staining is common in primary cutaneous melanoma, and LI has been shown to be an independent prognostic factor in melanoma. Its prognostic significance in melanocytic tumors of uncertain malignant potential (MELTUMP) has not been well characterized.
METHODS
This study included 32 patients with provisional diagnoses of MELTUMP. Lesions were evaluated for tumor thickness, the presence of ulceration, mitotic figures, mitotic figures at the base, tumor infiltrating lymphocytes (TILs), as well as peritumoral and intratumoral lymphatic density. Dual immunohistochemical staining was used to microscopically detect lymphatic endothelium (podoplanin) containing melanoma cells (S-100), with the aid of multispectral imaging in select cases. Univariate analysis was performed to identify associations between clinical and pathologic variables and melanoma related events.
RESULTS
The 32 patients had a median of 111 months follow-up. Two patients subsequently died of melanoma-related disease, one died of unknown causes, five developed nodal metastases, and the remainder showed no evidence of progressive disease. LI was identified in 8/32 cases (25%) by dual immunohistochemical stains, including both cases in which patients died of melanoma-related disease, one patient with bulky nodal metastasis, one of four patients with microscopic nodal metastases, and in four patients who showed no evidence of progressive disease. The presence of lymphatic invasion was associated with melanoma metastases or melanoma related death (p= 0.05).
CONCLUSION
The presence of lymphatic invasion by dual immunohistochemistry in MELTUMPs is associated with a poorer prognosis, specifically with melanoma metastasis and may therefore serve as a useful prognostic factor for risk stratifying patients with these diagnostically challenging lesions.
Introduction
A subset of bulky melanocytic lesions obscure the boundary between benign nevus and malignant melanoma and have long perplexed dermatopathologists due to their morphology and biologic behavior, often eluding consensus in their diagnosis as well as their nomenclature 1–3. While characterized as “borderline melanomas,” “minimal deviation melanoma,” “dermal-based borderline melanocytic tumor,” 4–6 and “atypical” counterparts to conventional nevi such as Spitz, blue, or deep penetrating nevi (in addition to many others), our preferred term for these lesions is “melanocytic tumors of uncertain malignant potential” (MELTUMP), as it aptly captures the diagnostic and prognostic challenge they represent 2,7–10. MELTUMP is a provisional diagnosis; although one may favor a benign or malignant characterization, a definitive diagnosis is not always possible at initial presentation, and long term (or perhaps life-long) clinical follow up remains the only true evidence of biologic behavior. These lesions often require expert consultation and frequently prompt aggressive management that would accompany a melanoma diagnosis.
Melanoma has a well-known propensity for lymph node metastasis 11,12. Lymphangiogenesis and lymphatic invasion (LI, defined as the presence of melanoma cell(s) within a lymphatic vessel) have been under increasing investigation in melanoma given the recent availability of antibodies specific for lymphatic endothelial cells 13,14. We and others have shown that LI detected by immunohistochemistry in primary melanomas is common, ranging from 16% to 47% 13,15, whereas blood vascular invasion is uncommon, ranging from 1% to 3% 16,17. We recently presented evidence of lymphangiogenesis in areas with regression in the radial growth phase adjacent to vertical growth phase (VGP) lesions, and the presence of LI in the area of radial growth phase regression may, at least in part, explain the association of regression with poorer prognosis 18. More recently, we showed that lymphatic invasion is an independent adverse prognostic factor and significantly increases the risk of metastasis in melanoma 19. We sought to determine the presence of LI by immunohistochemistry in MELTUMPs, with the hypothesis that the presence of LI may serve as a negative prognostic marker of disease in these patients. Additionally, lymphatic density (LD), both peritumorally and intratumorally, was assessed to study whether the extent of lymphangiogenesis in these lesions was associated with prognosis.
Materials and Methods
Twenty-three cases with a provisional diagnosis of MELTUMP were identified from one of the co-author’s consult cases (DEE). Diagnostic criteria for MELTUMP were discussed previously7,9,10. Nine additional cases with available residual tumor tissues were provided by another co-author (LC)2. All cases were reviewed by two pathologists (XX, RMA) and were confirmed to be MELTUMP lesions at initial presentation. Cases were also subcategorized as “Spitzoid” (with morphology resembling Spitz tumor), “DPN-like” (resembling a deep penetrating nevus), or “nevoid” (resembling a banal or dysplastic nevus). No “blue-nevus like” lesions were present in this cohort. Clinical follow up was obtained via consultant physicians and patients through a protocol approved by the Institutional Review Board of the University of Pennsylvania. Lesions were evaluated for tumor thickness and the presence of ulceration, mitotic figures, mitotic figures at the base, and tumor infiltrating lymphocytes (TILs). Dual immunohistochemical staining was used to detect lymphatic endothelium (podoplanin, D2-40) and melanoma cells (S-100 protein). The presence of LI was analyzed microscopically, with the aid of multispectral imaging (MSI) (27). Lymphatic invasion was defined as S-100 protein-positive cells with melanoma cytology within a podoplanin-positive lymphatic space. Peritumoral and intratumoral lymphatic density were also assessed. Peritumoral lymphatic density (LD) was defined as the number of lymphatic spaces in a “hotspot” in five high-power (400x) fields within 2 mm of the tumor edge. Intratumoral LD was defined as the number of lymphatic spaces in a “hotspot” in five high-power fields within the tumor.
Immunohistochemical assays were performed on 5 μm formalin-fixed, paraffin-embedded tissue sections and staining was done on a DakoCytomation Autostainer using the EnVision+ HRP DAB system (DakoCytomation) according to manufacturer’s recommendations. The D2-40 antibody (mouse monoclonal, 1:25 dilution; Signet Laboratories) that specifically detects a fixation resistant epitope on podoplanin was used to decorate lymphatic endothelium. Melanocytes were identified using S-100 protein antibody (rabbit polyclonal, 1:50; DakoCytomation). The antibody to lymphatic endothelium was visualized with the brown chromogen DAB (3, 3-diaminobenzidine; DakoCytomation) and antibodies to melanoma cells with the red chromogen Nova Red (Vector Laboratories). IHC-stained slides cut from unstained slides or tissue blocks were reviewed by 2 pathologists (RMA, XX), who were blinded to clinical outcome. LI (present or absent) was defined as the presence anywhere within the primary tumor of S-100 positive cell(s) with morphologic features of melanocytic tumor in lumens highlighted by podoplanin staining. Questionable instances were confirmed or refuted by use of MSI. Disagreements were resolved by consensus reading.
For MSI analysis, slides were examined using a Leica DMRA2 microscope (Leica Microsystems Inc.) equipped with planapochromatic lenses. Potential foci of LI were imaged at 200× through a liquid crystal filter using the Nuance Multispectral Imaging System (Cambridge Research and Instrumentation Inc.). Spectral data were acquired from 420 to 720 nm, and spectral unmixing was accomplished by Nuance software v1.42 and pure spectral libraries of individual chromogens (slides stained with only DAB, Nova red, or hematoxylin). Nonspecific background staining was subtracted from each image individually. To visualize several spectral markers simultaneously, images were then evaluated using unmixed images generated by the Nuance system.
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc.). Unpaired t-tests were used to evaluate correlations between LI and the tumor characteristics. The following cutoffs or distinctions were used for log-rank survival analysis: LI absent or present, male of female gender, thickness of >2 mm, brisk or non-brisk/absent TILs, mitoses at base present or absent, peritumoral LD median (>7), and intratumoral LD median (>5). Multivariate analysis was also performed.
Results
The median age of 32 patients was 31.5 (range 2–67), there were 16 males and 16 females, and the average tumor thickness was 2.46 mm (Table 2). 22 lesions were categorized as “Spitzoid,” 9 as “nevoid,” and 1 as “DPN-like.” Clinical follow up ranged from 4 to 276 months, with a median of 111 months. Of the 32, two patients subsequently died of melanoma-related disease, one died of unknown causes, five developed nodal metastases (one with bulky disease), and the remainder showed no evidence of progressive disease. Lymphatic invasion was not recognized in any of the cases except through the use of double immunohistochemical staining. Lymphatic invasion by our staining method was found in 8/32 cases (25%), including both cases in which patients died of melanoma-related disease (2/2, 100%), one patient with bulky nodal metastasis (1/1, 100%), one of four patients with microscopic nodal metastases (1/4), and in four patients who showed no evidence of progressive disease (Figures 1–3). The presence of lymphatic invasion correlated with more aggressive clinical outcomes, defined as either developing nodal metastases, distant metastases, or melanoma-related death by unpaired t-test (p= 0.047). The incidence of a melanoma related metastasis or death in the LI group was 57% (4/7) versus 16% (4/25) in the no LI group. LI was also associated with a significant melanoma-specific survival difference by log-rank analysis (p=0.03). None of the other parameters evaluated (age, tumor thickness, mitoses, mitoses at the base, ulceration, TILs, intratumoral, or peritumoral LD) demonstrated a statistically significant correlation with outcome or significant survival difference by unpaired t-test or log-rank analyses (Table 2). Multivariate statistical analyses were performed; however none of the results were statistically significant. No significant correlation could be made between histologic subtype of MELTUMP and lymphatic invasion, age, or other tumor characteristics.
Table 2.
Tumor characteristics (including lymphatic invasion (LI) and lymphatic density (LD)) and Correlation with Metastasis and Melanoma-specific death (MSD)
| Tumor Characteristic | Frequency | Percentage | Metastasis and MSD P value |
|---|---|---|---|
| Presence of LI | 0.05 | ||
| Present | 8 | 25 | |
| Absent | 24 | 75 | |
| Age | 0.64 | ||
| 0–25 | 14 | 44 | |
| 25–50 | 7 | 22 | |
| >50 | 11 | 34 | |
| Gender | 0.23 | ||
| Male | 16 | 50 | |
| Female | 16 | 50 | |
| Thickness (> 2mm) | 0.48 | ||
| 0–1 mm | 6 | 19 | |
| 1–4 mm | 18 | 56 | |
| >4 mm | 8 | 25 | |
| Presence of Mitotic Figures (per 1mm2) | 0.86 | ||
| 0 | 13 | 57 | |
| 1 | 4 | 17 | |
| >1 | 6 | 26 | |
| Presence of Mitoses at Base of Lesion | 0.69 | ||
| Present | 4 | 17 | |
| Absent | 19 | 83 | |
| Presence of TILs | 0.32 | ||
| None | 6 | 26 | |
| Non-brisk | 11 | 48 | |
| Brisk | 6 | 26 | |
| Peritumoral LD (>7) | 0.89 | ||
| Intratumoral LD (>7) | 0.52 | ||
Fig. 1. Case 13.
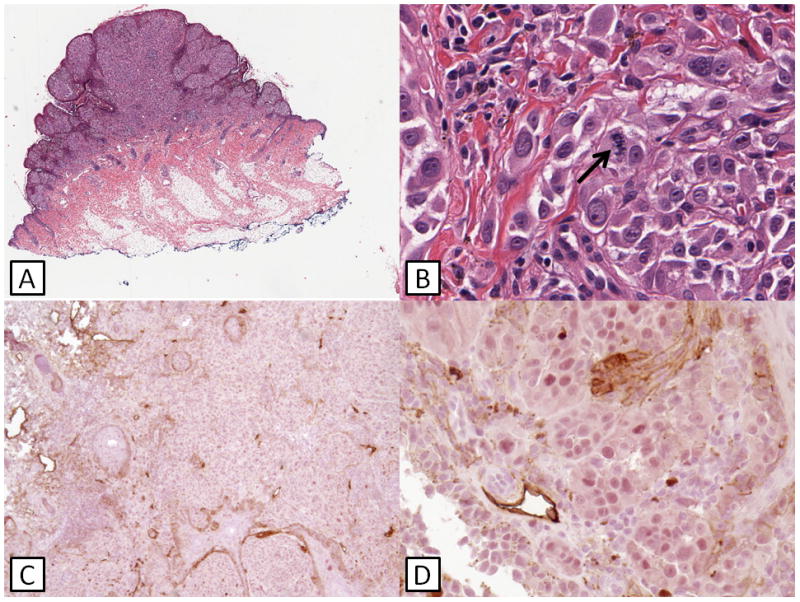
(A) A polypoid, exophytic lesion is seen. (B) Spitzoid morphology is present with prominent cytologic atypia and mitotic activity (arrow). (C) and (D) Double immunohistochemical staining shows the absence of lymphatic invasion. This patient is alive and well at follow-up with no evidence of progressive disease.
Fig. 3. Case 31.
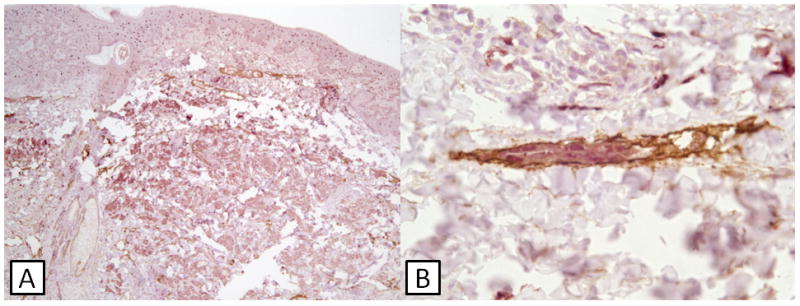
(A) Double staining shows S100-positive tumor cells extending into the deep dermis. A collection of tumor cells present within lymphatic endothelium is present (black rectangle). (B) Higher magnification of area within black rectangle depicting lymphatic invasion. This patient developed bulky lymph node metastases.
Discussion
The purpose of this study was an attempt to validate the use of evaluating lymphatic invasion by dual immunohistochemisty as a useful tool in ambiguous melanocytic lesions with vertical growth phase (MELTUMPs). Additionally, we assessed lymphatic density to see if this property correlated with outcome or other tumor characteristics. This particular subset of melanocytic lesions is fraught with controversy as well as minimal diagnostic reproducibility and agreement among dermatopathologists. There is some question whether the concept of “uncertainty” or “ambiguity” in melanocytic lesions is valid and can coexist with the pathologist’s burden to make definitive diagnoses 20,21. However, it is in these present authors’ collective opinion that there are particular lesions where one cannot make a definitive diagnosis based on accepted histopathologic criteria, and thus it is uncertain as to how a lesion will progress clinically. In a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, Austria, Cerroni and colleagues (including MCM and DEE) looked at 57 ambiguous melanocytic lesions to both assess the diagnostic reproducibility of these lesions as well potential features that may help in the categorization of these tumors. Diagnostic consensus was found to be relatively uncommon, even among a group of international experts in melanocytic tumors, as 15.8% of the cases were either classified by the majority of the panelists as uncertain or the diagnoses were split equally between benign and malignant 2. Additionally, among the several histopathologic criteria examined in this exercise, only three were found statistically different in the two groups with favorable and unfavorable behavior based on clinical follow up: presence of mitoses, mitoses near the base, and inflammatory infiltrate. Our study evaluated these three criteria to test if we could reproduce the same correlations with outcome; however we failed to find statistically significant associations. Our cohort had fewer cases with clinical follow up and fewer with unfavorable outcomes or aggressive behavior, as Dr. Cerroni’s cohort comprised 57 cases with clinical follow up, and several patients with bulky nodal metastases, visceral metastases, and melanoma related death. Nine cases from that cohort are also part of our study; however, no tumor characteristics were factored into the analysis of our cohort other than LI and LD, age, and sex. The difference between the studies is likely due to the number of cases and heterogeneous nature of MELTUMP cases.
Our statistically significant findings include an association between lymphatic invasion by our method and “aggressive behavior,” as defined by nodal metastases and melanoma-specific death. Another interesting statistically significant correlation is the presence of TILs with increased levels of lymphatic density. This phenomenon appears to link lymphocytic infiltrate with lymphangiogenesis in melanoma. This finding parallels our previous finding of the presence of lymphangiogenesis in the area of radial growth phase regression, suggesting that lymphocytes or other inflammatory cells may secrete cytokines inducing lymphangiogenesis. Nevertheless, the underlying mechanism of the phenomenon is still unclear and we are currently investigating this in our research laboratory.
Lymphatic invasion was present in both cases of melanoma-related death, suggesting high sensitivity of LI in MELTUMPs to detect unequivocal malignant behavior. In both cases, lymphatic vessels were difficult to appreciate on standard H and E staining. Lymphatic invasion was also present in one case with bulky nodal metastasis (1/1, 100%), and in one of four patients with microscopic nodal metastases (1/4, 25%). The significance of tumor deposits in lymph nodes is also a controversial matter in MELTUMPs. Unfortunately, since the cases were received in consultation from different regions, treatment of these lesions was not consistent; some patients received local wide-excision and never returned to the dermatologist whereas others underwent sentinel lymph node biopsy and subsequent lymphadenectomy. While sentinel lymph node (SLN) biopsy was performed in several of these cases, and is a useful prognostic tool in the management of those with melanoma, the role of (SLN) biopsy in MELTUMPs has not yet been established. Recent studies have looked at concurrent tumor deposits in lymph nodes of MELTUMPs, mostly of atypical Spitzoid lesions, and show that these lesions rarely progress to overt malignancy. A study of atypical spitzoid tumors at one institution showed that roughly half of 67 cases contained tumor deposits in lymph nodes while only one patient of the subset died of progressive disease 22. Other studies have presented similar findings 15,22–25. It appears that lymph node involvement in MELTUMP-like lesions occurs frequently and is perhaps indicative of low malignant potential, and the incidence of subsequent deadly disease is low. Nevertheless, most of these studies have relatively short follow-up time. The fact remains that melanoma can afflict both young and old, and appear nevoid, spitzoid, DPN-like or resemble any other benign counterpart, and metastasis could manifest several decades later in life 26,27. For a young child, one to two decades of disease-free follow up does not necessarily preclude the potential for disease progression into adulthood. What lymphatic invasion by dual immunohistochemistry may offer is definitive evidence of lymphovascular invasion, which excludes the possibility of a mechanical migration or colonization of a lymph node by a benign nevus, which may hold more prognostic relevance based on our data. Furthermore, since SLN biopsy is not currently standard of care in these lesions and can potentially cause morbidity especially with completion lymphadenectomy, our LI assay could potentially provide a surrogate marker for sentinel lymph node positivity. Further study would be needed to validate these assertions.
Finally, the study illustrates the difficulty in categorizing a subset of lesions which are heterogeneous but share a common diagnostic uncertainty due to histopathologic features that straddle benign and malignant characterizations. As molecular technology, currently array-CGH and FISH (and perhaps later next generation sequencing modalities), may provide useful supplemental data, currently no one histopathologic or ancillary criterion can establish a diagnosis. Unfortunately the only reliable indicator of malignancy in melanocytic lesions is the development of clinical or distant metastases. Based on our findings, our method of lymphatic invasion by dual immunohistochemistry may provide a relatively cheap, non-invasive, prognostic adjunct in determining which lesions are capable of distant metastasis and fatal outcomes.
Fig. 2. Case 2.
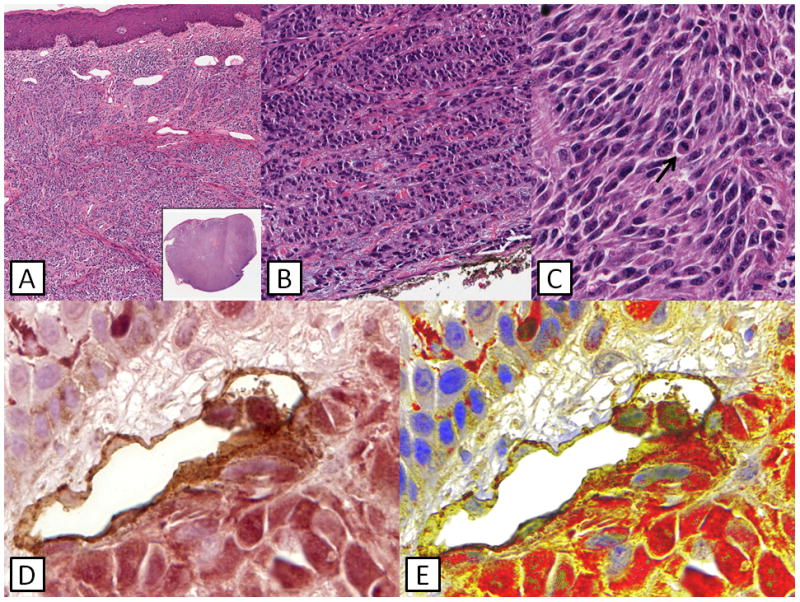
(A) Large nodular lesion composed of large fascicles of tumor cells. (B) Evidence of maturation seen at the base of the lesion. (C) Higher power shows hyperchromatic spindled and epithelioid tumor cells with readily identifiable mitotic figures (black arrow). (D) Double staining shows the presence of S100-positive tumor cells within D240-positive endothelium consistent with lymphatic invasion. (E) Presence of LI confirmed by multispectral imaging. This patient developed visceral melanoma metastases and expired two years after presentation.
Table 1.
Patient demographics and tumor characteristics
| Case | Age/Sex | Site | Subtype | LI | Intratumoral LD | Peritumoral LD | Mitoses (per 1mm2) | Mitoses at base | Thickness (mm) | TILs | Ulceration | Clinical Status | Followup (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 F | Back | N | No | 7 | 8 | 1 | No | 2.6 | Brisk | No | NED | 144 |
| 2 | 58 F | Vulva | S | Yes | 12 | Cannot assess | 4 | Yes | 17 | None | No | DM | 60 |
| 3 | 20 M | Right upper back | S | No | 35 | 14 | 0 | No | 0.6 | Brisk | No | NED | 112 |
| 4 | 56 M | Back | S | No | 0 | 1 | 0 | No | 0.8 | None | No | NED | 108 |
| 5 | 67 M | Eyelid | N | Yes | 0 | 4 | 0 | No | 0.7 | Brisk | Yes | DM | 74 |
| 6 | 53 M | Left upper eyelid | N | No | 4 | Cannot assess | 0 | No | 0.7 | None | No | NED | 94 |
| 7 | 37 F | Right medial calf | S | No | 5 | 1 | 0 | No | 1.2 | None | No | NED | 107 |
| 8 | 2 M | Right cheek | S | No | 0 | 1 | 2 | No | 1.8 | None | No | NED | 144 |
| 9 | 56 F | Cheek | N | No | 0 | 2 | 1 | No | 2 | Non-brisk | No | NED | 114 |
| 10 | 51 M | Right back | S | No | 2 | 2 | 0 | No | 0.6 | Non-brisk | No | DUC | 94 |
| 11 | 17 M | Back | N | No | 13 | Cannot assess | 2 | No | 3.5 | None | No | NED | 111 |
| 12 | 38 F | Right Shin | N | Yes | 15 | 11 | 0 | No | 1.5 | Non-brisk | No | NED | 150 |
| 13 | 2M | Right cheek | S | No | 9 | 27 | 0 | No | 2.2 | Non-brisk | No | NED | 122 |
| 14 | 12 F | Left anterior thigh | S | No | 5 | Cannot assess | 0 | No | 1.6 | Non-brisk | No | NED | 114 |
| 15 | 7 M | Left shoulder | S | Yes | 17 | Cannot assess | 9 | Yes | 2.2 | Brisk | No | NED | 94 |
| 16 | 23 F | Right lateral scalp | S | Yes | 16 | 15 | 1 | No | 2.2 | Non-brisk | No | ANM | 14 |
| 17 | 37 F | Back | S | No | 32 | 13 | 0 | No | 1.9 | Brisk | No | ANM | 13 |
| 18 | 59 M | Right forearm | D | No | 0 | 1 | 2 | Yes | 2 | Non-brisk | No | NED | 202 |
| 19 | 8 M | Right forearm | N | No | 17 | Cannot assess | 0 | No | 1.5 | Non-brisk | No | NED | 120 |
| 21 | 7 M | Scalp | S | No | 5 | 7 | 0 | No | 4.3 | Brisk | No | NED | 107 |
| 22 | 41 F | Left lower leg | S | No | 22 | Cannot assess | 1 | No | 1.4 | Non-brisk | No | NED | 109 |
| 23 | 52 F | Left medial shin | S | No | 8 | 12 | 0 | No | 0.6 | Non-brisk | No | NED | 111 |
| 24 | 10 F | Shoulder | S | Yes | 9 | 25 | * | * | 4.2 | * | No | NED | 168 |
| 25 | 56 M | Arm | S | No | 5 | 3 | * | * | 4 | * | No | NED | 204 |
| 26 | 21 M | Arm | S | No | 7 | 3 | * | * | 1.1 | * | No | NED | 120 |
| 27 | 28 F | Trunk | S | No | 26 | 17 | * | * | 4.3 | * | No | NED | 84 |
| 28 | 34 F | Shoulder | S | No | 4 | Cannot assess | * | * | 3.6 | * | No | NED | 120 |
| 29 | 58 M | Ear | N | No | 0 | 15 | * | * | 5.4 | * | No | NED | 276 |
| 30 | 28 F | Leg | N | No | 6 | 8 | * | * | 1.6 | * | Yes | ANM | 96 |
| 31 | 18 F | Trunk | S | Yes | 8 | 10 | * | * | 6.4 | * | No | ANM | 29 |
| 32 | 19 M | Leg | S | No | 10 | 6 | * | * | 5.4 | * | No | ANM | 4 |
KEY
S-Spitzoid
N-Nevoid
D-DPN-like
NED-No evidence of disease
ANM-Alive with nodal metastasis
DM-Died of melanoma
DUC-Died of unknown cause
cases contributed by LC-data not available for analysis
Acknowledgments
Funding: This work was supported by grants CA-093372 and AR-054593 from the National Institute of Health to XX.
The authors would like to thank all consultant physicians for their original consultations and their assistance, particularly Dr. Peter Olson and Dr. Andrea Galassi.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
Contributor Information
Ronnie M. Abraham, Department of Pathology, University of Pennsylvania, Philadelphia, PA, USA.
Giorgos Karakousis, Department of Surgery, University of Pennsylvania, Philadelphia, PA, USA.
Geza Acs, Departments of Anatomic Pathology and Women’s Oncology, Moffitt Cancer Center, Tampa, FL, USA.
Amy F. Ziober, Department of Pathology, University of Pennsylvania, Philadelphia, PA, USA.
Lorenzo Cerroni, Department of Dermatology, Medical University of Graz, Graz, Austria.
Martin C. Mihm, Jr., Department of Pathology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
David E. Elder, Department of Pathology, University of Pennsylvania, Philadelphia, PA, USA.
Xiaowei Xu, Email: xug@mail.med.upenn.edu, Department of Pathology, University of Pennsylvania, 3400 Spruce St., 6 Founders Building, Philadelphia, PA 19104, USA, Tel: (215) 662-6503, Fax: (215) 349-5910.
References
- 1.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–20. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 2.Cerroni L, Barnhill R, Elder D, et al. Melanocytic tumors of uncertain malignant potential: results of a tutorial held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol. 2010;34:314–26. doi: 10.1097/PAS.0b013e3181cf7fa0. [DOI] [PubMed] [Google Scholar]
- 3.Barnhill RL, Argenyi Z, Berwick M, et al. Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma (“malignant blue nevus”) Am J Surg Pathol. 2008;32:36–44. doi: 10.1097/PAS.0b013e3181573aaf. [DOI] [PubMed] [Google Scholar]
- 4.Magro CM, Crowson AN, Mihm MC, Jr, et al. The dermal-based borderline melanocytic tumor: a categorical approach. J Am Acad Dermatol. 2010;62:469–79. doi: 10.1016/j.jaad.2009.06.042. [DOI] [PubMed] [Google Scholar]
- 5.Reed RJ. Minimal deviation melanoma. Monogr Pathol. 1988:110–52. [PubMed] [Google Scholar]
- 6.Reed RJ. Minimal deviation melanoma. Borderline and intermediate melanocytic neoplasia. Clin Lab Med. 2000;20:745–58. [PubMed] [Google Scholar]
- 7.Elder DE. Tumorigenic melanocytic proliferations. New York: Demos Medical Pub; 2010. [Google Scholar]
- 8.Elder DE, Murphy GF, et al. Armed Forces Institute of Pathology (U.S.) Melanocytic tumors of the skin. Washington: Armed Forces Institute of Pathology: Available from the American Registry of Pathology; 1991. [Google Scholar]
- 9.Elder DE, Xu X. The approach to the patient with a difficult melanocytic lesion. Pathology. 2004;36:428–34. doi: 10.1080/00313020412331283905. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Elder DE. A practical approach to selected problematic melanocytic lesions. Am J Clin Pathol. 2004;121 (Suppl):S3–32. doi: 10.1309/YP99JBAUDXVEPVPG. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 12.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–17. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 13.Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massi D, Puig S, Franchi A, et al. Tumour lymphangiogenesis is a possible predictor of sentinel lymph node status in cutaneous melanoma: a case-control study. J Clin Pathol. 2006;59:166–73. doi: 10.1136/jcp.2005.028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochran AJ, Binder S, Morton DL. The role of lymphatic mapping and sentinel node biopsy in the management of atypical and anomalous melanocytic lesions. J Cutan Pathol. 2010;37 (Suppl 1):54–9. doi: 10.1111/j.1600-0560.2010.01509.x. [DOI] [PubMed] [Google Scholar]
- 16.Doeden K, Ma Z, Narasimhan B, et al. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J Cutan Pathol. 2009;36:772–80. doi: 10.1111/j.1600-0560.2008.01166.x. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Gimotty PA, Guerry D, et al. Lymphatic invasion revealed by multispectral imaging is common in primary melanomas and associates with prognosis. Hum Pathol. 2008;39:901–9. doi: 10.1016/j.humpath.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun SJ, Gimotty PA, Hwang WT, et al. High lymphatic vessel density and lymphatic invasion underlie the adverse prognostic effect of radial growth phase regression in melanoma. Am J Surg Pathol. 2011;35:235–42. doi: 10.1097/PAS.0b013e3182036ccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Chen L, Guerry D, et al. Lymphatic invasion is independently prognostic of metastasis in primary cutaneous melanoma. Clin Cancer Res. 2012;18:229–37. doi: 10.1158/1078-0432.CCR-11-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeBoit PE. Minimal deviation melanoma: concept or quagmire? Adv Dermatol. 1997;13:289–304. [PubMed] [Google Scholar]
- 21.Mones JM, Ackerman AB. “Atypical” Spitz’s nevus, “malignant” Spitz’s nevus, and “metastasizing” Spitz’s nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:310–33. doi: 10.1097/00000372-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ludgate MW, Fullen DR, Lee J, et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer. 2009;115:631–41. doi: 10.1002/cncr.24047. [DOI] [PubMed] [Google Scholar]
- 23.Busam KJ, Murali R, Pulitzer M, et al. Atypical spitzoid melanocytic tumors with positive sentinel lymph nodes in children and teenagers, and comparison with histologically unambiguous and lethal melanomas. Am J Surg Pathol. 2009;33:1386–95. doi: 10.1097/PAS.0b013e3181ac1927. [DOI] [PubMed] [Google Scholar]
- 24.Lohmann CM, Coit DG, Brady MS, et al. Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. Am J Surg Pathol. 2002;26:47–55. doi: 10.1097/00000478-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Murali R, Sharma RN, Thompson JF, et al. Sentinel lymph node biopsy in histologically ambiguous melanocytic tumors with spitzoid features (so-called atypical spitzoid tumors) Ann Surg Oncol. 2008;15:302–9. doi: 10.1245/s10434-007-9577-3. [DOI] [PubMed] [Google Scholar]
- 26.Barnhill RL. Childhood melanoma. Semin Diagn Pathol. 1998;15:189–94. [PubMed] [Google Scholar]
- 27.Scalzo DA, Hida CA, Toth G, et al. Childhood melanoma: a clinicopathological study of 22 cases. Melanoma Res. 1997;7:63–8. doi: 10.1097/00008390-199702000-00010. [DOI] [PubMed] [Google Scholar]


