Abstract
During nervous system development the neurotransmitter identity changes and coexpression of several neurotransmitters is a rather generalized feature of developing neurons. In the mature nervous system, different physiological and pathological circumstances recreate this phenomenon. The rules of neurotransmitter respecification are multiple. Among them, the goal of assuring balanced excitability appears as an important driving force for the modifications in neurotransmitter phenotype expression. The functional consequences of these dynamic revisions in neurotransmitter identity span a varied range, from fine-tuning the developing neural circuit to modifications in addictive and locomotor behaviors. Current challenges include determining the mechanisms underlying neurotransmitter phenotype respecification and how they intersect with genetic programs of neuronal specialization.
1. Introduction
Neurotransmitters are the mediators of the most prominent form of communication in the nervous system. The identity of the neurotransmitter involved in a given chemical synapse is crucial in multiple ways. The matching of neurotransmitter in the presynaptic cell with its receptor in the postsynapse is essential to successful transmission. Whether the neurotransmitter participates in inhibitory or excitatory synapses changes the functional properties of the underlying circuit. In addition, depending on the particular pair of neurotransmitter/receptor the qualitative and quantitative features of the synapse differ due to the distinct kinetics and signaling cascades imprinted in the identity of the synaptic partners. Hence, the understanding of mechanisms that determine neurotransmitter specification is paramount. Neurotransmitter signaling is apparent before synaptogenesis arguing for a role of neurotransmitters beyond synaptic transmission. Here we review the origin of neurotransmitter phenotype determination, the ontogenesis of neurotransmitter signaling, the changes in neurotransmitter specification, and discuss the relevance of this neurotransmitter respecification to the function of the nervous system. We particularly focused the review on the studies that support the concept that neurotransmitter phenotype expression is dynamic and sensitive to changes in developmental and environmental cues.
2. Ontogeny of neurotransmitter phenotype expression and signaling
Neurotransmitters like GABA, dopamine and noradrenaline are present in the ectoderm of late blastula and early neural plate stage Xenopus embryos and regulate neuronal differentiation (Rowe et al., 1993). The pituitary adenylate cyclase activating peptide is also expressed in the mouse embryonic neural tube (Waschek et al., 1998). Serotonin released from notochord is uptaken by floor plate cells and regulates changes in cell shape and cell movement important for neural tube closure (Lauder, 1988; Wallace, 1982). Progenitor mitotic cells express the cholinergic phenotype in the olfactory, lateral and third ventricle of the embryonic mouse brain before the time of last cell division and onset of neuronal differentiation (Schambra et al., 1989). All these studies demonstrate the presence of neurotransmitters at early embryonic stages, before neuronal differentiation is accomplished. Because neurotransmitters are expressed in cells that are not fully differentiated neurons, the specialized structure of the synapse is not present yet at these early developmental stages but other forms of release seem to operate in the immature nervous system for neurotransmitter signaling. GABA and glutamate are released in a SNARE- and calcium-independent manner and signal in embryonic and neonatal hippocampal neurons before synapse formation (Demarque et al., 2002). Glycine is also diffusely released from radial cells in the embryonic mouse spinal cord and enhances the spontaneous calcium-mediated activity of immature neurons (Scain et al., 2010). Volume acetylcholine transmission between starburst amacrine cells mediates retinal calcium wave propagation during mouse development (Ford and Feller, 2012). These alternative modes of neurotransmitter release are not exclusive to the developing nervous system but are also apparent in progenitor niches of the adult brain. Pools of dividing neural progenitors of the postnatal subventricular zone in the mouse brain are sensitive to the nonsynaptic release of GABA, which regulates neural stem cell proliferation and migration (Liu et al., 2005; Pathania et al., 2010).
Many studies have demonstrated that neurotransmitter receptors are also expressed at early stages of nervous system development by assessing their expression and functionality. Glutamate, GABA, dopamine, serotonin, purinergic and muscarinic acetylcholine receptors are expressed in the proliferating retina of many different species (Martins and Pearson, 2008). The activation of GABAA or AMPA receptors depolarizes ventricular zone cells of the embryonic rat neocortext and regulates cell proliferation (LoTurco et al., 1995). GABAA receptors are also present in precursors of rat cerebellar granule cells and regulate their proliferation (Fiszman et al., 1999). Knockdown of the embryonic α2 glycine receptor subunit alters the proliferation rate of zebrafish spinal neuron progenitors and thus decreases the number of spinal interneurons (McDearmid et al., 2006). These studies illustrate that even at the early stages of neural progenitor proliferation the neurotransmitter signaling is functional in many different nervous system structures and species. Other developmental processes also witness the early neurotransmitter expression and are regulated by neurotransmitter signaling. Migration of granule cells is modulated by NMDA receptors in the mouse developing cerebellum (Komuro and Rakic, 1993) and GABA receptors modulate embryonic rat cortical cell migration (Behar et al., 1996) and cortical interneuron migration in mice (Bortone and Polleux, 2009).
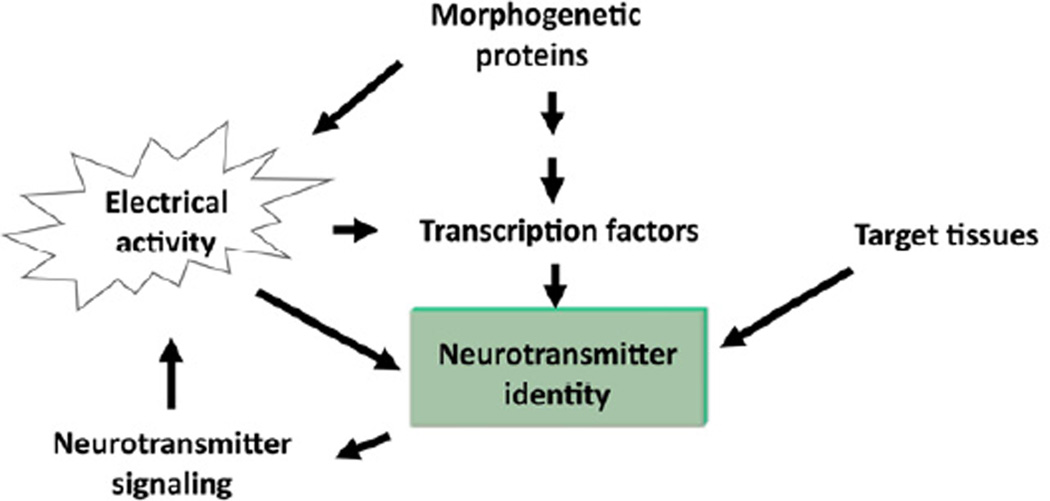
The differentiation of neurons involves the acquisition of morphological and functional characteristics. Many of these features are specified within the neural progenitor from which the neuron emerges. In the developing spinal cord, a progressive commitment to specific neuronal features is achieved by a combinatorial transcription factor code triggered by morphogenetic proteins such as Sonic hedgehog (Shh) and Bone Morphogenetic Proteins (BMPs) (Jessell, 2000). Is the expression of neurotransmitters and neurotransmitter receptors part of the specification program? Expression of certain transcription factors in developing neurons is necessary and sufficient to drive the expression of specific neurotransmitter phenotypes (Figure 1). For instance, the LIM homeodomain-containing transcription factor Lmx1b is required for the acquisition of the serotonergic phenotype in mice and links the Nkx2.2-mediated progenitor specification with the Pet1-dependent terminal differentiation (Ding et al., 2003). The choice of glutamatergic versus GABAergic phenotypes in the spinal cord dorsal horn is determined by the expression of the transcription factors Tlx1/3 and Pax2, respectively (Cheng et al., 2004). Moreover, Tlx knockout mice show an increase in the number of GABAergic neurons in the dorsal horn, suggesting that Tlx genes repress the expression of the GABAergic phenotype (Cheng et al., 2004). Reciprocally, the postmitotic homeobox gene Lbx1 upstream of Pax2, represses the glutamatergic phenotype and promotes the specification of GABAergic dorsal horn neurons (Cheng et al., 2005).
Figure 1. Regulation of neurotransmitter phenotype expression.
The specification of neural progenitors by morphogenetic proteins triggers a genetic program that results in expression of transcription factors in the emerging neurons necessary for the definition of the neurotransmitter identity. This is not a rigid program because it is intersected by other developmental and environmental cues, including pre and postsynaptogenic electrical activity and factors released from target tissues. In turn, the neurotransmitter signaling feedback loops into the process of neurotransmitter specification.
Despite the strong correlation between the expression of certain transcription factors and the neurotransmitter phenotype in a specific neuronal subtype, the fact that neurotransmitters are present before the specialization is achieved suggests that the acquisition of neurotransmitter identity is dynamic and may escape the deterministic genetic program of neuronal specialization.
3. Interrelation between the genetic program and dynamically regulated signaling pathways
The neural fate program triggered by morphogenetic proteins is indeed modulated by intracellular signaling pathways that are in turn dynamically regulated and are sensitive to many environmental and developmental cues. In the developing mouse and chick spinal cord, the transcription factor Olig2 drives two neural cell phenotypes as different as oligodendrocytes and motor neurons depending on its phosphorylation status, presumably regulated by PKA activity (Li et al., 2011). Shh and BMPs regulate calcium-mediated electrical activity of embryonic spinal neurons and contribute to a gradient of excitability along the dorsoventral axis of the developing Xenopus spinal cord (Belgacem and Borodinsky, 2011; Swapna and Borodinsky, 2012). In turn, electrical activity modifies neuronal differentiation. BMPs decrease calcium-mediated electrical activity by recruiting p38 MAPK, which negatively modulates calcium spikes. Reciprocally, the higher levels of calcium spike activity in the ventral spinal cord, prevent the expansion of the BMP-induced dorsal commissural interneuron phenotype in ventral domains (Swapna and Borodinsky, 2012). In contrast, Shh enhances calcium spike activity in embryonic spinal neurons by recruiting Gαi protein and IP3 receptor-operated stores. In turn, this enhanced activity mediates the increase in number of GABAergic spinal neurons induced by ectopic Shh (Belgacem and Borodinsky, 2011). The mechanisms by which genetically-driven programs are modulated by dynamically regulated signaling pathways can be multiple but they must converge at some point in the modulation of the expression and/or activity of transcription factors that drive the neurotransmitter specification. For instance, the expression of Lmx1b, driver of the serotonergic phenotype, is regulated by levels of calcium spike activity in Xenopus hindbrain (Demarque and Spitzer, 2010). The expression of the GABAergic/glutamatergic transcription factor selector, Tlx3, is also regulated by levels of electrical activity in the developing Xenopus spinal cord (Marek et al., 2010). Ca2+ spikes phosphorylate cJun that binds to Tlx3 CRE site and represses its expression. This represses the specification of the glutamatergic phenotype hence promoting the prevalence of the GABAergic phenotype (Marek et al., 2010). These examples of electrical activity-dependent neurotransmitter specification respond to a homeostatic paradigm through which the developing nervous system responds to enhancement of electrical activity by increasing the number of inhibitory neurotransmitter-expressing neurons and to suppression of electrical activity by increasing the number of excitatory neurotransmitter-expressing neurons (Belgacem and Borodinsky, 2011; Borodinsky et al., 2004; Demarque and Spitzer, 2010; Dulcis and Spitzer, 2008; Marek et al., 2010).
4. Overlapping neurotransmitter phenotypes during development: Neurotransmitter respecification
Several studies have investigated the developmental course of neurotransmitter expression. GABAergic and glutamatergic phenotypes are detected at neural plate stages in Xenopus embryos. Interestingly, there is overlap in the expression of these two neurotransmitters in individual spinal cells. The coexpression of the GABAergic and glutamatergic phenotypes is lost at larval stages (Root et al., 2008). Similarly, a dual glutamatergic and dopaminergic phenotype is evident in rat ventral mesencephalon at embryonic day 15, which is lost by embryonic day 19 retaining the dopaminergic phenotype in exclusivity (Dal Bo et al., 2008). In 24 h old zebrafish embryos, a third of Pax2-expressing spinal interneurons are both GABAergic and glycinergic (Batista and Lewis, 2008) and even a small number of GABAergic cells also express the glutamatergic phenotype (Higashijima et al., 2004). The incidence of the triple and dual phenotypes decreases as development progresses (Higashijima et al., 2004). In the developing auditory brainstem of the gerbil and rat the developmentally regulated coexistence of the GABAergic and glycinergic phenotypes is also apparent (Kotak et al., 1998; Nabekura et al., 2004). Moreover, these immature glycinergic/GABAergic presynaptic neurons in the rat lateral superior olive also release glutamate (Gillespie et al., 2005). The overlap of GABAergic and glutamatergic phenotypes is also transiently apparent in the developing rat cerebellar Purkinje cells (Gras et al., 2005) and in granule cells of the rodent dentate gyrus. These mossy fibers functionally express a dominant GABAergic phenotype during neonatal stages (Safiulina et al., 2006), they adopt the glutamatergic phenotype later on (Gutierrez et al., 2003; Walker et al., 2001) until the third postnatal week when mostly the glutamatergic phenotype prevails (Gutierrez et al., 2003). This overlap in neurotransmitter expression is not exclusive to the neurotransmitters referenced above. For instance, during development the early arriving axons that will innervate the sweat glands are noradrenergic but they transition and switch to the cholinergic phenotype as innervation progresses (Landis and Keefe, 1983; Schotzinger and Landis, 1988, 1990) and coexpression of the cholinergic and noradrenergic phenotypes is transiently apparent (Landis and Keefe, 1983).
The consideration of the rather promiscuous expression of two or more neurotransmitters in developing neural progenitors and immature neurons compared to fully mature neurons, suggest that there is pruning of neurotransmitter expression as development progresses. Alternatively, the observed coexpression corresponds to the transition between the initial phenotype towards the mature one. In fact, both scenarios are present. An example of the transient coexistence of multiple neurotransmitters that leads to a switch in neurotransmitter expression is well represented by the neurons in which the target tissue triggers the expression of a different neurotransmitter than the one initially specified. The initial noradrenergic phenotype, transitioning to the dual noradrenergic/cholinergic, ending with the cholinergic phenotype of sympathetic neurons that innervate the sweat glands, is a paradigmatic example (Landis and Keefe, 1983; Schotzinger and Landis, 1988, 1990). The mechanisms underlying this neurotransmitter respecification involve a retrograde signaling triggered by the neurotrophic cytokines of the CNTF family expressed in the sweat glands that induce the switch from noradrenergic to cholinergic phenotype (Habecker et al., 1995; Stanke et al., 2006) in a p38-dependent manner (Loy et al., 2011). Neurotrophins are important regulators of neurotransmitter expression. The p75 neurotrophin receptor regulates the development and relative number of medial septal region basal forebrain cholinergic and GABAergic neurons in a non-cell autonomous manner (Lin et al., 2007). Neurotrophins like BDNF can even mediate dynamic and fast changes in the identity of the synapse between sympathetic neurons and cardiac myocytes by controlling the release of excitatory noradrenaline or inhibitory acetylcholine presynaptically coexpressed (Yang et al., 2002).
The expression of multiple neurotransmitters in the early stages of development followed by a pruning and specialization on a single neurotransmitter phenotype appears to be the most common situation (Berube-Carriere et al., 2009; Gillespie et al., 2005; Nabekura et al., 2004; Root et al., 2008). The mechanisms underlying these transitions are not fully understood with some but significant exceptions which seem to point towards a feedback loop, dependent on neurotransmitter signaling (Figure 1), Spontaneous electrical activity is regulated by neurotransmitter signaling and its blockade perturbs the progression in neurotransmitter phenotype specification during spinal cord development (Root et al., 2008). Other developing systems are also sensitive to neurotransmitter signaling for the regulation of neurotransmitter phenotype expression; glutamate signaling regulates the appearance of the cholinergic phenotype in developing hypothalamic neurons (Belousov et al., 2001; Liu et al., 2008).
5. Neurotransmitter respecification in the mature nervous system
The developing nervous system is paradigmatic in its capacity to change, to transform itself and to rectify. Neurons are generated in high numbers and then trimmed down to the appropriate population size. Synapses are formed and then some are stabilized while others are eliminated. Expression of neurotransmitter receptor subunits is dynamically regulated conferring different synaptic properties to the developing neuronal networks (Borodinsky et al., 2012). Considering this rather generalized neurotransmitter respecification during development, the question is whether this phenomenon occurs in the mature nervous system as well. Indeed, adult rat hippocampal glutamatergic granule cells grown in vitro, can be induced to recapitulate the dual GABAergic/glutamatergic phenotype characteristic of the developing cells, by sustained enhancement of glutamatergic transmission or by BDNF (Gomez-Lira et al., 2005). The added GABAergic phenotype is also apparent in hippocampal slices of epileptic rats and in BDNF-treated slices from naive rats (Gomez-Lira et al., 2005). More recently, the evaluation of the coexistence of the glutamatergic and GABAergic phenotypes in the adult rat brain demonstrated that VGAT, the vesicular GABA transporter, is present in synaptosomes from hippocampal and cerebellar mossy fibers, typical glutamatergic and excitatory synapses. Reciprocally, VGLUT2, the vesicular glutamate transporter, is present in synaptosomes of cerebellar basket cell terminals, classical inhibitory and GABAergic terminals (Zander et al., 2010). The dual dopaminergic/glutamatergic phenotype in the embryonic nucleus accumbens that is more prominent at early developmental stages is enhanced postnatally (P15 rats) after neonatal (P4) injury with 6-hydroxydopamine cerebroventricular administration to lesion dopaminergic neurons (Dal Bo et al., 2008). The change in neurotransmitter specification continues in the adult (P90) and the acquired glutamatergic phenotype in dopaminergic neurons is mostly lost in rats injured at neonatal stages (Berube-Carriere et al., 2009).
6. Function of neurotransmitter respecification: Homeostasis of nervous system excitability
What are the roles of the changes in neurotransmitter specification? Recent studies have started to answer this question by experimentally interfering with neurotransmitter respecification and assessing the functional consequences (Figure 2). In conditional VGLUT2 knockout mice lacking the transporter only in dopaminergic neurons, stimulation of locomotor activity by cocaine or amphetamine is impaired (Birgner et al., 2010; Hnasko et al., 2010). This seems to be due to the enhancement of dopamine vesicular storage by glutamate, which is missing from the projections of ventral tegmental area neurons in the knockout animals (Hnasko et al., 2010). Remarkably, absence of VGLUT2 in dopamine neurons also results in what seems to be an opposite behavioral outcome compared to the lack of stimulated locomotor activity, an enhanced reward consumption and reward-associated memory (Alsio et al., 2011). This unexpected change in addictive-like behavior seems to be a result of a compensatory mechanism, an upregulation of dopamine receptors in the striatum of the conditional VGLUT2 knockout mice (Alsio et al., 2011).
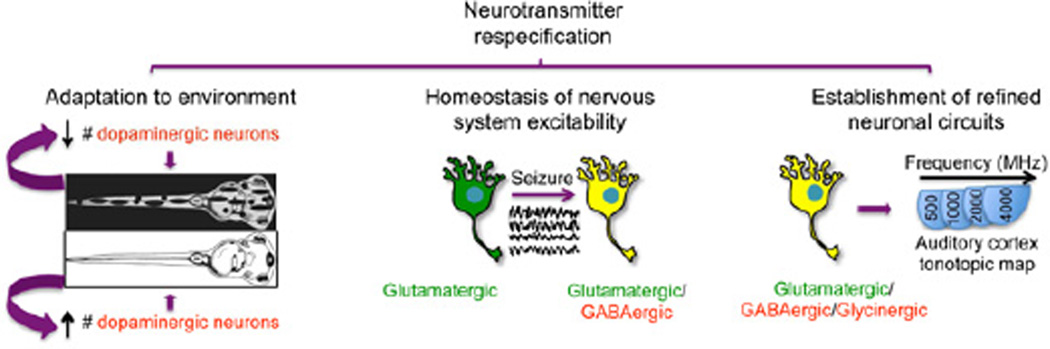
Figure 2. Roles of neurotransmitter respecification.
Activity-dependent changes in specification of the dopaminergic phenotype in the ventral suprachiasmatic nucleus underlie the camouflage behavior of Xenopus laevis larva (left (Dulcis and Spitzer, 2008)). Electrically- or convulsant-induced seizures in rats promote the expression of the GABAergic phenotype in glutamatergic pyramidal hippocampal neurons counteracting the imposed perturbation in the circuit activity (middle (Gomez-Lira et al., 2005)). The developmental coexpression of the glutamatergic phenotype in glycinergic/GABAergic neurons of the olive nucleus is necessary for the establishment of appropriate tonotopic maps in the auditory cortex (right (Noh et al., 2010)).
In the developing auditory system where the triple GABAergic, glycinergic and glutamatergic phenotype is transiently expressed, the elimination of the glutamatergic phenotype severely jeopardizes the refinement of the developing neuronal circuit (Noh et al., 2010). The developmental strengthening that occurs in wild type synapses between the medial nucleus trapezoid body and the lateral superior olive is reduced in VGLUT3 knockout mice. On the other hand, synapse elimination between neurons of these two structures that is also needed to fine-tune the developing circuit is also impaired in these mutant animals. This defective progression of changes in synaptic function at the onset of hearing leads to a deficient and perturbed tonotopic organization (Noh et al., 2010). In addition, the elimination of the glutamatergic phenotype from these GABAergic/glycinergic developing connections induces an imbalance among all the inputs that contribute to the excitation/inhibition status of the lateral superior olive (Noh et al., 2010).
The balance in the excitability of the developing nervous system appears to be a common denominator in the regulation of the neurotransmitter phenotype. Changes in illumination in the Xenopus laevis larva that lead to changes in activation of the retinohypothalamic projections induce changes in the number of dopaminergic neurons (Dulcis and Spitzer, 2008). The changes follow a homeostatic paradigm, when activity is enhanced, more dopaminergic inhibitory neurons in the ventral suprachiasmatic nucleus are specified inhibiting melanotrope cells and inducing a lighter skin and the background camouflage behavior (Dulcis and Spitzer, 2008). The specification of the serotonergic phenotype of the Xenopus hindbrain is also dependent on levels of spontaneous activity and has behavioral consequences (Demarque and Spitzer, 2010). When activity is enhanced by overexpression of voltage-gated Na+ channels, the number of serotonergic neurons in the hindbrain decreases and leads to an increase in the duration of episodes of the fictive swimming. In contrast, suppressing activity by overexpression of an inward rectifier K+ channel leads to an increase in the number of serotonergic neurons and a decrease in the fictive swimming episode duration (Demarque and Spitzer, 2010). This homeostatic regulation of neurotransmitter specification is also apparent in the developing spinal cord (Belgacem and Borodinsky, 2011; Borodinsky et al., 2004) and is accompanied by correlated changes in neurotransmitter receptor expression in target cells (Borodinsky and Spitzer, 2007).
7. Concluding remarks
A vast number of studies, some of which were presented herein, support and generalize the phenomenon of a dynamic neurotransmitter phenotype specification in the developing and mature nervous system. Neurotransmitter expression can be transient and is regulated by developmental and environmental cues. These changes in neurotransmitter expression have significant consequences and need to be incorporated in the repertoire of mechanisms underlying plasticity and compensation to the changing environment. Achieving homeostasis of excitability in the nervous system is a challenging endeavor. It is likely that a different combination of mechanisms operate under different circumstances to ensure balance. Modifications in neurotransmitter specification appear to be critical for the establishment of appropriate neuronal circuits during development and hence play a pivotal role in nervous system function.
Highlights.
Neurotransmitter specification is dynamic in the developing and adult nervous system
Changes in neurotransmitter phenotype expression follow a homeostatic paradigm
Neurotransmitter respecification is crucial for the establishment of refined circuits
Plasticity of neurotransmitter phenotype expression underlies adaptive behaviors
Acknowledgements
I.S., Y.H.B. were supported and E.B.S. is supported by the Shriners Hospital for Children Postdoctoral Fellowship. The research in the lab is supported by the Klingenstein Foundation Award in Neuroscience 2008, Basil O’Connor Award #5-FY09-131, March of Dimes, NSF 1120796, NIH-NINDS R01NS073055 and SHC 86500-NCA grants to L.N.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alsio J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LE, Kullander K, Levesque D, Wallen-Mackenzie A. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista MF, Lewis KE. Pax2/8 act redundantly to specify glycinergic and GABAergic fates of multiple spinal interneurons. Dev Biol. 2008;323:88–97. doi: 10.1016/j.ydbio.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16:1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci U S A. 2011;108:4482–4487. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belousov AB, O'Hara BF, Denisova JV. Acetylcholine becomes the major excitatory neurotransmitter in the hypothalamus in vitro in the absence of glutamate excitation. J Neurosci. 2001;21:2015–2027. doi: 10.1523/JNEUROSCI.21-06-02015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube-Carriere N, Riad M, Dal Bo G, Levesque D, Trudeau LE, Descarries L. The dual dopamine-glutamate phenotype of growing mesencephalic neurons regresses in mature rat brain. J Comp Neurol. 2009;517:873–891. doi: 10.1002/cne.22194. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Greves M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallen-Mackenzie A. VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 2010;107:389–394. doi: 10.1073/pnas.0910986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Belgacem YH, Swapna I. Electrical activity as a developmental regulator in the formation of spinal cord circuits. Curr Opin Neurobiol. 2012;22:624–630. doi: 10.1016/j.conb.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci U S A. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- Dal Bo G, Berube-Carriere N, Mendez JA, Leo D, Riad M, Descarries L, Levesque D, Trudeau LE. Enhanced glutamatergic phenotype of mesencephalic dopamine neurons after neonatal 6-hydroxydopamine lesion. Neuroscience. 2008;156:59–70. doi: 10.1016/j.neuroscience.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Demarque M, Spitzer NC. Activity-dependent expression of Lmx1b regulates specification of serotonergic neurons modulating swimming behavior. Neuron. 2010;67:321–334. doi: 10.1016/j.neuron.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behaviour. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman ML, Borodinsky LN, Neale JH. GABA induces proliferation of immature cerebellar granule cells grown in vitro. Brain Res Dev Brain Res. 1999;115:1–8. doi: 10.1016/s0165-3806(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci. 2012;29:61–71. doi: 10.1017/S0952523811000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C, Vinatier J, Amilhon B, Guerci A, Christov C, Ravassard P, Giros B, El Mestikawy S. Developmentally regulated expression of VGLUT3 during early post-natal life. Neuropharmacology. 2005;49:901–911. doi: 10.1016/j.neuropharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. Co-existence and co-release of classical neurotransmitters : ex uno plures. New York, NY: Springer; 2009. [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the "glutamatergic" granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habecker BA, Tresser SJ, Rao MS, Landis SC. Production of sweat gland cholinergic differentiation factor depends on innervation. Dev Biol. 1995;167:307–316. doi: 10.1006/dbio.1995.1025. [DOI] [PubMed] [Google Scholar]
- Higashijima S, Mandel G, Fetcho JR. Distribution of prospective glutamatergic, glycinergic, and GABAergic neurons in embryonic and larval zebrafish. J Comp Neurol. 2004;480:1–18. doi: 10.1002/cne.20278. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, Palmiter RD, Rayport S, Edwards RH. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260:95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC, Keefe D. Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as morphogens. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- Li H, de Faria JP, Andrew P, Nitarska J, Richardson WD. Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron. 2011;69:918–929. doi: 10.1016/j.neuron.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Hinterneder JM, Rollor SR, Birren SJ. Non-cell-autonomous regulation of GABAergic neuron development by neurotrophins and the p75 receptor. J Neurosci. 2007;27:12787–12796. doi: 10.1523/JNEUROSCI.3302-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Popescu IR, Denisova JV, Neve RL, Corriveau RA, Belousov AB. Regulation of cholinergic phenotype in developing neurons. J Neurophysiol. 2008;99:2443–2455. doi: 10.1152/jn.00762.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Loy B, Apostolova G, Dorn R, McGuire VA, Arthur JS, Dechant G. p38alpha and p38beta mitogen-activated protein kinases determine cholinergic transdifferentiation of sympathetic neurons. J Neurosci. 2011;31:12059–12067. doi: 10.1523/JNEUROSCI.0448-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek KW, Kurtz LM, Spitzer NC. cJun integrates calcium activity and tlx3 expression to regulate neurotransmitter specification. Nat Neurosci. 2010;13:944–950. doi: 10.1038/nn.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Liao M, Drapeau P. Glycine receptors regulate interneuron differentiation during spinal network development. Proc Natl Acad Sci U S A. 2006;103:9679–9684. doi: 10.1073/pnas.0504871103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci. 2004;7:17–23. doi: 10.1038/nn1170. [DOI] [PubMed] [Google Scholar]
- Noh J, Seal RP, Garver JA, Edwards RH, Kandler K. Glutamate co-release at GABA/glycinergic synapses is crucial for the refinement of an inhibitory map. Nat Neurosci. 2010;13:232–238. doi: 10.1038/nn.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58:865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SJ, Messenger NJ, Warner AE. The role of noradrenaline in the differentiation of amphibian embryonic neurons. Development. 1993;119:1343–1357. doi: 10.1242/dev.119.4.1343. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scain AL, Le Corronc H, Allain AE, Muller E, Rigo JM, Meyrand P, Branchereau P, Legendre P. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci. 2010;30:390–403. doi: 10.1523/JNEUROSCI.2115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra UB, Sulik KK, Petrusz P, Lauder JM. Ontogeny of cholinergic neurons in the mouse forebrain. J Comp Neurol. 1989;288:101–122. doi: 10.1002/cne.902880109. [DOI] [PubMed] [Google Scholar]
- Schotzinger RJ, Landis SC. Cholinergic phenotype developed by noradrenergic sympathetic neurons after innervation of a novel cholinergic target in vivo. Nature. 1988;335:637–639. doi: 10.1038/335637a0. [DOI] [PubMed] [Google Scholar]
- Schotzinger RJ, Landis SC. Acquisition of cholinergic and peptidergic properties by sympathetic innervation of rat sweat glands requires interaction with normal target. Neuron. 1990;5:91–100. doi: 10.1016/0896-6273(90)90037-g. [DOI] [PubMed] [Google Scholar]
- Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- Swapna I, Borodinsky LN. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proc Natl Acad Sci U S A. 2012;109:16336–16341. doi: 10.1073/pnas.1202818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Wallace JA. Monoamines in the early chick embryo: demonstration of serotonin synthesis and the regional distribution of serotonin-concentrating cells during morphogenesis. Am J Anat. 1982;165:261–276. doi: 10.1002/aja.1001650304. [DOI] [PubMed] [Google Scholar]
- Waschek JA, Casillas RA, Nguyen TB, DiCicco-Bloom EM, Carpenter EM, Rodriguez WI. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci U S A. 1998;95:9602–9607. doi: 10.1073/pnas.95.16.9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]
- Zander JF, Munster-Wandowski A, Brunk I, Pahner I, Gomez-Lira G, Heinemann U, Gutierrez R, Laube G, Ahnert-Hilger G. Synaptic and vesicular coexistence of VGLUT and VGAT in selected excitatory and inhibitory synapses. J Neurosci. 2010;30:7634–7645. doi: 10.1523/JNEUROSCI.0141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]