Abstract
Chronic activation of the NF-κB pathway is associated with progressive neurodegeneration in Parkinson’s disease (PD). Given the role of neuronal RING finger protein 11 (RNF11) as a negative regulator of the NF-κB pathway, in this report we investigated the function of RNF11 in dopaminergic cells in PD-associated neurodegeneration. We found that RNF11 knock-down in an in vitro model of PD mediated protection against 6-OHDA-induced toxicity. In converse, over-expression of RNF11 enhanced 6-OHDA-induced dopaminergic cell death. Furthermore, by directly manipulating NF-κB signaling, we showed that the observed RNF11-enhanced 6-OHDA toxicity is mediated through inhibition of NF-κB-dependent transcription of TNF-α, antioxidants GSS and SOD1, and anti-apoptotic factor BCL2. Experiments in an in vivo 6-OHDA rat model of PD recapitulated the in vitro results. In vivo targeted RNF11 over-expression in nigral neurons enhanced 6-OHDA toxicity, as evident by increased amphetamine-induced rotations and loss of nigral dopaminergic neurons as compared to controls. This enhanced toxicity was coupled with down-regulation of NF-κB transcribed GSS, SOD1, BCL2, and neurotrophic factor BDNF mRNA levels, in addition to decreased TNF-α mRNA levels in ventral mesenchephalon samples. In converse, knockdown of RNF11 was associated with protective phenotypes and increased expression of above-mentioned NF-κB transcribed genes. Collectively, our in vitro and in vivo data suggest that RNF11-mediated inhibition of NF-κB in dopaminergic cells exaggerates 6-OHDA toxicity by inhibiting neuroprotective responses while loss of RNF11 inhibition on NF-κB activity promotes neuronal survival. The decreased expression of RNF11 in surviving cortical and nigral tissue detected in PD patients, thus implies a compensatory response in the diseased brain to PD-associated insults. In summary, our findings demonstrate that RNF11 in neurons can modulate susceptibility to 6-OHDA toxicity through NF-κB mediated responses. This neuron-specific role of RNF11 in the brain has important implications for targeted therapeutics aimed at preventing neurodegeneration.
Keywords: Parkinson’s disease, NF-κB, E3 ligase, 6-hydroxydopamine, 6-OHDA, neurodegeneration, human tissue, AAV, antioxidants
Introduction
Parkinson’s disease (PD) is a progressive neurological disorder with motor abnormalities as the cardinal clinical symptom (Coelho and Ferreira, 2012; Fahn, 2003). A pathological hallmark of PD is degeneration of dopaminergic neurons in the substantia nigra (SN) (Crossman, 1989), however the underlying disease-causing mechanisms remain to be elucidated. Recently, human clinical imaging, epidemiological studies and genetic associations have highlighted and confirmed a role for neuroinflammation in PD (Chen et al., 2005; Frank-Cannon et al., 2009; Gerhard et al., 2006; Hald and Lotharius, 2005; Hamza et al., 2010; Hirsch et al., 2005; Ton et al., 2006) as well as raise the possibility that chronic inflammatory responses may promote progressive degeneration of dopaminergic neurons.
Inflammatory responses in the central nervous system are primarily mediated by microglia through activation of NF-κB, a signaling pathway that elevates levels of inflammatory cytokines (ie., TNF-α), apoptotic factors and oxidative stress. Interestingly, NF-κB, a transcription factor, is expressed in neurons and glia (O'Neill and Kaltschmidt, 1997) and has divergent roles both as a promoter and inhibitor of neurodegeneration (Panet et al., 2001; Tansey and Goldberg, 2010). NF-κB is activated in response to various toxic stimulations and persistent NF-κB activation has been associated with progressive neurodegeneration in PD (Glass et al., 2010; Hunot et al., 1997; Mogi et al., 2007). To maintain cellular homeostasis, activation of NF-κB pathway is tightly controlled with several layers of regulation (Ruland, 2011). One such regulator is RING finger protein 11 or RNF11 (Pranski et al., 2012a; Shembade et al., 2009).
RNF11 acts as a negative regulator of NF-κB signaling pathway through its associations with the A20 ubiquitin-editing protein complex. This complex is comprised of A20, an NF-κB transcribed gene (Krikos et al., 1992); Tax1 (human T-cell leukemia virus type I) binding protein 1 (TAX1BP1), a regulator of A20; and Itch, an E3 ligase (Shembade et al., 2007; Shembade et al., 2008; Shembade et al., 2009). RNF11 is predominantly expressed in neurons (Anderson et al., 2007) and is essential for regulation of neuronal NF-κB signaling (Pranski et al., 2012a). Given that RNF11 mRNA is reduced in PD SN (Noureddine et al., 2005) and activated NF-κB has been observed in dopaminergic nigral neurons in PD tissue (Ghosh et al., 2007; Hunot et al., 1997), we hypothesized that RNF11 in dopaminergic neurons may modulate NF-κB signaling and possibly alter cell survival. This hypothesis was tested by genetic manipulation of RNF11 expression in both cellular and rat 6-hydroxydopamine (6-OHDA) models of PD. Our results suggest that loss of RNF11-mediated inhibition of NF-κB signaling in dopaminergic cells is protective against 6-OHDA toxicity through NF-κB transcribed cytokines, antioxidants and anti-apoptotic factors. This report highlights the importance of neuronal RNF11 as a negative modulator of NF-κB responses with major implications for targeted therapeutics in PD.
Material and methods
Cell culture
PC12 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were cultured in Dulbecco's modified Eagle medium (Mediatech, Manassas, VA, USA), 10% heat-inactivated horse serum (Gibco, Invitrogen, Grand Island, NY, USA), 5% fetal clone serum (Hyclone Laboratories, Logan, UT, USA), and 1% penicillin/streptomycin. Primary cortical neurons were prepared from wild-type C57BL/6 mice at embryonic day 18 as previously described (Davis et al., 2010). Cells were dissociated by trituration through a Pasteur pipette and plated on 0.25 mg/ml poly-L-lysine-coated dishes in neurobasal medium (Invitrogen, Carlsbad, CA, USA) containing B-27 supplement (Invitrogen), 2 mM L-glutamine and 1% penicillin/streptomycin. Cytosine arabinoside was added at a final concentration of 5 µM on day 3 in vitro to control proliferation of non-neuronal cells. After 8 days in vitro, neuron-enriched cultures were used for experiments. All cultures were maintained at 37°C in 5% CO2.
Antibodies and reagents
Antibodies used were: GFAP (MAB360, Millipore), GFP (Rockland, Gilbertsville, PA, USA), Iba1 (ab5076, Abcam), Itch (611198; BD Transduction Laboratories, San Diego, CA, USA), MAP2 (M061755, BD Biosciences, San Diego, CA, USA), Olig2 (MABN50, Millipore), phospho-p65 (3036S; Cell Signaling Technology, Beverly, MA, USA), p65 (C22B4; Cell Signaling Technology), rabbit polyclonal RNF11 (described in (Anderson et al., 2007)), TAX1BP1 (ab22049, Abcam), Traf6 (ab13853, Abcam), tyrosine hydroxylase (TH, Sigma-Aldrich, St. Louis, MO, USA), and V5 (MCA1360; AbD Serotec, Oxford, UK). Appropriate biotin or fluorescent conjugated secondary antibodies were purchased from Vector (Burlingame, CA, USA), Invitrogen (Carlsbad, CA, USA), Jackson ImmunoResearch Laboratories (West Grove, PA, USA), and ThermoFisher Scientific (Waltham, MA, USA). The following reagents were utilized: 6-OHDA (Sigma-Aldrich), Bay 11-7085 (Santa Cruz Biotech, Santa Cruz, CA, USA), D-amphetamine (Sigma-Aldrich), and recombinant TNF-α (R&D Systems, Minneapolis, MN, USA).
Plasmids and transfections
Human RNF11 cDNA was originally subcloned into pcDNA3.1(+) (Invitrogen) using Kpn1and Not1 restriction sites as described previously (Anderson et al., 2007). Wild-type RNF11 was cut out of pcDNA and into pFUGW with BamHI and Asc1. A V5 sequence was added to the N-terminus of the RNF11 sequence and was PCR-amplified into the plasmid. V5-RNF11, shScramble, and shRNF11 were subcloned into the pAAV vector using Age1 and Kpn1 sites. The NF-κB Luciferase vector (pGL4.32[luc2P/NF-KB/Hygro]) and internal control Renilla vector (pGL4.74[hRluc/TK]) vector were purchased from Promega (Madison, WI, USA) as described previously (Pranski et al., 2012a). The NF- κB Luciferase vector contains a (GGGAATTTCC)5 NF-κB response element protein promoter. Transient transfections of PC12 cells were performed using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s protocol.
RNA interference and virus production
Adeno-associated viruses (AAV, serotype 2) for V5-RNF11, shRNF11, and shScramble constructs were produced by the Emory University Viral Vector Core facility.
Luciferase reporter assays
As described previously (Pranski et al., 2012a), PC12 cells were transfected with the luciferase and renilla plasmids. All stimulations of cells were preceded by a 1 hour serum starvation period. Cells were stimulated for 6 hours before cell lysates were prepared in 1X Passive Lysis Buffer (Promega). Luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. Firefly luciferase values were normalized according to Renilla luciferase values. Luciferase results are presented as fold change relative to the untreated control sample. Control samples with luciferase, Renilla luciferase, or vector transfection were performed to confirm specificity of luminescence.
Cell death assays
Cell viability was measured using the CellTiter 96 Non-radioactive Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. Absorbance was measured at 570 nM with a reference wavelength at 650 nM. After sample absorbance was corrected for background absorbance, cell death values were calculated as the percentage of control absorbance.
Extraction of RNA from cells
Total RNA was isolated from cells using a standard TRIzol reagent (Invitrogen) extraction protocol. RNA was converted to cDNA using a High Capacity cDNA Reverse Transcription Kit (Ambion, Austin, TX, USA).
Quantitative real-time PCR (qRT-PCR)
Real-time PCR was performed with a 7500 Fast real time-PCR System (Applied Biosystems, Foster City, CA, USA) using 20ng/µl cDNA, TaqMan Universal PCR Master Mix II (Applied Biosystems), and gene-specific TaqMan probes (Applied Biosystems). The following primers were used: human A20 (Hs00234712_m1), rat BCL2 (Rn00586772_m1), rat BDNF (Rn02531967_s1), rat CD45 (Rn00709901_m1), rodent GAPDH (ABI 4308313), rat GSS (Rn00564188_m1), human GUSB (Hs99999908_m1), rat NF-κB (Rn01399583_m1), mouse RNF11 (Mn00450014_m1), rat RNF11 (Rn01417031_m1), human RNF11 (Hs00702517_s1), rat SOD1 (Rn00566938_m1), rat TH (Rn00562500_m1), and rat TNF-α (Rn99999017_m1). For each RNA sample, each primer set was run in triplicate. For rat tissue and cell culture data, results were normalized to the housekeeping gene GAPDH and evaluated by the 2ΔΔCt method. RNA levels are expressed relative to the naive animal. For human tissue, results were normalized to the housekeeping gene GUSB and evaluated by comparative cycle threshold method.
Immunocytochemistry
Primary cortical neurons were grown on coverslips coated with poly-L-lysine (Sigma-Aldrich). After being manipulated, cells were fixed with 2% paraformaldehyde and immunostained as described previously (Anderson et al., 2007) with an antibody against p65. Cell nuclei were stained with Hoechst 333258 (Molecular Probes/Invitrogen). The fluorophore-conjugated secondary antibody used was goat anti-mouse Rhodamine Red-X (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). For p65 analysis, coverslips were imaged at 40× magnification using an Olympus BX51 Fluorescence Microscope (Olympus America, Inc, Melville, NY, USA), and co-localization of p65 and 4′,6-diamidino-2-phenylindole in GFP-positive cells was quantified as described previously (Volpicelli et al., 2001). For each condition, at least 50 cells were quantified. The percentage of p65-positive pixels above threshold intensity which overlapped with Hoechst staining was calculated for each cell. An area near the perimeter of the cell was used as background. For each experiment, the fold change in percentage of p65-positive pixels was normalized to unstimulated cells.
Animal studies
Young male adult Sprague Dawley rats (approximately 250 g) were purchased from Charles River Laboratories (Wilmington, MA, USA) and housed in pathogen-free climate-controlled facilities at the Animal Resources Center at Emory University. All animal studies were approved by the Institutional Animal Care and Use Committee at Emory University.
Stereotaxic injections
Sprague Dawley rats (n=20 per group) were anesthetized with 80 mg/kg ketamine and 8 mg/kg xylazene and placed in stereotaxic frame. Their eyes were covered in ophthalmic ointment. The scalp was sterilized before the skull was exposed and incised. We followed a previously published protocol to target the AAV to the nigra (Bartus et al., 2011). A unilateral injection of AAV2 (2 µl of 1.6 × 109 vg/µl) was given at a rate of 1 µl/2.5 min into the nigra of the right hemisphere (anteroposterior (AP), −5.3 mm from bregma; mediolateral (ML), +2.3 mm from midline; and dorsoventral (DV), −7.2 mm from below the surface of dura). The intrastriatal 6-OHDA injection was performed 2 weeks after intranigral AAV2 injection. We followed a previously published protocol with a decreased concentration of 6-OHDA to induce a moderate retrograde lesion in the nigrostriatal pathway (Kirik et al., 1998). A unilateral injection of 10 µg of 6-OHDA (2 µl of 5 µg/µl) was given at a rate of 1 µl/2.5 min into the striatum of the right hemisphere (AP, −1.2 mm from bregma; ML, +3.4 mm from midline; and DV, −5.0 mm from below the surface of dura).
Rotational behavior analysis
At 3 weeks after 6-OHDA lesion, amphetamine-induced rotational behavior was monitored as published previously (Kirik et al., 1998). Animals received 5 mg/kg D-amphetamine intraperitoneally. One hour after injection animals were monitored for rotation behavior for 10 minutes. Rotational asymmetry was scored as a complete rotation toward the lesion (ipsilateral). The net rotational asymmetry was expressed as rotations/minute.
Microdissection and harvest of tissue for qRT-PCR
At 4 weeks after 6-OHDA lesion, animals were deeply anesthetized with isofluorane and decapitated. The brain was rapidly harvested and the ventral mesencephalic region was microdissected from both hemispheres on an ice-cold Petri dish. Samples were flash-frozen in liquid nitrogen and stored at −80°C until processed for RNA extraction.
Perfusion and tissue processing for histology
At 4 weeks after 6-OHDA lesion, animals were deeply anesthetized with isofluorane and intracardially perfused with 4% paraformaldehyde in PBS, pH 7.4. Brains were post-fixed for 48 hours in the same paraformaldehyde solution and cryoprotected in 10% sucrose for 24 hours and 30% sucrose for 24 hours. Serial coronal sections (40 µm thickness) through the entire striatum and substantia nigra were collected and stored in anti-freeze solution for further analysis.
Brightfield immunohistochemistry
50 µm thick coronal sections were stained as previously described (Betarbet et al., 2000). Briefly, sections were blocked with 10% normal goat serum and 10% Triton-X in TBS. Sections were incubated with primary antibodies for 72 hours at 4 deg C followed by appropriate biotinylated secondary antibody. The avidin-biotin complex method was used to detect the antigen signal (ABC elite kit, Vector). Peroxidase activity was developed using Sigma FAST 3,3`-diaminobenzadine. Tissue sections were dehydrated in a graded series of ethanols, immersed in histoclear, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA, USA). Primary antibody was omitted for negative control reactions. Immunostained sections were examined using an Olympus BX51 Fluorescence Microscope (Olympus America, Inc., Melville, NY, USA) and images were processed using Adobe Photoshop 7.0 software (San Jose, CA, USA).
Fluorescence immunohistochemistry of mammalian tissue
Brain sections were stained using a standard immunofluorescence protocol. Briefly, sections were blocked with 10% normal horse serum and 10% Triton-X in TBS. Sections were incubated with a combination of primary antibodies overnight at 4°C followed by appropriate fluorescent conjugated secondary antibodies. Sections were incubated with Hoechst 333258 to counter stain nuclei. Primary antibodies were omitted as a negative control. Images were captured using a Zeiss LSM 510 laser scanning confocal microscope. For final output, images were processed using Adobe Photoshop 7.0 software.
Quantification of double-transduced cells in rat tissue
After staining sections for TH and GFP, the percentage of double-transduced cells was determined by fluorescence microscopy on five coronal sections of three animals. For each animal, at least 350 cells were counted. Values are given as mean percentage of the three animals ± standard deviation.
Quantification of colocalization coefficients in rat tissue
After staining sections for the appropriate antibodies, the degree of colocalization was determined by calculating the overlap coefficient according to Manders in single confocal images using the “colocalization” feature of Imaris 7.6.0, 64-bit version (Bitplane AG) as described in (Mazzon et al., 2012). Automated thresholding to correct the background was used. For each condition, three to five coronal sections of three animals were analyzed. Values are reported as mean Mander’s coefficient for three animals ± standard deviation.
Stereological analysis of nigral dopaminergic neurons
The optical fractionator probe of Stereoinvestigator software (MicroBrightField, Version 10.04, MBF Bioscience, Williston, VA, USA) was used to obtain an unbiased estimate of TH-immunoreactive neurons in the SN pars compacta (SNpc) as described previously (Hutson et al., 2011). Every 4th nigral sections were processed for TH immunohistochemistry using brightfield immunohistochemistry. The boundary of the SNpc was defined according to previous demarcations in the rat (German and Manaye, 1993). Cells were counted blinded with a 40× objective using a Zeiss Imager.M2 microscope (Thornwood, CO, USA) through the extent of the SNpc. Stereologic parameters were as follows: counting frame, 50×50 µm; optical dissector, 18 µm; grid size, 140×140 µm. Every fourth section was stained for TH and yielded a target coefficient of error (Gundersen's m = 1) of < 0.10. A dopaminergic neuron was defined as a TH-immunoreactive cell body with a clearly visible unstained nucleus.
Extraction of RNA from tissue
Total RNA was isolated from approximately 100 mg of human or rodent brain tissue that was homogenized and sonicated before using an RNeasy Lipid Tissue kit (Qiagen, Valencia, CA, USA). cDNA was created using a High Capacity cDNA reverse transcription kit (Ambion).
Human tissue
Human brain tissues used for qRT- PCR analysis were derived from 46 autopsy brains, which were pathologically evaluated for Alzheimer’s disease (AD) and PD, from the Emory University Brain Bank (Atlanta, GA, USA). The neuropathologic diagnosis of definite AD was made according to criteria of the Consortium to Establish a Registry for Alzheimer's Disease (Mirra et al., 1991). The neuropathologic diagnosis of PD was based on the presence of nigral degeneration and Lewy bodies. Control cases had no clinical history or neuropathologic diagnosis of neurologic disease. Four groups were compared: 14 subjects aged 62 to 79 years (mean = 71) with clinically and pathologically confirmed PD, 17 subjects aged 58 to 84 years (mean = 70) with clinically and pathologically confirmed AD, 8 subjects aged 67 to 79 years (mean = 73) with a clinical diagnosis of dementia with Lewy bodies and neuropathologic findings of AD with concomitant Lewy pathology, and 17 control subjects aged 52 to 92 years (mean = 71). Human brain tissue used for immunohistochemical analysis of diseased tissue was derived from a subset of these cases with available paraformaldehyde fixed tissue. 50 µm thick coronal sections, through the frontal cortex from control and PD cases, were stained for Itch, RNF11, and TAX1BP1 using brightfield immunohistochemistry as previously described (Betarbet et al., 2000).
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 4.03 software (GraphPad Software, Inc, La Jolla, CA, USA). Statistical significance was set at P < 0.05. For mRNA analysis of human case material, primary neurons, and rat tissue, rotational analysis, and stereology, differences among means were analyzed using one-way analysis of variance (ANOVA). When ANOVA showed significant differences, comparisons between means were tested by either Dunnett’s multiple comparison or Bonferroni selected multiple comparison post hoc tests. Differences among means were analyzed using two-way ANOVA for p65 analysis, PC12 cell mRNA analysis, and cell death assays. When ANOVA showed significant differences, comparisons between means were tested by either Dunnett’s multiple comparison or Bonferroni selected multiple comparison post hoc tests. All experimental results are presented as means ± SEM for at least three independent experiments. * P < 0.05; ** P < 0.01, *** P < 0.001 unless otherwise noted.
Results
Previously we established the role of neuronal RNF11 as a component of the A20 ubiquitin-editing complex in regulating TNF-α-induced canonical NF-κB activity in human neuroblastoma cells and primary neuronal cultures (Pranski et al., 2012a). Here we investigated the functional role of RNF11 in PD pathogenesis. In PD patients, NF-κB activation has been reported in dopaminergic cells of the SN (Ghosh et al., 2007; Hunot et al., 1997), thus we examined the effects of manipulation of RNF11 in nigral dopaminergic cells in in vitro and in vivo 6-OHDA models of PD.
In vitro knockdown of RNF11 in dopaminergic cells imparts neuroprotection against 6-OHDA-induced toxicity
PC12 cells were used for the in vitro system since these cells have a dopaminergic phenotype and are responsive to 6-OHDA (Elkon et al., 2004; Zhu et al., 2012). We transfected PC12 cells with the following pAAV plasmids: shRNF11 that directs the expression of small hairpin RNA (shRNA) targeted against RNF11, shScramble that directs the expression of a scrambled version of shRNF11, and V5-RNF11 that directs the over-expression of V5-tagged RNF11. We monitored cell death after 18 hours of 6-OHDA exposure as described previously (Zhu et al., 2012). In response to 6-OHDA exposure, we observed a dose-dependent cell death response in each of the cell lines (Fig. 1A). We observed increased susceptibility to cell death in pAAV-V5-RNF11-transfected cells and decreased susceptibility in pAAV-shRNF11-transfected cells when compared to control pAAV vector or pAAV-shScramble-transfected cells, implicating RNF11 as a modulator of 6-OHDA toxicity. 6-OHDA has been previously reported to induce NF-κB activation (Tarabin and Schwaninger, 2004) and RNF11 is a known regulator of NF-κB signaling (Pranski et al., 2012a). Transcriptional activation of NF-κB signaling for both 6-OHDA and RNF11 were confirmed through luciferase reporter assays (SI Fig. 1A). To measure activity of NF-κB signaling in PC12 cells, cells were transiently co-transfected with plasmids for control empty pAAV, pAAV-shScramble, pAAV-shRNF11, and pAAV-V5-RNF11, and plasmids containing an NF-κB response element driving expression of firefly luciferase or the T7 promoter driving expression of Renilla luciferase. Cells were exposed to 0 or 100 µM of 6-OHDA for 6 hours. NF-κB-dependent firefly luciferase activity levels were normalized to control Renilla luciferase activity, and the level of NF-κB activity was calculated as the fold change between stimulated and unstimulated samples. Stimulation with 6-OHDA caused a 11.1-fold increase in NF-κB activity in the pAAV-shRNF11cells, which was significantly different from the observed 2.8-fold increase in NF-κB activity in control cells (p < 0.001) and pAAV-shScramble cells (p < 0.001) (SI Fig. 1A). No difference was observed between pAAV-shScramble cells and control cells. These results indicate that reduced expression of RNF11 increases NF-κB activation induced by 6-OHDA in PC12 cells.
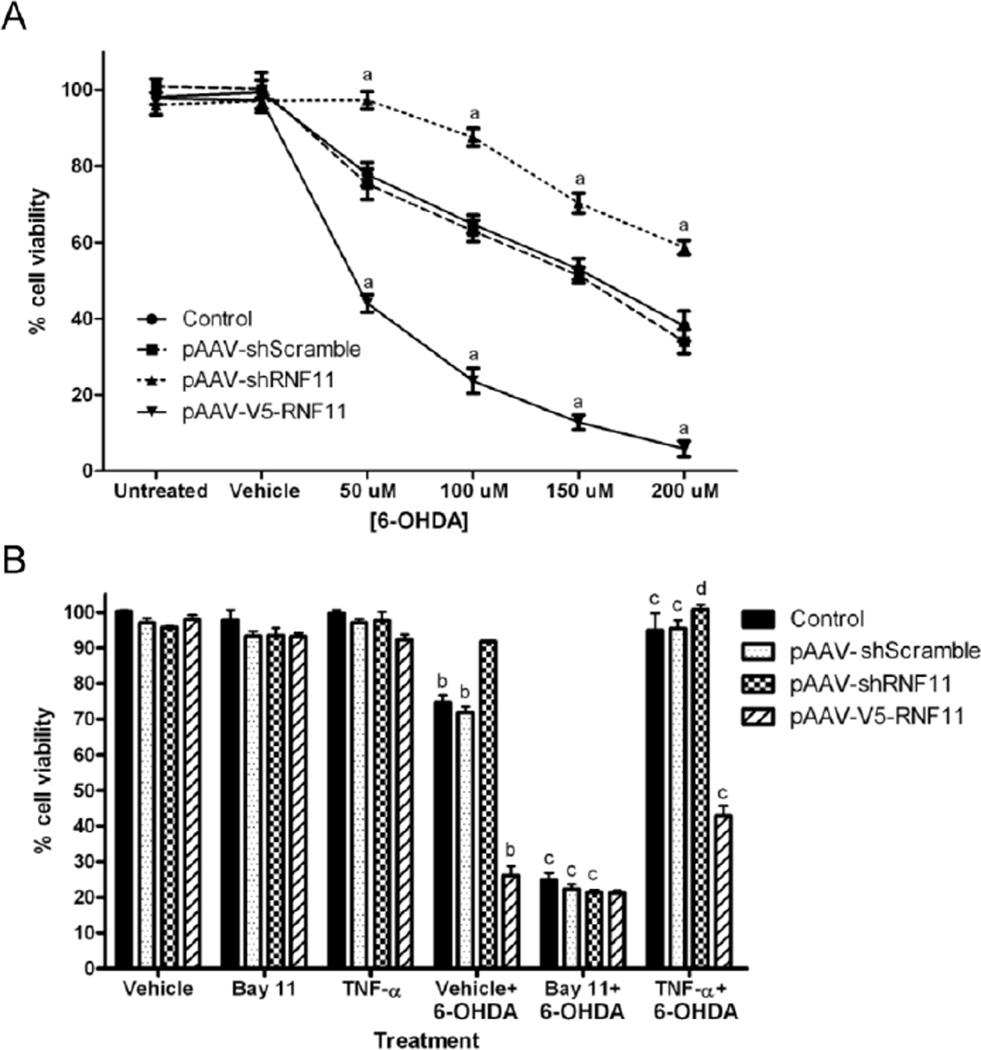
Figure 1. In vitro knockdown of RNF11 in dopaminergic cells imparts neuroprotection against 6-OHDA-induced toxicity.
A. PC12 cells were transfected with empty pAAV vector (control), shScramble, shRNF11, or V5-RNF11 pAAV constructs and exposed to media (untreated), saline (vehicle), or 50–200 µM 6-OHDA for 18 hours before measuring cell viability. B. PC12 cells were transfected with empty pAAV vector (control), shScramble, shRNF11, or V5-RNF11 pAAV constructs and exposed to saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 18 hours before measuring cell viability. Values expressed are mean percentage of viable cells using untreated control cells as the standard. Results were analyzed by two-way ANOVA with Bonferroni post-tests. a, P < 0.001 compared to control cells at the same dosage of 6-OHDA, b, P < 0.001 compared to vehicle treated cells with the same transfected plasmid, c, P < 0.001 compared to vehicle and 6-OHDA treated cells with the same transfected plasmid, d, P < 0.01 compared to vehicle and 6-OHDA treated cells with the same transfected plasmid.
The induction of activation of NF-d expression in this system was also confirmed through an orthogonal technique (Pranski et al., 2012a) using immunocytochemistry to examine the translocation of an NF-κB transcription factor, p65, into the nucleus as a marker of activation of NF-κB signaling (SI Fig. 1B). Activation of TNF receptors and associated complex association by canonical NF-κB signaling releases p65 and other transcription factors from the IκB kinase (IKK) complex and allows them to translocate to the nucleus and drive NF-κB-dependent transcription (Liu and Chen, 2011). We observed increased co-localization of p65 with Hoechst 333258 (a nuclear stain) at 30 minutes after 6-OHDA stimulation in all transfection conditions. Furthermore, we observed a persistent increased co-localization of Hoechst 333258 with p65 in pAAV-shRNF11 transfected cells in comparison to pAAV-shScramble transfected cells at 120 minutes (pAAV-shScramble: 0.92-fold change, pAAV-shRNF11: 1.83-fold change) (P < 0.001), which was not observed with pAAV-V5-RNF11 transfected cells (pAAV-V5-RNF11: 1.07-fold change). Therefore, we hypothesized that RNF11-mediated NF-κB activation was responsible for the observed differences in 6-OHDA-induced cell death. To test this idea, we pretreated cells with an NF-κB activator or inhibitor prior to exposure with 6-OHDA and measured cell death and alterations in mRNA expression of NF-κB and its target genes. To inhibit NF-κB signaling, we used Bay 11-7085, a molecule that blocks the phosphorylation of IκB and the subsequent dissolvement of the complex rendering NF-κB transcription factors inactive (Pierce et al., 1997). The ability of Bay 11-7085 to ablate NF-κB signaling was confirmed by luciferase reporter assays (SI Fig. 1A) and by examining the translocation of p65 into the nucleus (SI Fig. 1C). PC12 cells were transiently co-transfected with constructs for control empty pAAV, pAAV-shScramble, pAAV-shRNF11, and pAAV-V5-RNF11, as well as plasmids containing an NF-κB response element driving expression of firefly luciferase or the T7 promoter driving expression of Renilla luciferase. Cells were exposed to 0 or 20 µM of Bay 11-7085 for 1 hour and luciferase readouts were measured after 6 hours of stimulation with vehicle or 6-OHDA. Exposure to Bay11-7085 prevented an increase in NF-κB activity in all transfection conditions compared to TNF-α or 6-OHDA treated cells (p < 0.001) (SI Fig. 1A). Similarly, with Bay 11-7085 pretreatment, we did not observe any significant increase in translocation of p65 into the nucleus in any condition suggesting that the inhibitor was blocking canonical NF-κB signaling. With Bay 11-7085 pretreatment alone, we observed no changes in cell viability (Fig. 1B). However, pretreatment of Bay 11-7085 followed by exposure to 100 µM 6-OHDA increased susceptibility to cell death in the control, pAAV-shScramble, and pAAV-shRNF11-transfected cells (control: 24.89%, pAAV-shScramble: 22.27%, pAAV-shRNF11: 21.36% cell viability) to that observed in the pAAVV5-RNF11-transfected cells with 100 µM 6-OHDA treatment alone (26.16% cell viability). We also activated NF-κB signaling prior to exposure with 6-OHDA in order to possibly bestow additional protection. To activate NF-κB signaling, we pretreated PC12 cells with recombinant 10 ng/mL TNF-α. TNF-α pretreatment did not alter transcriptional activation of NF-κB signaling observed in luciferase reporter assays (SI Fig. 1A), not did it alter p65 translocation observed in response to 6-OHDA with the different RNF11 pAAV constructs (SI Fig. 1D). With TNF-α pretreatment, we observed no changes in cell viability (Fig. 1B). However, pretreatment of TNF-α followed by exposure to 6-OHDA was found to increase protection to 6-OHDA-induced cell death in all cell lines; the viability of control and pAAV-shScramble-transfected cells was similar to pAAV-shRNF11-transfected cells (control: 94.97%, pAAV-shScramble: 95.47%, pAAV-shRNF11: 100.9%, pAAV-V5-RNF11: 42.90% cell viability).
Protection against 6-OHDA-induced cell death in PC12 cells is dependent upon NF-κB up-regulation of antioxidants and anti-apoptotic factors
To determine the NF-κB transcribed genes contributing to the observed differences in 6-OHDA cell toxicity following RNF11 manipulations, parallel samples were analyzed for mRNA expression by qRT-PCR. We examined the mRNA expression of a NF-κB subunit (p50), cytokine (TNF-α), oxidative antioxidants (GSS, SOD1), brain derived neurotrophic factor (BDNF), and anti-apoptosis or pro-survival (BCL2) factor using qRT-PCR. Glutathione synthase (GSS) is the enzyme responsible for catalyzing the reaction producing glutathione, while superoxide dismutase 1 (SOD1) is an enzyme that catalyzes the reaction to prevent oxidative damage by ·O2−. Meanwhile, BDNF is a molecule important for neurogenesis and survival of nigral dopaminergic neurons (Nagahara and Tuszynski, 2011; Stahl et al., 2011).
Loss of RNF11 enhanced 6-OHDA induced NF-κB activation
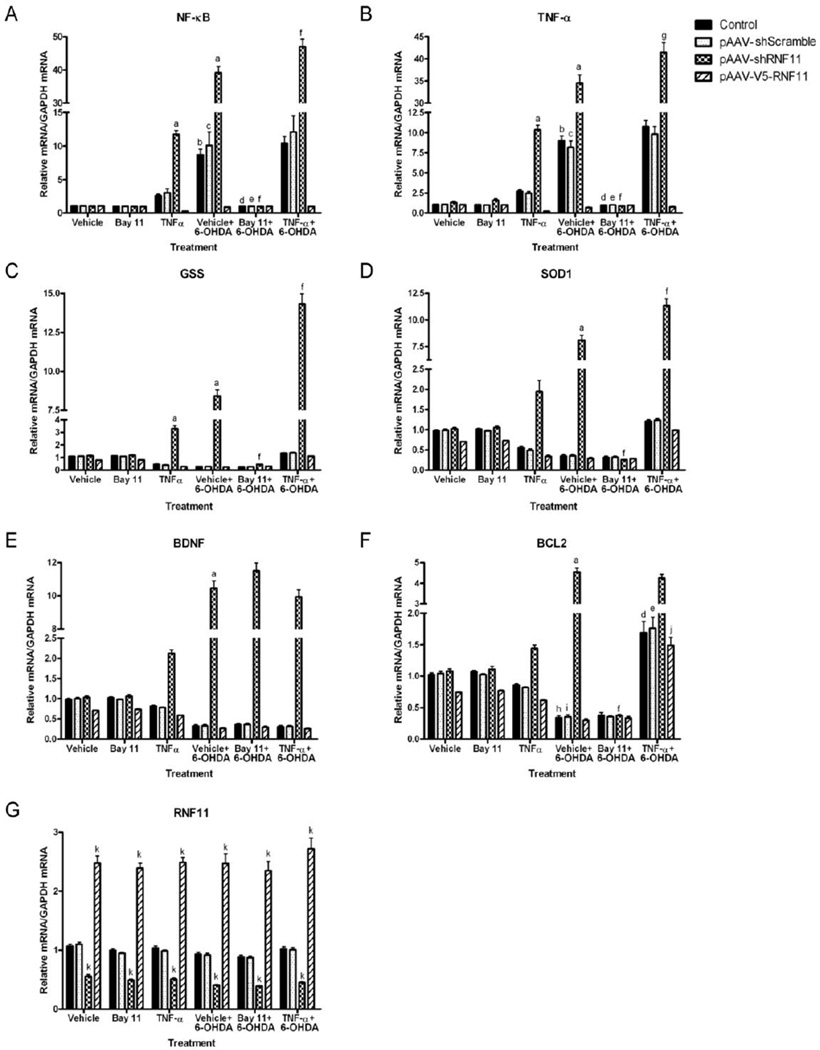
Exposure to 6-OHDA induced NF-κB activation as measured by mRNA levels of NF-κB (p50 subunit) and TNF-α in pAAV-shScramble-transfected cells (Fig. 2A–B, Tables 1 and 2). This activation was enhanced in pAAV-shRNF11-transfected cells and was not observed in pAAV-V5-RNF11-transfected cells, confirming the role of RNF11 as a negative modulator of NF-κB pathway. Additionally, we observed decreased mRNA expression of GSS, SOD1, BDNF, and BCL2 in all of the cell lines except for pAAV-shRNF11-transfected cells following 6-OHDA exposure (Fig. 2C–F, Tables 3–6). pAAV-shRNF11-transfected cells expressed significantly increased levels of GSS, SOD1, BDNF, and BCL2 following 6-OHDA exposure suggesting the important role of these NF-κB transcribed factors (Pahl, 1999) in the observed differences in 6-OHDA-induced cell death. Lastly, RNF11 mRNA levels were as expected in the different cell lines, with a 53% knockdown in the pAAV-shRNF11-transfected cells and an approximately 150% increase with pAAV-V5-RNF11 transfection (Fig. 2G, Table 7). Moreover, exposure to 6-OHDA did not alter RNF11 mRNA expression by qRT-PCR. These in vitro studies validated our hypothesis that RNF11-mediated NF-κB inhibition in dopaminergic cells can impact vulnerability to 6-OHDA toxicity through NF-κB transcribed genes.
Figure 2. Protection against 6-OHDA-induced cell death in PC12 cells is dependent upon NF-κB up-regulation of antioxidants and anti-apoptotic factors.
PC12 cells were transfected with empty pAAV vector (control), shScramble, shRNF11, or V5-RNF11 pAAV constructs and exposed to saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 18 hours. Total RNA was extracted and examined by qRT-PCR for expression of the p50 subunit of NF-κB (A), TNF-α (B), GSS (C), SOD1 (D), BDNF (E), BCL2 (F), and RNF11 (G) mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by two-way ANOVA with Bonferroni post-tests. a, P < 0.001 compared to vehicle treated shRNF11 cells, b, P < 0.001 compared to vehicle treated control cells, c, P < 0.001 compared to vehicle treated shScramble cells, d, P < 0.001 compared to vehicle pretreated and 6-OHDA treated control cells, e, P < 0.001 compared to vehicle pretreated and 6-OHDA treated shScramble cells, f, P < 0.001 compared to vehicle pretreated and 6-OHDA treated shRNF11 cells, g, P < 0.01 compared to vehicle pretreated and 6-OHDA treated shRNF11 cells, h, P < 0.01 compared to vehicle treated control cells, i, P < 0.01 compared to vehicle treated shScramble cells, j, P < 0.001 compared to vehicle pretreated and 6-OHDA treated V5-RNF11 cells, k, P < 0.001 compared to control cells with the same pretreatment and treatment conditions. Bars without letters denoting significance are not significantly different from vehicle treated cells with the same transfected plasmid (Bay 11, TNF-α, and vehicle pretreatment and 6-OHDA treatment conditions) or from vehicle pretreated and 6-OHDA treated cells with the same transfected plasmid (Bay 11 or TNF-α pretreatment and 6-OHDA treatment conditions).
Table 1.
Mean relative mRNA levels of NF-κB (p50 subunit) in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 1.051 | 1.069 | 1.023 | 1.049 |
| Bay 11-7085 + Vehicle | 0.976 | 0.997 | 0.959 | 0.944 |
| TNF-α + Vehicle | 2.605 | 3.024 | 11.749 a | 0.262 |
| Vehicle + 6-OHDA | 8.685 b | 10.081 c | 39.165 a | 0.874 |
| Bay 11-7085 + 6-OHDA | 0.983 d | 0.999 e | 0.956 f | 0.981 |
| TNF-α + 6-OHDA | 10.422 | 12.098 | 46.998 f | 0.961 |
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle treated control cells,
P < 0.001 compared to vehicle treated pAAV-shScramble cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated control cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shScramble cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shRNF11 cells.
Table 2.
Mean relative mRNA levels of TNF-α in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 1.051 | 1.070 | 1.293 | 1.038 |
| Bay 11-7085 + Vehicle | 1.034 | 0.993 | 1.576 | 1.013 |
| TNF-α + Vehicle | 2.688 | 2.453 | 10.360 a | 0.202 |
| Vehicle + 6-OHDA | 8.961 b | 8.178 c | 34.534 a | 0.674 |
| Bay 11-7085 + 6-OHDA | 0.983 d | 1.001 e | 0.890 f | 0.971 |
| TNF-α + 6-OHDA | 10.754 | 9.8136 | 41.441 g | 0.741 |
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle treated control cells,
P < 0.001 compared to vehicle treated pAAV-shScramble cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated control cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shScramble cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAVshRNF11 cells,
P < 0.01 compared to vehicle pretreated and 6-OHDA treated pAAV-shRNF11 cells.
Table 3.
Mean relative mRNA levels of GSS in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 1.079 | 1.098 | 1.135 | 0.783 |
| Bay 11-7085 + Vehicle | 1.125 | 1.081 | 1.170 | 0.811 |
| TNF-α + Vehicle | 0.459 | 0.410 | 3.289 a | 0.281 |
| Vehicle + 6-OHDA | 0.288 | 0.295 | 8.412 a | 0.241 |
| Bay 11-7085 + 6-OHDA | 0.259 | 0.265 | 0.454 b | 0.313 |
| TNF-α + 6-OHDA | 1.348 | 1.373 | 14.300 b | 1.096 |
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shRNF11 cells.
Table 6.
Mean relative mRNA levels of BCL2 in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 1.021 | 1.040 | 1.075 | 0.741 |
| Bay 11-7085 + Vehicle | 1.065 | 1.023 | 1.108 | 0.768 |
| TNF-α + Vehicle | 0.852 | 0.819 | 1.440 | 0.614 |
| Vehicle + 6-OHDA | 0.337 a | 0.352 b | 4.547 c | 0.297 |
| Bay 11-7085 + 6-OHDA | 0.378 | 0.353 | 0.376 d | 0.333 |
| TNF-α + 6-OHDA | 1.688 e | 1.763 f | 4.246 | 1.489 g |
P < 0.01 compared to vehicle treated control cells,
P < 0.01 compared to vehicle treated pAAV-shScramble cells,
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated control cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shScramble cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-V5-RNF11 cells.
Table 7.
Mean relative mRNA levels of RNF11 in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 1.070 | 1.102 | 0.560 a | 2.476 a |
| Bay 11-7085 + Vehicle | 0.997 | 0.948 | 0.490 a | 2.391 a |
| TNF-α + Vehicle | 1.037 | 0.986 | 0.510 a | 2.487 a |
| Vehicle + 6-OHDA | 0.930 | 0.917 | 0.409 a | 2.471 a |
| Bay 11-7085 + 6-OHDA | 0.883 | 0.871 | 0.388 a | 2.347 a |
| TNF-α + 6-OHDA | 1.023 | 1.009 | 0.450 a | 2.718 a |
P < 0.001 compared to control cells with the same pretreatment and treatment conditions.
Bay 11-7085 inhibited 6-OHDA induced NF-κB activation similar to RNF11
To confirm and extend these results, parallel samples that were pretreated with Bay 11-7085 and TNF-α were also examined by qRT-PCR. While Bay 11-7085 did not alter RNF11 mRNA levels (Fig. 2G, Table 7), the induced expression of the p50 subunit of NF-κB and TNF-α mRNA following 6-OHDA exposure was completely abolished (Fig. 2A–B, Tables 1 and 2). Bay 11-7085 pretreatment did not alter mRNA expression of BDNF in pAAV-shRNF11-transfected cells (Fig. 2E, Table 5). In contrast, the increased mRNA levels of GSS, SOD1, and BCL2 observed in pAAV-shRNF11-transfected cells were eliminated with Bay 11-7085 pretreatment, with all cell types being identical to pAAV-V5-RNF11-transfected cells (Fig. 2C, 2D, 2F, Tables 3, 4, 6). The results of this experiment suggest that the protection granted with knockdown of RNF11 is related to the NF-κB dependent up-regulation of antioxidants and anti-apoptotic factors, and not BDNF.
Table 5.
Mean relative mRNA levels of BDNF in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 0.980 | 0.998 | 1.032 | 0.712 |
| Bay 11-7085 + Vehicle | 1.023 | 0.982 | 1.063 | 0.737 |
| TNF-α + Vehicle | 0.818 | 0.786 | 2.127 | 0.589 |
| Vehicle + 6-OHDA | 0.320 | 0.328 | 10.458 a | 0.268 |
| Bay 11-7085 + 6-OHDA | 0.352 | 0.360 | 11.503 | 0.294 |
| TNF-α + 6-OHDA | 0.304 | 0.311 | 9.935 | 0.254 |
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells.
Table 4.
Mean relative mRNA levels of SOD1 in PC12 cells.
| Control | pAAV-shScramble | pAAV-shRNF11 | pAAV-V5-RNF11 | |
|---|---|---|---|---|
| Vehicle + Vehicle | 0.971 | 0.988 | 1.021 | 0.704 |
| Bay 11-7085 + Vehicle | 1.013 | 0.973 | 1.053 | 0.729 |
| TNF-α + Vehicle | 0.551 | 0.492 | 1.947 | 0.337 |
| Vehicle + 6-OHDA | 0.346 | 0.354 | 8.094 a | 0.289 |
| Bay 11-7085 + 6-OHDA | 0.311 | 0.318 | 0.255 b | 0.281 |
| TNF-α + 6-OHDA | 1.213 | 1.235 | 11.332 b | 0.986 |
P < 0.001 compared to vehicle treated pAAV-shRNF11 cells,
P < 0.001 compared to vehicle pretreated and 6-OHDA treated pAAV-shRNF11 cells.
TNF-α enhanced 6-OHDA induced NF-κB activation similar to reduced RNF11
TNF-α also did not alter RNF11 mRNA levels (Fig. 2G, Table 7). Only modest increases in the expression of the p50 subunit of NF-κB and TNF-α mRNA were observed in all cell lines, with only a significant increase in the pAAV-shRNF11 cells when compared to cells exposed to 6-OHDA alone (Fig. 2A–B, Tables 1 and 2). Also, we observed significant increases in expression of GSS, SOD1, and BCL2 mRNA in all cell lines with TNF-α pretreatment (Fig. 2C, 2D, 2F, Tables 3, 4, 6), while expression of BDNF mRNA remained similar following 6-OHDA exposure regardless of pretreatment conditions (Fig. 2E, Table 5). Consistent with our results in the Bay 11-7085 pretreatment experiments, this experiment confirms that loss of RNF11-mediated NF-κB inhibition in dopaminergic cells and subsequent upregulation of antioxidants and anti-apoptotic factors contribute to the protection we observe against 6-OHDA-induced cell death.
Validation of AAV2-mediated targeted knockdown and over-expression of RNF11 in vitro and in vivo
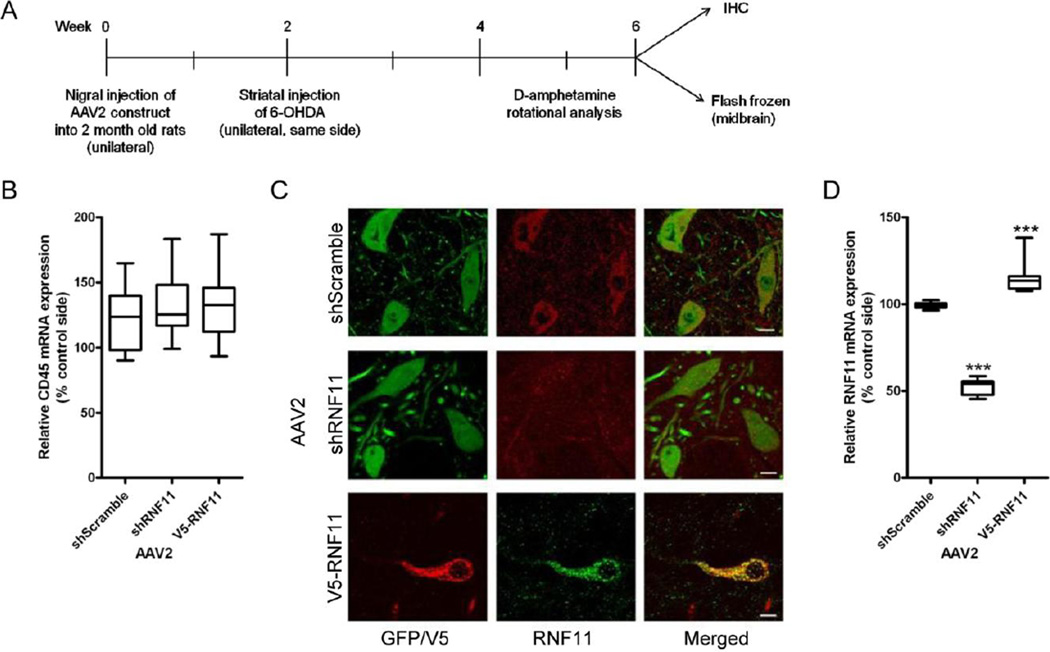
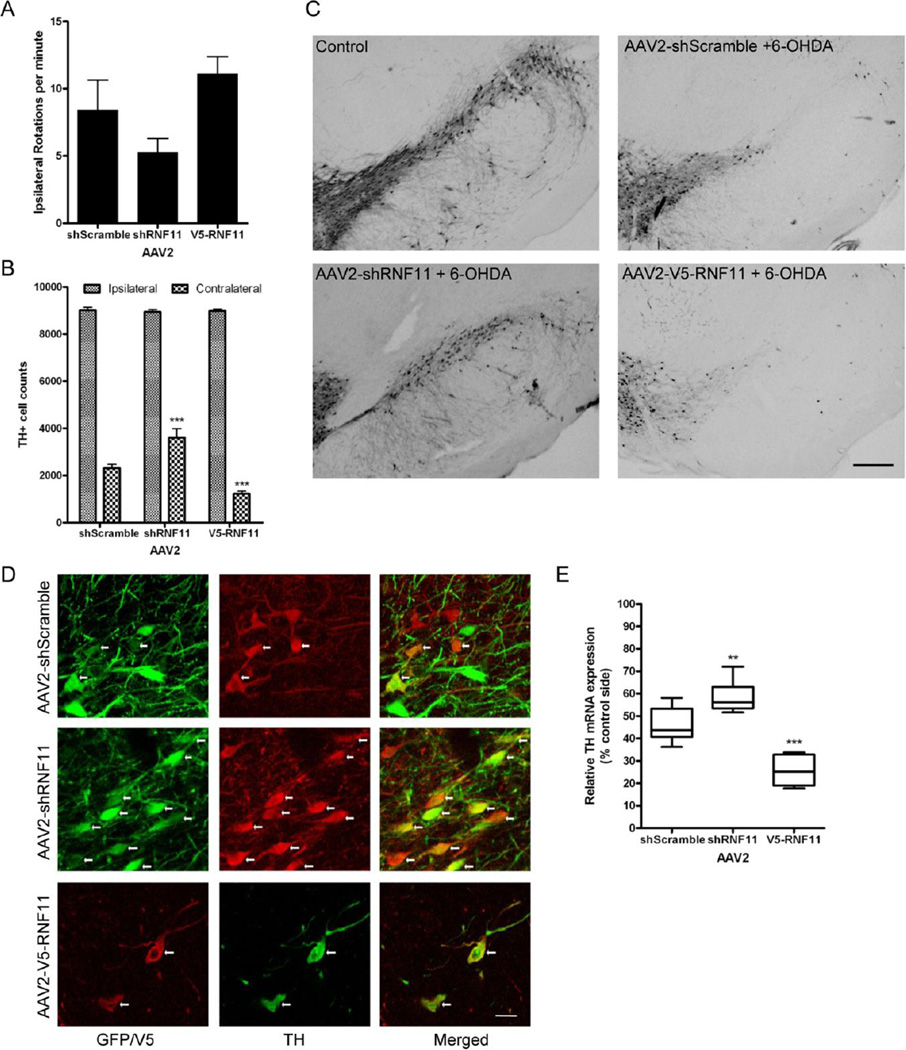
Subsequent to finding the contribution of RNF11-mediated NF-κB activation on dopaminergic cell death, we investigated the effects of RNF11 on dopaminergic neuronal loss, a phenotype essential to PD pathology and movement dysfunction, in a rodent model of PD. These experiments enabled us to determine RNF11’s functional role in PD-related degeneration in an in vivo system. The design for the experimental paradigm (Fig. 3A) was to alter RNF11 expression in nigral dopaminergic neurons, followed by intrastriatal administration of neurotoxin 6-OHDA two weeks later. Animals were examined after four weeks through immunohistochemistry or qRT-PCR. To manipulate RNF11 expression, we generated and tested the ability of AAV2 to specifically alter neuronal expression within the SNpc as previously described (Bartus et al., 2011). The three viruses used were AAV2 expressing a scramble shRNA sequence (AAV2-shScramble-GFP), AAV2 expressing shRNA targeted against RNF11 (AAV2-shRNF11-GFP), and transduction of AAV2 over-expressing V5-RNF11 (AAV2-V5-RNF11) using the same constructs from our in vitro experiments. The AAV2-V5-RNF11 was developed without GFP due to the size constraints associated with AAV.
Figure 3. Validation of AAV2-mediated targeted knockdown and over-expression of RNF11 in vivo.
A. Schematic for animal experiments. AAV2 constructs (GFP-shScramble, GFP-shRNF11, V5-RNF11) were unilaterally injected into the substantia nigra pars compacta of two month old male rats. Two weeks later, a unilateral striatal lesion was induced by injecting 6-OHDA (10 µg) into the striatum of rats. Three weeks after lesion, rotational behavior induced by an intraperotoneal injection of 5 mg/kg D-amphetamine was measured in all animals as a physiological measure of striatal dopamine depletion. Four weeks after lesion, animals were anesthetized and brains were either fixed for immunohistochemical analysis or microdissected and flash frozen for qRT-PCR analysis. B. Total RNA was extracted from flash frozen ventral midbrain samples and examined by qRT-PCR for expression of CD45 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. C. Representative images of tag expression (GFP or V5) and RNF11 at the level of the substantia nigra in AAV2-injected hemisphere demonstrating knock down (middle panel) and over-expression (bottom panel) of RNF11 in nigral neurons. Scale bar: 10 µM. D. Total RNA was extracted from flash frozen ventral midbrain samples and examined by qRT-PCR for expression of RNF11 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by one-way ANOVA with Dunnett’s post-tests. ***, P < 0.001 compared to AAV2-shScramble animals.
In vitro confirmation
First, we confirmed the ability of AAV2 to manipulate RNF11 expression in primary neuronal cultures. In primary neurons, transduction of AAV2-shRNF11-GFP decreased RNF11 mRNA levels by approximately 60% by qRT-PCR when compared to untransduced as well as following transduction of AAV2-shScramble-GFP, while transduction of AAV2-V5-RNF11 increased RNF11 mRNA levels by approximately 50% (P < 0.001 for both) (SI Fig. 2A). Additionally, we found that transduction of AAV2-shScramble-GFP did not significantly alter RNF11 mRNA levels when compared to untransduced cells. Next, we verified that manipulation of RNF11 levels by AAV2 produced the expected changes in NF-κB signaling as previously reported (Pranski et al., 2012a) using immunocytochemistry to examine the translocation of p65 (SI Fig. 2B). We observed increased co-localization of p65 with Hoechst 333258 at 120 minutes after 6-OHDA stimulation in AAV2-shRNF11-GFP transduced cells in comparison to AAV2-shScramble-GFP transduced cells (AAV2-shScramble-GFP: 1.08-fold change, AAV2-shRNF11-GFP: 2.18-fold change) and no significant increase in co-localization of p65 with Hoechst 333258 at any time point examined in AAV2-V5-RNF11 transduced cells in comparison to AAV2-shScramble-GFP transduced cells (AAV2-V5-RNF11: 30 minutes: 1.31-fold change, 120 minutes: 1.02-fold change) (P < 0.001). These experiments confirmed that AAV2 was altering RNF11 mRNA levels that resulted in negative modulation of the NF-κB signaling pathway.
In vivo confirmation
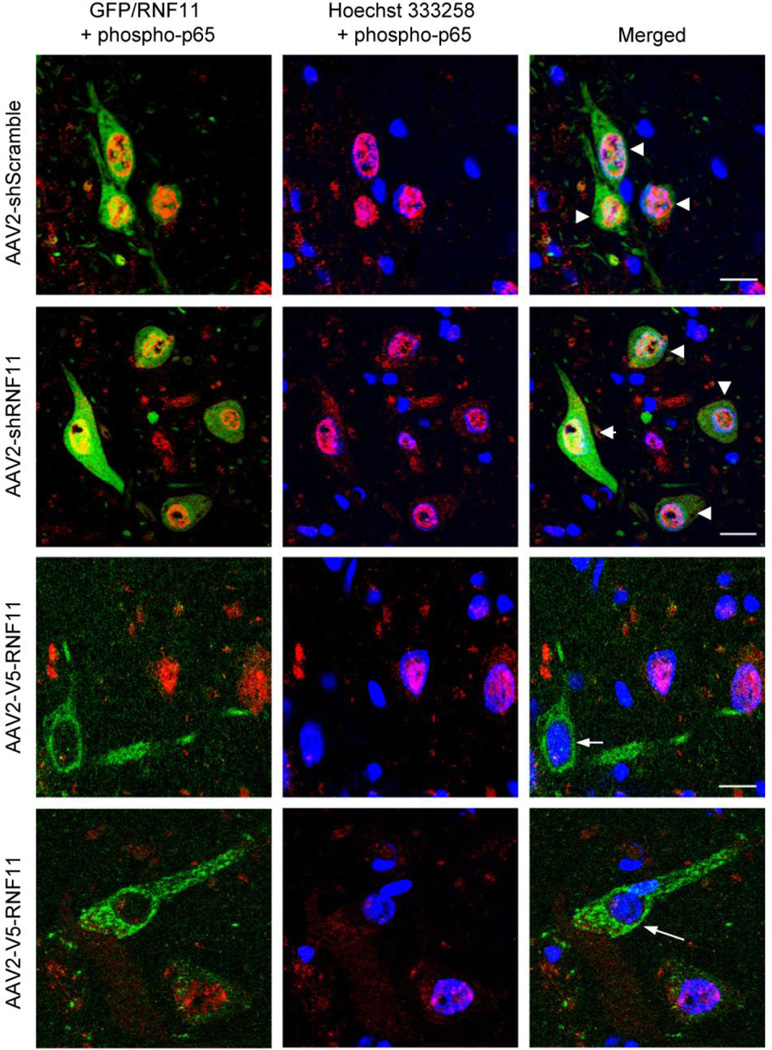
For targeted manipulation of RNF11 expression in dopaminergic neurons, a unilateral intranigral injection of AAV2 was performed. As described previously, the three AAV2 used were AAV2-shScramble-GFP, AAV2-shRNF11-GFP, and AAV2-V5-RNF11. Thus, the three groups of rats used for this set of experiments were (1) shScramble, (2) shRNF11, and (3) V5-RNF11. The AAV2 serotype was used since it has been shown previously to primarily transduce neurons (as opposed to glia) within the SNpc (Bartus et al., 2011; Decressac et al., 2012; Gasmi et al., 2007; Mulcahy et al., 2012). We evaluated transduction efficiency in the SN two weeks post AAV2 GFP injections. We observed extensive infection of dopaminergic neurons, as evident from GFP and TH co-localization throughout the extent of the SN on the ipsilateral hemisphere (SI Fig. 3A–B, Mander’s coefficient = 0.92±0.7). At the site of injection, most of the TH-positive neurons were double labeled for GFP (88.9%±2.3) similar to previous reports (Decressac et al., 2012; Mulcahy et al., 2012). Some GFP-positive cells were also scattered in the SN pars reticulata. Additionally, we observed GFP-positive immunoreactivity in the ipsilateral striatum suggesting that our construct was not only being expressed throughout the cell body but also extended to the processes and terminals of the nigral dopaminergic neurons (SI Fig. 3C) as reported previously(Decressac et al., 2012; Mulcahy et al., 2012). GFP was not detected on the contralateral side at all. The transduced cells in the nigra were nearly all MAP2-positive neurons (SI Fig. 3D, Mander’s coefficient = 0.95±0.8), with little to no detectable infection of Iba1-positive microglia (SI Fig. 3E, Mander’s coefficient = 0.09±0.6) or GFAP-positive astrocytes (images not shown, Mander’s coefficient = 0.05±1.0). Furthermore, we did not observe an up-regulation of microglia in response to the AAV2 injections similar to other reports (Roca et al., 2011). However 6-OHDA administration increased Iba1-positive microglia on the ipsilateral side compared to the contralateral uninjected side (data not shown). The activation of microglia was quantified using qRT-PCR to measure relative CD45 expression in ventral mesencephalic (VM) brain samples. CD45 is a specific marker of microglia activation in response to inflammation and injury (González-Scarano and Baltuch, 1999; Kreutzberg, 1996). CD45 mRNA level on the ipsilateral side was significantly upregulated at 4 weeks following 6-OHDA injections as compared to the contralateral uninjected side in all the three groups. However, no significant difference in CD45 expression was observed between the three groups (Fig. 3B) in the 6-OHDA injected side suggesting that microglial activation following 6-OHDA exposure was similar in all the three groups. Most importantly, AAV2 was able to manipulate RNF11 expression in vivo as expected. We qualitatively observed appreciable differences in RNF11 immunoreactivity in cells expressing the different viruses (Fig. 3C), which mirrored the mRNA expression results. Using qRT-PCR to measure relative RNF11 expression in VM brain samples, we saw an approximately 50% decrease in the AAV2-shRNF11-GFP animals and an approximately 20% increase in the AAV2-V5-RNF11 animals in comparison to AAV2-shScramble-GFP animals (Fig. 3D, Table 8). Moreover, we observed qualitative differences in the localization of phospho-p65 (a marker of NF-κB activation) in neurons in the SNpc between the three groups of animals (Fig. 4). While both the AAV2-shRNF11-GFP and AAV2-shScramble-GFP-injected animals had co-localization of phospho-p65 with Hoechst 333258 in SNpc neurons that also expressed GFP (Fig 4, arrowheads), neurons expressing high levels of RNF11 in the SNpc of AAV2-V5-RNF11-injected animals did not have co-localization of phospho-p65 with Hoechst 333258 (see cells indicated by white arrows) suggesting absence of NF-κB activation. These results show that AAV2 is able to manipulate RNF11 mRNA levels in vivo as expected, that AAV2 targeted TH-positive neurons, and that RNF11 manipulations can alter NF-κB signaling in vivo as expected, allowing us to confidently use these reagents to manipulate expression levels of RNF11 in 6-OHDA rat model of PD.
Table 8.
Mean relative mRNA levels in AAV2-injected ventral midbrain samples (% of control side).
| AAV2- shScramble |
AAV2- shRNF11 |
AAV2- V5-RNF11 |
|
|---|---|---|---|
| TH | 46.6 | 58.4 b | 26.0 a |
| NF-κB (p50 subunit) | 118.9 | 137.9 a | 111.2 c |
| TNF-α | 127.0 | 142.6 b | 116.8 c |
| GSS | 79.5 | 302.1 a | 66.1 c |
| SOD1 | 60.3 | 201.7 a | 49.5 |
| BDNF | 77.0 | 131.0 a | 59.8 a |
| BCL2 | 247.5 | 379.8 a | 142.9 a |
| RNF11 | 99.2 | 52.7 a | 114.9 a |
| CD45 | 122.2 | 132.4 | 132.3 |
P < 0.001,
P < 0.01,
P < 0.05 compared to AAV2-shScramble animals.
Figure 4. Validation of RNF11 as a negative modulator of NF-κB signaling in vivo.
Representative images of virus expression (GFP or RNF11, in green) and phospho-p65 (in red) at the level of the substantia nigra in AAV2-injected hemisphere. Note the colocalization (arrowheads) of Hoechst 333258 (in blue) and phospho-p65 in the AAV2-shScramble and AAV2-shRNF11 animals (first and second rows) and minimal colocalization in AAV2-V5-RNF11 animals (third and fourth rows) in virus-positive nigral neurons as indicated by white arrows. Scale bar: 20 µM.
In vivo targeted knockdown of nigral dopaminergic RNF11 imparts neuroprotection against 6-OHDA-induced toxicity and attenuates rotational behavior
6-OHDA is a hydroxylated analog of dopamine and is commonly used as a neurotoxin to induce dopaminergic degeneration (Blum et al., 2001) primarily through reactive oxygen species-dependent apoptosis (Holtz et al., 2006; Lotharius et al., 1999). In these experiments, a unilateral intrastriatal preterminal injection of 6-OHDA was performed ipsilateral to the AAV2 injection while the contralateral hemisphere served as an internal control. As a physiological measure of 6-OHDA-induced lesion of the nigrostriatal pathway, we tested amphetamine-induced rotation behavior three weeks after striatal 6-OHDA injections. We found 6-OHDA induced amphetamine (5 mg/kg, i.p.) rotations in the AAV2-shScramble-GFP animals were attenuated in the AAV2-shRNF11-GFP animals, but slightly increased in the AAV2-V5-RNF11 animals (AAV2-shScramble-GFP: 8.4, AAV2-shRNF11-GFP: 5.3, AAV2-V5-RNF11: 11.1 rotations/minute) (Fig. 5A), suggesting that 6-OHDA induced lesion (ie., loss of striatal dopamine) may be reduced in the AAV2-shRNF11-GFP group versus the AAV2-shScramble-GFP group while the lesion may be more extensive in the RNF11 over-expressing group. These suggested behavioral phenotypes implied that knockdown of RNF11 may be protective while RNF11 over-expression could potentiate 6-OHDA toxicity.
Figure 5. In vivo targeted knockdown of nigral dopaminergic RNF11 imparts neuroprotection against 6-OHDA-induced toxicity and attenuates rotational behavior.
A. Three weeks after lesion, rotational behavior induced by an intraperitoneal injection of 5 mg/kg D-amphetamine was measured in all animals as a physiological measure of striatal dopamine depletion and expressed as the number of ipsilateral turns per minute. While a one-way ANOVA between groups was significant (P = 0.01), no significant differences between groups were found using Dunnett’s multiple comparison test. Values expressed are group mean ± SEM. B. Stereological estimates of nigral dopaminergic neuron number (TH-immunoreactive neurons) after 6-OHDA injection on the unlesioned (contralateral) and lesioned (ipsilateral) sides. Values were expressed as the group mean ± SEM; ***, P < 0.001, significantly different from AAV2-shScramble animals. C. Representative sections of TH-immunoreactivity from an uninjected hemisphere (control) or hemispheres receiving injections of shScramble-GFP, shRNF11-GFP, or V5-RNF11 AAV2 and 6-OHDA. Scale bar: 200 µM. D. Representative images of co-localization (indicated by white arrows) of expression of GFP or V5 and TH at the level of the substantia nigra in AAV2-injected hemisphere. Note the numerous GFP/TH-positive cells in AAV2-shRNF11 group. Scale bar: 20 µM. E. Total RNA was extracted from flash frozen ventral midbrain samples and examined by qRT-PCR for expression of TH mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by one-way ANOVA with Dunnett’s post-tests. ***, P < 0.001, **, P < 0.01 from AAV2-shScramble animals.
The RNF11-induced differences in 6-OHDA-induced lesions in the three animal groups, indicating differential loss of nigral dopaminergic neurons, was confirmed by both qualitative and quantitative analysis of nigral dopaminergic cell loss and TH mRNA levels in the three groups of rats. Similar to previous reports (Kirik et al., 1998), striatal injections of 6-OHDA resulted in retrograde loss of nigral dopaminergic cells in all three groups of rats. To quantify and differentiate the loss of dopaminergic cells, the number of TH-positive soma was estimated within the SNpc using unbiased stereology. We found that AAV2-shRNF11-GFP injection in the SN rescued 15% of the nigral dopaminergic neurons from 6-OHDA-induced cell death, while AAV2-V5-RNF11 injection in the SN enhanced 6-OHDA-induced death of nigral dopaminergic neurons by 12%, (Fig. 5B–C) as compared to control group with AAV2-shScramble-GFP injection (P < 0.001).
Qualitative analysis of fluorescence immunohistochemistry and double labeling for GFP or V5 and TH demonstrated the presence of more GFP-TH positive cells in the AAV2-shRNF11-GFP group in comparison to the AAV2-shScramble-GFP group (Fig. 5D). Additionally, very few V5/TH-positive cells and processes were present in the SN in the AAV2-V5-RNF11 group (Fig. 5D), further confirming the differences in 6-OHDA toxicity in the three groups. Moreover, these double-labeling experiments demonstrated that AAV2-V5-RNF11 resulted in loss of dopaminergic cells and not just loss of TH expression as evident from absence of both V5 and TH-positive immunoreactivity in the AAV2-V5-RNF11 group.
Lastly, the statistically significant differences in stereological counts of TH-positive neurons between the three groups were further emphasized following analysis of relative TH mRNA levels in the VM by qRT-PCR. In comparison to the unlesioned side, a 12% increase in TH mRNA in the AAV2-shRNF11-GFP group and a 20% decrease in TH mRNA in the AAV2-V5-RNF11 group was observed compared to the AAV2-shScramble group-GFP (Fig. 5E, Table 8). The slight discrepancy in the TH mRNA data versus the stereological cell counts could possibly be due to the inclusion of ventral tegmental area (VTA) in the VM samples; VTA neurons were more compromised in the AAV2-V5-RNF11 group (Fig. 5C).
Thus, the protection of dopaminergic neurons in the SNpc achieved with nigral delivery of targeted knockdown of neuronal RNF11 correlated with attenuated ipsiversive circling behavior induced by amphetamine (Fig. 5). In reverse, the enhanced dopaminergic degeneration in the SNpc with nigral delivery of RNF11 over-expression correlated with increased ipsiversive circling behavior induced by amphetamine. This correlation of the behavioral data with TH mRNA levels, quantitative analysis of TH cells, and double-labeling experiments in the SN confirmed the differences in 6-OHDA-induced lesions in the three groups, suggesting that targeted manipulation of RNF11 expression in nigral dopaminergic neurons can impact 6-OHDA-induced toxicity.
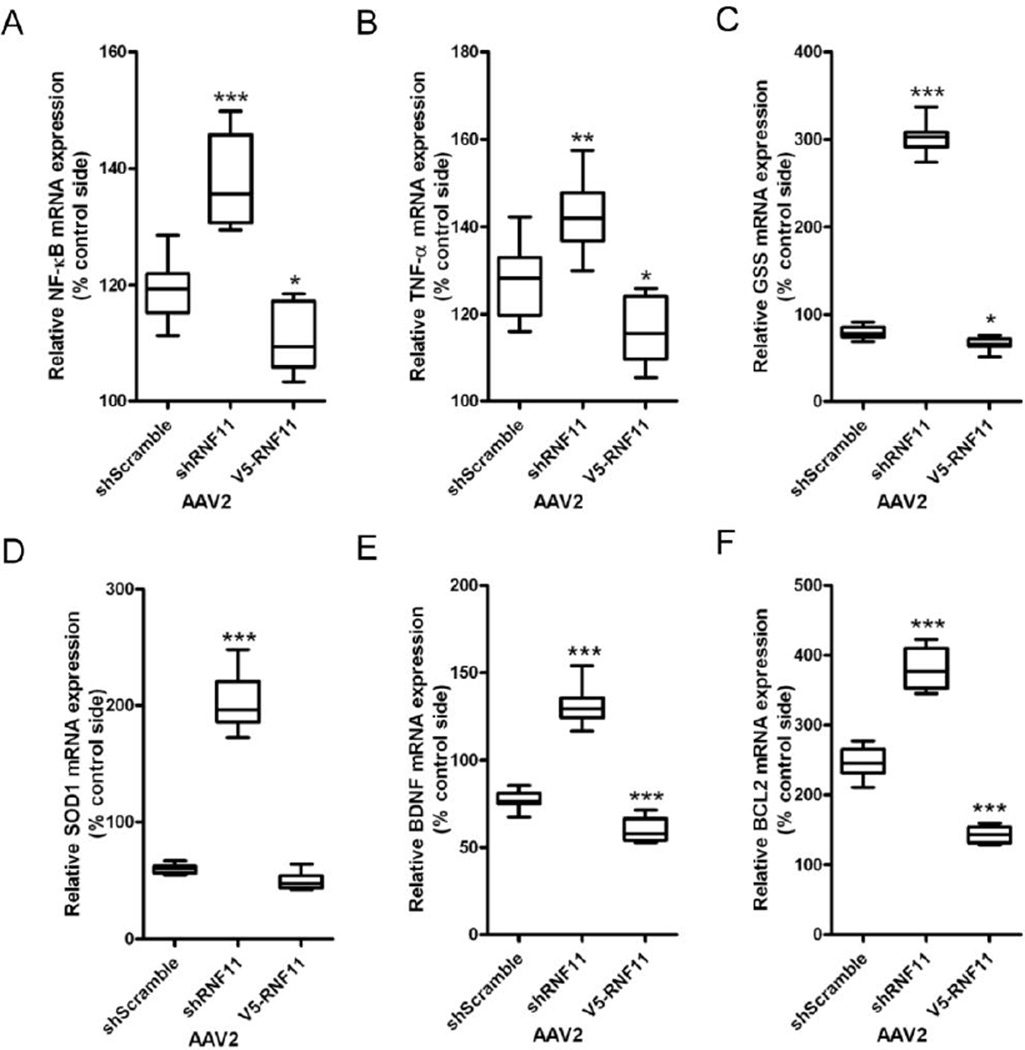
In vivo targeted knockdown of nigral dopaminergic RNF11 increases NF-κB signaling
Since RNF11 is a negative modulator of NF-κB pathway (Pranski et al., 2012a), we hypothesized that the differences in protection against 6-OHDA toxicity conferred by RNF11 was due to differential NF-κB activation in the three experimental groups as an extension of our in vitro studies. Therefore we examined the expression level of NF-κB and its target genes in VM tissue from rat brain. This also enabled us to determine the NF-κB-mediated responses that could be accountable for the differences in 6-OHDA-induced dopaminergic degeneration. Again, we examined the mRNA expression of a NF-κB subunit (p50), cytokine (TNF-α), brain derived neurotrophic factor (BDNF), oxidative antioxidants (GSS, SOD1), and anti-apoptosis or pro-survival (BCL2) factor using qRT-PCR. The mRNA level for the p50 subunit of NF-κB and TNF-α was elevated in the 6-OHDA lesioned hemisphere in all groups, with the largest increase observed in the AAV2-shRNF11-GFP group and minimum increase in the AAV2-V5-RNF11 group (Fig. 6A–B, Table 8). The gene expression data confirmed the immunohistochemical observation (Fig. 4) that NF-κB activity is reduced in AAV2-V5-RNF11 group. mRNA expression for GSS, SOD1, and BDNF was decreased on the lesioned side of the AAV2-shScramble-GFP group (Fig. 6C–E, Table 8). A more substantial decrease in GSS, SOD1, and BDNF mRNA expression was seen in the lesioned AAV2-V5-RNF11 group, while a significant increase in SOD1 and BDNF mRNA expression was observed in lesioned AAV2-shRNF11-GFP hemisphere. Lastly, we measured the mRNA levels of BCL2. While increased BCL2 mRNA expression was seen in all the lesioned animals, a significant increase was observed in the AAV2-shRNF11-GFP group and a significant decrease was observed in the AAV2-V5-RNF11 group in comparison to the AAV2-shScramble-GFP group (Fig. 6F, Table 8). From these results, we concluded that the degree of vulnerability to 6-OHDA conferred by the targeted manipulation of RNF11 in dopaminergic neurons correlated negatively with NF-κB mRNA expression levels and expression of its target genes including TNF-α, antioxidants, neurotrophic factors, and anti-apoptotic factors. Thus, knockdown of RNF11 related to increased NF-κB activity and dopaminergic cell survival, while RNF11 overexpression inhibited NF-κB activity and increased dopaminergic cell loss.
Figure 6. In vivo targeted knockdown of nigral dopaminergic RNF11 increases NF-κB signaling.
Total RNA was extracted from flash frozen ventral midbrain samples and examined by qRT-PCR for expression of the p50 subunit of NF-κB (A), TNF-α (B), GSS (C), SOD1 (D), BDNF (E), and BCL2 (F) mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by one-way ANOVA with Dunnett’s post-tests.*, P < 0.05, **, P < 0.01, ***, P < 0.001 from AAV2-shScramble animals.
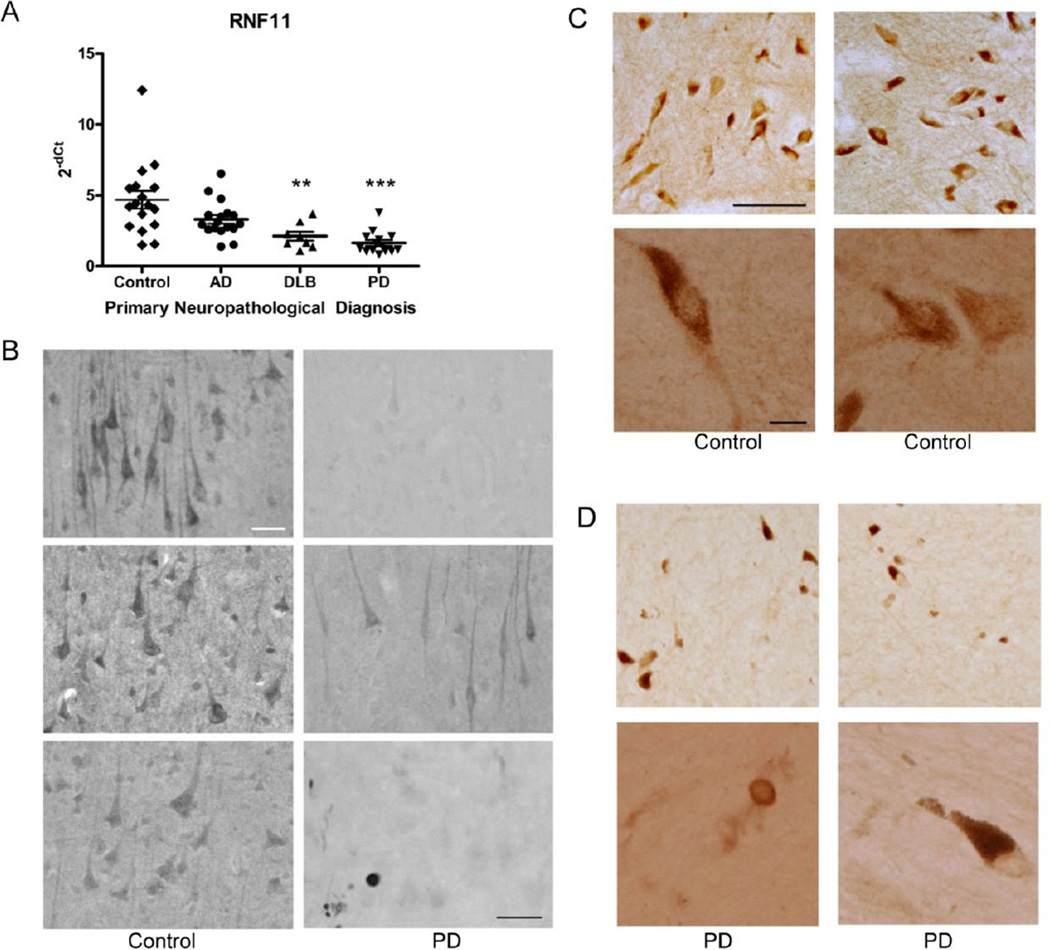
Reduction of RNF11 mRNA expression in Parkinson’s disease
Given that A20 ubiquitin-editing protein complex is a crucial regulator of the canonical NF-κB pathway (Verstrepen et al., 2010) and that chronic activation of NF-κB is associated with PD neurodegeneration (Glass et al., 2010; Hunot et al., 1997; Mattson and Meffert, 2006), we hypothesized that for aberrant NF-κB pathway activity, expression of one or more of the A20 ubiquitin-editing protein complex components may be altered in PD patients. Therefore we examined the distribution of the various components of the A20 ubiquitin-editing protein complex in PD brain tissue. Previously, we demonstrated that RNF11, Itch and TAX1BP1, components of the A20 ubiquitin-editing protein complex, are constitutively and highly expressed in neurons as compared to glial cells in various regions of normal human brain (Pranski et al., 2012b). Moreover, mRNA expression of A20, a NF-κB-inducible protein, was also detected in all the brain regions examined (Pranski et al., 2012b). Given the vulnerability of cortical neurons and spread of pathology to neocortical regions during the later stages of PD (Braak et al., 2006), we chose to examine neocortical tissue from diseased brain along with age-matched controls. To determine if alterations in expression were specific to PD we also examined samples from AD and dementia with Lewy bodies (DLB) cases. We first examined the expression of A20 and RNF11 mRNA by qRT-PCR and observed that the mRNA level of RNF11 was significantly decreased in DLB (P < 0.01) and PD (P < 0.001) patients compared to control cases (control: 4.696, AD: 3.313, DLB: 2.125, PD: 1.643 mean relative cycle threshold) (Fig. 7A). In contrast, the mRNA level of A20 did not significantly differ between PD, AD and DLB groups (SI Fig. 3A). Reduced RNF11 mRNA has been reported previously in PD SN (Noureddine et al., 2005).
Figure 7. Down-regulation of RNF11 mRNA and protein expression in Parkinson’s disease brain tissue.
A. Total RNA was extracted from neocortical tissue of control, Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and Parkinson’s disease (PD) cases and examined by real-time PCR for expression of RNF11 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GUSB levels as an internal control. **, P < 0.01; ***, P < 0.001 from control. B. Frontal cortex from control and PD cases was analyzed by immunohistochemistry using an antibody against RNF11. Scale bar: 50 µM. Bottom right panel, scale bar: 25 µM. C, D. Substantia nigra from control (C) and PD cases (D) was analyzed by immunohistochemistry using an antibody against RNF11. Top row in each, scale bar: 100 µM. Bottom row in each, scale bar: 25 µM.
Reduction of RNF11 protein expression in Parkinson’s disease
In order to confirm these results with an orthogonal approach, we examined the protein expression of components of the A20 ubiquitin-editing protein complex by immunohistochemistry in PD and matched control cases. As previously shown, detection of A20 by immunohistochemistry is not possible with the available commercial antibodies (Pranski et al., 2012b). Therefore, we examined the expression of the other crucial members of the A20 ubiquitin-editing protein complex: RNF11, Itch, and TAX1BP1. A striking difference in intensity of RNF11 immunoreactive neurons in the frontal cortex was observed between control and PD tissue (Fig. 7B). The staining for RNF11 in PD tissue was substantially diminished in comparison to control cases, although faint RNF11 immunoreactivity could be observed in a portion of neurons in PD tissue. No obvious difference in Itch (SI Fig. 3B) or TAX1BP1 (data not shown) immunoreactivity was observed in cortical tissue between control and PD samples. Additionally, we examined the expression of RNF11 in the SN of control and PD tissue. In control cases, RNF11 had a neuronal, somatodendritic pattern of expression similar to that previously reported (Fig. 7C) (Anderson et al., 2007; Pranski et al., 2012b). However, similar to the cortex, a prominent reduction in intensity of RNF11 immunoreactivity was observed in SN neurons in PD cases (Fig. 7D). As reported previously (Anderson et al., 2007), RNF11 immunoreactivity was observed in Lewy body-like inclusions in the cortex and SN (Fig. 7B, bottom right panel, Fig. 7D bottom left).
Together our in vivo and in vitro data provide strong evidence that RNF11 modulated NF-κB activation in dopaminergic cells is imperative for survival mechanisms against 6-OHDA toxicity and that the down-regulation of RNF11 expression we observed in PD brain samples may represent a protective mechanism against a toxic environment.
Discussion
Understanding the regulation of NF-κB is highly relevant in PD, where activated NF-κB is observed in surviving nigral neurons (Ghosh et al., 2007; Hunot et al., 1997) and microglial cells (Ghosh et al., 2007), increased NF-κB level is detected in striatum and SN (Mogi et al., 2007) and persistent NF-κB activation is associated with increased inflammatory responses and degeneration of dopaminergic neurons in animal models of PD (Glass et al., 2010; Hirsch and Hunot, 2009; Mattson and Meffert, 2006; Tansey and Goldberg, 2010). In this study, we investigated the role of RNF11, a negative regulator of NF-κB (Pranski et al., 2012a; Shembade et al., 2009), in PD-associated neurodegeneration by manipulation of RNF11 expression in in vivo and in vitro models of PD. Our studies demonstrated that in dopaminergic cells, RNF11 (1) is a negative regulator of NF-κB and (2) can modulate susceptibility to 6-OHDA toxicity through NF-κB mediated responses, including induction of TNF-α, antioxidants and anti-apoptotic factors. Interestingly, RNF11 over-expression enhanced dopaminergic cell death through decreased NF-κB responses while RNF11 knock-down substantially protected against 6-OHDA toxicity via enhanced NF-κB activation. Together, our in vivo and in vitro studies provide compelling evidence that RNF11 can modulate NF-κB activation in dopaminergic neurons with critical influences on PD-associated neurodegeneration.
RNF11, as a negative regulator of the canonical NF-κB signaling pathway (Pranski et al., 2012a; Shembade et al., 2009), is expected to have a crucial role in most NF-κB-mediated cellular function including inflammatory responses, similar to A20 (Lee et al., 2000). In this study we chose to examine the influence of RNF11 on vulnerability of dopaminergic cells to 6-OHDA, a PD toxin. 6-OHDA has been extensively used in cell culture systems (Cova et al., 2012; Grau and Greene, 2012; Lu et al., 2012) and in animals to model PD (Betarbet et al., 2002; Bezard et al., 2012). Moreover, 6-OHDA has been used in various cell culture systems to demonstrate that activation of NF-κB signaling pathway (Liang et al., 2007; Zhang et al., 2011) and TNF-α (Lee et al., 2009; McCoy et al., 2006; McCoy and Tansey, 2008) are toxic to nigral dopaminergic neurons. The unique aspect of our experimental paradigm, however, was that we manipulated NF-κB activation through expression of RNF11 in the nigral neurons specifically targeted by 6-OHDA. Interestingly, our in vivo and in vitro experiments demonstrated that RNF11-mediated inhibition of NF-κB exacerbated 6-OHDA toxicity while reduced expression of RNF11 and consequent increased NF-κB activation protected against 6-OHDA neurodegeneration.
This outcome was surprising to us since it is generally believed that in PD, NF-κB activation and increased expression of TNF-α are toxic for dopaminergic neurons and promote neurodegeneration (Cao et al., 2010; Shembade et al., 2007; Verstrepen et al., 2010). Moreover, inhibitors of the NF-κB pathway have been shown to confer neuroprotection in PD models (Flood et al., 2011; Ghosh et al., 2007; Tran et al., 2008) by diminishing inflammatory responses. An inflammatory response is commonly defined as a response mounted by immune cells, including microglial cells of the central nervous system, that includes NF-κB activation and subsequent transcription of numerous cytokines (ie., TNF-α) and chemokines that perpetuate this response (Flood et al., 2011; Glass et al., 2010). Indeed, the importance of microglial NF-κB activation and TNF-α in PD is well documented (Aoki et al., 2009; Kaltschmidt et al., 2005; Minghetti et al., 2005; Wilms et al., 2007). Modulation of microglial NF-κB through various factors including regulator of G-protein signaling 10 (RGS10), nuclear receptor related 1 protein (NURR1), Fcγ receptors, and cluster of differentiation 200 (CD200) receptors (Cao et al., 2010; Lee et al., 2011; Saijo et al., 2009; Wang et al., 2007) have specifically shown that it is microglial NF-κB activation and the subsequent increase in TNF-α that are harmful to dopaminergic neurons. In addition, others have demonstrated that increased microglial activation and TNF-α levels are detrimental to dopaminergic cell survival in various PD models (ie., alpha-synuclein and toxin models) (Aoki et al., 2009; Ferger et al., 2004; McCoy et al., 2006). Thus neurons appear to be passive targets of immune offensives following microglial NF-κB activation.
Our study, however, suggests that NF-κB modulators such as RNF11 can influence neurons, including dopaminergic neurons, to mount a ‘protective response’ characterized by NF-κB activation and TNF-α when exposed to an insult. Though we have demonstrated this phenomenon for the first time in a PD model, there are numerous reports that show activation of neuronal NF-κB and increased TNF-α expression confers protection against neuronal toxicity (Kaltschmidt et al., 2005; Mattson and Meffert, 2006). For instance, pretreatment with TNF-α conferred protection in hippocampal neurons exposed to metabolic and excitotoxic insults (Cheng et al., 1994; Mattson et al., 1997; Tamatani et al., 1999). This protection was dependent upon NF-κB activation which increased production of anti-apoptotic proteins such as BCL2, consistent with what we found. Similar protective effects of NF-κB activation are observed in cortical and sensory neurons (Fernyhough et al., 2005; Marchetti et al., 2004). Likewise, similar protection with NF-κB activation is found in disease models. With AD, there is increased neuronal vulnerability to amyloid β with inhibition of NF-κB that can be reversed with TNF-α-mediated NF-κB activation (Barger et al., 1995). It is hypothesized that NF-κB activation is protective when observed in AD patients, as NF-κB activation around plaques diminishes with disease progression (Kaltschmidt et al., 1999). Similarly, inhibitors of NF-κB enhanced apoptosis in response to neurotoxins in dopaminergic cells (Taglialatela et al., 1997), hippocampal neurons (Albensi and Mattson, 2000), and sympathetic neurons (Maggirwar et al., 1998). Further, TNF-α null mice show increased neuronal death after ischemia (Bruce et al., 1996) and TNFR knockout mice have exacerbated cortical neuron death in a traumatic brain injury model (Sullivan et al., 1999).
This protective role of neuronal NF-κB activation is not entirely unexpected given the diverse roles of NF-κB. Persistent NF-κB activation and subsequent increase in TNF-α have been linked to both cell survival and cell toxicity (Costanzo et al., 2011; Galardi et al., 2011; Gordon et al., 2011; Li et al., 2012; Mattson and Meffert, 2006) against multitude of cellular insults in different disease model systems, indicating that NF-κB signaling may induce different outcomes in a context-specific manner. Furthermore, NF-κB is expressed in both neurons and glia (O'Neill and Kaltschmidt, 1997). Additionally, NF-κB activation can regulate transcription of both pro- and anti-survival factors (Blum et al., 2001; McCoy and Tansey, 2008).
In fact it is the NF-κB transcribed pro-survival factors that seem to be the underlying cause for neuroprotection observed in our studies. Dopaminergic cells are particularly susceptible to oxidative stress (Graham et al., 1978), while neurotrophic and anti-apoptotic factors can confer protection against environmental and genetic risk factors associated with PD pathogenesis (Mangano et al., 2011; Nagahara and Tuszynski, 2011; Rieker et al., 2012; Stahl et al., 2011; Yin et al., 2012). We showed that in cases of increased protection against 6-OHDA-induced death, such as following neuronal RNF11 knockdown, there is an associated increase in expression of (1) antioxidants GSS and SOD1, (2) neurotrophic factor BDNF and (3) anti- apoptotic factor BCL2. Additionally, we showed that this increase is dependent on NF-κB activation, suggesting that NF-κB transcribed expression of antioxidants and anti-apoptotic factors may contribute to dopaminergic cell survival. BDNF, though still conferring protection, appears to be transcribed by an IκB independent pathway. Thus our in vitro and in vivo data lead us to conclude that RNF11-mediated NF-κB responses in dopaminergic cells can alter cell survival in PD models of degeneration.
We would like to acknowledge that in our in vivo experiments, RNF11-mediated neuroprotective or neurotoxic effects were modest though statistically significant. Transduction efficiency as well as extent of RNF11 suppression in vivo could impact the effects of RNF11 on neuronal survival observed in our studies. Moreover, given that PD is a multifactorial disorder (Hirsch, 1999; Jellinger, 2001; Tatton, 2000; Tatton and Chalmers-Redman, 1998), NF-κB is one of the many pathways involved in 6-OHDA-induced toxicity (Liang et al., 2007) similar to numerous mechanisms involved in PD pathogenesis that could together influence neuronal survival.
Normal expression of A20 ubiquitin-editing protein complex components, including RNF11, is important for normal homeostasis in non-dividing neurons to maintain optimum NF-κB function since this pathway is important for synaptic plasticity and neuronal stability (Boersma et al., 2011; Imielski et al., 2012; Mattson and Camandola, 2001). However, in neurodegenerative disease such as PD, alterations in any of the A20 complex components could modulate NF-κB signaling to alleviate or exaggerate stress and toxicity in cell-specific context. Our finding that PD patients have reduced RNF11 protein expression in cortical and nigral neurons suggest persistence of NF-κB signaling in these neurons. Indeed activated NF-κB has been reported in nigral neurons in PD cases (Ghosh et al., 2007; Hunot et al., 1997). Together with our in vitro and in vivo data, it may appear that reduced RNF11 expression levels in surviving neurons may be a protective and compensatory response to PD-associated stressors, including glia-mediated inflammatory responses, oxidative stress, and mitochondrial dysfunction (Kreutzberg, 1996). Further investigation to better understand the modulation of NF-κB signaling by RNF11, in contexts of diverse central nervous system cell types (including glial cells) and specific insults, is critical for our understanding of the contribution of RNF11 to PD pathogenesis. Additionally, it will be important to explore factors that regulate expression of RNF11, such as miR-19 RNA (Gantier et al., 2012) to create a more targeted therapeutic intervention for PD. For now, our work has characterized RNF11 as a negative regulator of NF-κB signaling in PD-related neurodegeneration and provides a foundation for future investigations into PD therapeutics.
Supplementary Material
A. PC12 cells were transiently co-transfected with pAAV constructs (empty pAAV control, pAAV-shScramble, pAAV-shRNF11, or pAAV-V5-RNF11), as well as luciferase and Renilla plasmids and stimulated with saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 6 hours. Values represent the fold change in NF-κ B-dependent activity as measured by the ratio of luciferase to Renilla luminescence in stimulated over unstimulated samples ± SEM in triplicate experiments. Results were analyzed by one-way ANOVA with Bonferroni post-tests. Statistical comparisons refer to pAAV-shScramble cells of the same treatment. B, C, D. PC12 cells were transiently transfected with empty pAAV control, pAAV-shScramble, pAAV-shRNF11, or pAAV-V5-RNF11. Cells were stimulated with saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 0, 30, or 120 minutes. Cells were immunostained for p65 and nuclear DNA (Hoechst 333258). Cells were analyzed for overlap of p65-positive and Hoechst 333258-positive pixels. The fold change in percentage overlap of p65- and Hoechst 333258-positive pixels was calculated relative to unstimulated conditions. Results were analyzed by two-way repeated measures ANOVA with Bonferroni post-tests. a, P < 0.001 compared to pAAV-shScramble cells at 30 minutes, b, P < 0.01 compared to pAAV-shScramble cells at 60 minutes, c, P < 0.001 compared to pAAV-shScramble cells at 60 minutes, d, P < 0.001 compared to pAAV-shScramble cells at 120 minutes.
A. Total RNA was extracted from murine primary neurons that were untransduced or transduced with shScramble, shRNF11, or V5-RNF11 AAV2 constructs, and examined by qRT-PCR for expression of RNF11 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by one-way ANOVA with Dunnett’s multiple comparisons post-tests. ***, P < 0.001. B. Murine primary neurons were transduced with shScramble, shRNF11, or V5-RNF11 AAV2 constructs and stimulated for 0, 30, or 120 min with TNF-α before being immunostained for p65 and Hoechst 333258. Transduced cells were analyzed for overlap of p65- and Hoechst 333258-positive pixels. The fold change in percentage overlap of p65- and Hoechst 333258-positive pixels was calculated relative to unstimulated conditions. Values represent means ± SEM of at least 50 cells in triplicate experiments. Results were analyzed by two-way ANOVA with Bonferroni selected multiple comparisons post-tests. ***, P < 0.001, **, P < 0.01 compared to AAV2-shScramble cells.
Animals were stereotaxically injected with AAV2 (tagged with GFP or V5) constructs in the nigra. Representative images of TH (red) and GFP (green) staining at the level of the substantia nigra (low magnification in A and high magnification in B) and striatum (C) in the uninjected and AAV2-injected hemispheres showing spread of AAV2 infection throughout the nigrostriatal pathway. Scale bar for A: 200 µM. Scale bar for B and C: 50 µM. D. Representative images of GFP- (green) and MAP2-positive (red) cells at the level of the substantia nigra in AAV2-injected hemisphere. Note the extensive co-localization of GFP with MAP2. Scale bar: 20 µM. E, Representative images of GFP- (green) and Iba1-positive (red) cells at the level of the substantia nigra in AAV2-injected hemisphere. Note the absence of co-localization. Scale bar: 20 µM.
A. Total RNA was extracted from neocortical tissue of control, Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and Parkinson’s disease (PD) cases and examined by real-time PCR for expression of A20 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GUSB levels as an internal control. B. Frontal cortex from control and PD cases was analyzed by immunohistochemistry using an antibody against Itch. Scale bar: 50 µM.
Highlights.
RNF11 modulates susceptibility to 6-OHDA toxicity through NF-κB signaling
Protection against 6-OHDA toxicity involves antioxidants and anti-apoptotic factors
Neuronal RNF11 acts as a negative regulator of NF-κB signaling in vivo
RNF11 expression is reduced in PD cortical and nigral tissue
Acknowledgments
We thank members of the Lah/Levey, Tansey, and Seyfried labs for constructive discussion regarding this manuscript, and the National Institutes of Health through the Alzheimer’s Disease Research Center grant (AG025688), NIEHS ES015777 (RSB), NIEHS ES012870 (ELP), and NINDS NS007480 (ELP). This research project was supported in part by the viral vector and microscopy cores of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AAV
adeno-associated virus
- AD
Alzheimer’s disease
- ANOVA
analysis of variance
- AP
anteroposterior
- BDNF
brain-derived neurotrophic factor
- CD200
Cluster of Differentiation 200
- DLB
dementia with Lewy bodies
- DV
dorsoventral
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSS
glutathione synthetase
- GUSB
beta-glucuronidase
- IKK
IκB kinase
- ML
mediolateral
- NF-κB
nuclear factor κB
- NURR1
nuclear receptor related 1 protein
- qRT-PCR
quantitative real-time PCR
- PD
Parkinson’s disease
- RGS10
regulator of G-protein signaling 10
- RNF11
RING finger protein 11
- SEM
standard error of the mean
- shRNA
small hairpin RNA
- SN
substantia nigra
- SNpc
SN pars compacta
- SOD1
superoxide dismutase 1
- TAX1BP1
Tax1 (human T-cell leukemia virus type I) binding protein 1
- TH
tyrosine hydroxylase
- TNF
tumor necrosis factor
- VM
ventral mesencephalic
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse(New York, N.Y.) 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Anderson LR, et al. PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J Neuropathol Exp Neurol. 2007;66:955–964. doi: 10.1097/nen.0b013e3181567f17. [DOI] [PubMed] [Google Scholar]
- Aoki E, et al. Role of nuclear transcription factor kappa B (NF-kappaB) for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice. Experimental and Molecular Pathology. 2009;86:57–64. doi: 10.1016/j.yexmp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Barger SW, et al. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, et al. Properly scaled and targeted AAV2-NRTN (neurturin) to the substantia nigra is safe, effective and causes no weight loss: Support for nigral targeting in Parkinson's disease. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Betarbet R, et al. Animal models of Parkinson's disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Betarbet R, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bezard E, et al. Animal models of Parkinson's disease: Limits and relevance to neuroprotection studies. Movement Disorders. 2012 doi: 10.1002/mds.25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Boersma MCH, et al. A Requirement for Nuclear Factor-κB in Developmental and Plasticity-Associated Synaptogenesis. The Journal of Neuroscience. 2011;31:5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, et al. Cognitive decline correlates with neuropathological stage in Parkinson's disease. J Neurol Sci. 2006;248:255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nature medicine. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- Cao S, et al. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson's disease. Molecular Neurodegeneration. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Cheng B, et al. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. 2012 doi: 10.1038/nrneurol.2012.126. advance online publication. [DOI] [PubMed] [Google Scholar]
- Costanzo A, et al. In: NF-κB, IκB Kinase and Interacting Signal Networks in Squamous Cell Carcinomas Signaling Pathways in Squamous Cancer. Glick AB, Waes CV, editors. New York: Springer; 2011. pp. 201–222. [Google Scholar]
- Cova L, et al. Neuroprotective effects of human mesenchymal stem cells on neural cultures exposed to 6-hydroxydopamine: implications for reparative therapy in Parkinson's disease. Apoptosis. 2012;17:289–304. doi: 10.1007/s10495-011-0679-9. [DOI] [PubMed] [Google Scholar]
- Crossman AR. Neural mechanisms in disorders of movement. Comp Biochem Physiol A. 1989;93:141–149. doi: 10.1016/0300-9629(89)90201-6. [DOI] [PubMed] [Google Scholar]
- Davis AA, et al. Deletion of M1 Muscarinic Acetylcholine Receptors Increases Amyloid Pathology In Vitro and In Vivo. The Journal of Neuroscience. 2010;30:4190–4196. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, et al. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson's disease. Exp Neurol. 2012;235:306–315. doi: 10.1016/j.expneurol.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Elkon H, et al. Oxidative stress, induced by 6-hydroxydopamine, reduces proteasome activities in PC12 cells: implications for the pathogenesis of Parkinson's disease. Journal of molecular neuroscience : MN. 2004;24:387–400. doi: 10.1385/JMN:24:3:387. [DOI] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's Disease as a Clinical Syndrome. Annals of the New York Academy of Sciences. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Ferger B, et al. Genetic ablation of tumor necrosis factor-alpha (TNF-α) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. Journal of neurochemistry. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, et al. Activation of nuclear factor-kappaB via endogenous tumor necrosis factor alpha regulates survival of axotomized adult sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1682–1690. doi: 10.1523/JNEUROSCI.3127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood PM, et al. Transcriptional Factor NF-kappaB as a Target for Therapy in Parkinson's Disease. Parkinsons Dis. 2011;2011 doi: 10.4061/2011/216298. 216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon T, et al. Does neuroinflammation fan the flame in neurodegenerative diseases? Molecular Neurodegeneration. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galardi S, et al. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Research. 2011;39:3892–3902. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantier MP, et al. A miR-19 regulon that controls NF-κB signaling. Nucleic Acids Research. 2012 doi: 10.1093/nar/gks521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson's disease. Neurobiology of disease. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gerhard A, et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiology of disease. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. The Journal of comparative neurology. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Ghosh A, et al. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proceedings of the National Academy of Sciences. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annual review of neuroscience. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Gordon JW, et al. Multiple Facets of NF-κB in the Heart. Circulation Research. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- Graham DG, et al. Autoxidation versus Covalent Binding of Quinones as the Mechanism of Toxicity of Dopamine, 6-Hydroxydopamine, and Related Compounds toward C1300 Neuroblastoma Cells in Vitro. Molecular Pharmacology. 1978;14:644–653. [PubMed] [Google Scholar]
- Grau CM, Greene LA. In: Use of PC12 Cells and Rat Superior Cervical Ganglion Sympathetic Neurons as Models for Neuroprotective Assays Relevant to Parkinson’s Disease Neurotrophic Factors. Skaper SD, editor. Humana Press; 2012. pp. 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Experimental Neurology. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hamza TH, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC. Mechanism and consequences of nerve cell death in Parkinson's disease. Journal of neural transmission. Supplementum. 1999;56:127–137. doi: 10.1007/978-3-7091-6360-3_7. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? The Lancet Neurology. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, et al. Neuroinflammatory processes in Parkinson's disease. Parkinsonism & Related Disorders. 2005;11:S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Holtz WA, et al. Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death evoked by parkinsonian mimetics. Journal of neurochemistry. 2006;99:54–69. doi: 10.1111/j.1471-4159.2006.04025.x. [DOI] [PubMed] [Google Scholar]
- Hunot S, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson CB, et al. Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. Journal of neurotrauma. 2011;28:1783–1801. doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imielski Y, et al. Regrowing the Adult Brain: NF-κB Controls Functional Circuit Formation and Tissue Homeostasis in the Dentate Gyrus. PLoS ONE. 2012;7:e30838. doi: 10.1371/journal.pone.0030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Cell death mechanisms in neurodegeneration. Journal of cellular and molecular medicine. 2001;5:1–17. doi: 10.1111/j.1582-4934.2001.tb00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, et al. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proceedings of the National Academy of Sciences. 1999;96:9409–9414. doi: 10.1073/pnas.96.16.9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, et al. Signaling via NF-κB in the nervous system. Biochimica et Biophysica Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Kirik D, et al. Characterization of Behavioral and Neurodegenerative Changes Following Partial Lesions of the Nigrostriatal Dopamine System Induced by Intrastriatal 6-Hydroxydopamine in the Rat. Experimental neurology. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krikos A, et al. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. Journal of Biological Chemistry. 1992;267:17971–17976. [PubMed] [Google Scholar]
- Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-K, et al. Regulator of G-Protein Signaling-10 Negatively Regulates NF-κB in Microglia and Neuroprotects Dopaminergic Neurons in Hemiparkinsonian Rats. The Journal of Neuroscience. 2011;31:11879–11888. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-K, et al. Neuroinflammation in Parkinson's Disease. Journal of Neuroimmune Pharmacology. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. Forkhead transcription factor FOXO3a activates NF-kB through B-cell lymphoma/leukemia (BCL10) and promotes tumor cell survival in serum-deprivation. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M111.291708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang ZQ, et al. NF-kappaB contributes to 6-hydroxydopamine-induced apoptosis of nigral dopaminergic neurons through p53. Brain Res. 2007;1145:190–203. doi: 10.1016/j.brainres.2007.01.130. [DOI] [PubMed] [Google Scholar]
- Liu S, Chen ZJ. Expanding role of ubiquitination in NF-kappaB signaling. Cell Res. 2011;21:6–21. doi: 10.1038/cr.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, et al. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D-Y, et al. Resistin protects against 6-hydroxydopamine-induced cell death in dopaminergic-like MES23.5 cells. Journal of Cellular Physiology. 2012 doi: 10.1002/jcp.24163. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, et al. Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:10356–10365. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano EN, et al. Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson's disease. Neurobiology of disease. 2011;43:99–112. doi: 10.1016/j.nbd.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Marchetti L, et al. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-D-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. The Journal of biological chemistry. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S. NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, et al. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. Journal of neuroscience research. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell death and differentiation. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Mazzon C, et al. Agrin is required for survival and function of monocytic cells. Blood. 2012;119:5502–5511. doi: 10.1182/blood-2011-09-382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, et al. Blocking Soluble Tumor Necrosis Factor Signaling with Dominant-Negative Tumor Necrosis Factor Inhibitor Attenuates Loss of Dopaminergic Neurons in Models of Parkinson's Disease. The Journal of Neuroscience. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, et al. Microglial activation in chronic neurodegenerative diseases: roles of apoptotic neurons and chronic stimulation. Brain Research Reviews. 2005;48:251–256. doi: 10.1016/j.brainresrev.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) Neurology. 1991;41:479. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mogi M, et al. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mulcahy P, et al. Development and characterisation of a novel rat model of Parkinson's disease induced by sequential intranigral administration of AAV-alpha-synuclein and the pesticide, rotenone. Neuroscience. 2012;203:170–179. doi: 10.1016/j.neuroscience.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Noureddine MA, et al. Genomic convergence to identify candidate genes for Parkinson disease: SAGE analysis of the substantia nigra. Mov Disord. 2005;20:1299–1309. doi: 10.1002/mds.20573. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends in neurosciences. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Panet H, et al. Activation of nuclear transcription factor kappa B (NF-kappaB) is essential for dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:391–398. doi: 10.1046/j.1471-4159.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Pierce JW, et al. Novel Inhibitors of Cytokine-induced IκBα Phosphorylation and Endothelial Cell Adhesion Molecule Expression Show Anti-inflammatory Effects in Vivo. Journal of Biological Chemistry. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Pranski EL, et al. Neuronal RING finger protein 11 (RNF11) regulates canonical NF-kappaB signaling. J Neuroinflammation. 2012a;9:67. doi: 10.1186/1742-2094-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranski EL, et al. Comparative distribution of protein components of the A20 ubiquitin-editing complex in normal human brain. Neurosci Lett. 2012b;520:104–109. doi: 10.1016/j.neulet.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieker C, et al. Ablation of serum response factor in dopaminergic neurons exacerbates susceptibility towards MPTP-induced oxidative stress. European Journal of Neuroscience. 2012;35:735–741. doi: 10.1111/j.1460-9568.2012.08003.x. [DOI] [PubMed] [Google Scholar]
- Roca V, et al. The degenerating substantia nigra as a susceptible region for gene transfer-mediated inflammation. Parkinson's disease. 2011;2011 doi: 10.4061/2011/931572. 931572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruland J. Return to homeostasis: downregulation of NF-[kappa]B responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- Saijo K, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, et al. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 2007;26:3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, et al. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol. 2008;9:254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- Shembade N, et al. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. Embo J. 2009;28:513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl K, et al. Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson's disease. Brain research. 2011;1378:105–118. doi: 10.1016/j.brainres.2010.12.090. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, et al. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglialatela G, et al. Inhibition of nuclear factor kappa B (NFkappaB) activity induces nerve growth factor-resistant apoptosis in PC12 cells. Journal of neuroscience research. 1997;47:155–162. [PubMed] [Google Scholar]
- Tamatani M, et al. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. The Journal of biological chemistry. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabin V, Schwaninger M. The role of NF-kappaB in 6-hydroxydopamine- and TNFalpha-induced apoptosis of PC12 cells. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:563–569. doi: 10.1007/s00210-004-0938-1. [DOI] [PubMed] [Google Scholar]
- Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM. Mitochondria in neurodegenerative apoptosis: an opportunity for therapy? Annals of Neurology. 1998;44:S134–S141. doi: 10.1002/ana.410440720. [DOI] [PubMed] [Google Scholar]
- Ton TG, et al. Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Movement Disorders. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- Tran TA, et al. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J Neuroinflammation. 2008;5:14. doi: 10.1186/1742-2094-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstrepen L, et al. Expression, biological activities and mechanisms of action of A20 (TNFAIP3) Biochem Pharmacol. 2010;80:2009–2020. doi: 10.1016/j.bcp.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, et al. Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. J Biol Chem. 2001;276:47590–47598. doi: 10.1074/jbc.M106535200. [DOI] [PubMed] [Google Scholar]
- Wang X-J, et al. CD200–CD200R Regulation of Microglia Activation in the Pathogenesis of Parkinson's Disease. Journal of Neuroimmune Pharmacology. 2007;2:259–264. doi: 10.1007/s11481-007-9075-1. [DOI] [PubMed] [Google Scholar]
- Wilms H, et al. Inflammation in Parkinsons Diseases and Other Neurodegenerative Diseases: Cause and Therapeutic Implications. Current Pharmaceutical Design. 2007;13:1925–1928. doi: 10.2174/138161207780858429. [DOI] [PubMed] [Google Scholar]
- Yin Y, et al. Modulation of neuronal survival factor MEF2 by kinases in Parkinson's disease. Frontiers in Physiology. 2012;3 doi: 10.3389/fphys.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson's disease. Journal of Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu TG, et al. Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neuroscience letters. 2012;506:175–179. doi: 10.1016/j.neulet.2011.10.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. PC12 cells were transiently co-transfected with pAAV constructs (empty pAAV control, pAAV-shScramble, pAAV-shRNF11, or pAAV-V5-RNF11), as well as luciferase and Renilla plasmids and stimulated with saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 6 hours. Values represent the fold change in NF-κ B-dependent activity as measured by the ratio of luciferase to Renilla luminescence in stimulated over unstimulated samples ± SEM in triplicate experiments. Results were analyzed by one-way ANOVA with Bonferroni post-tests. Statistical comparisons refer to pAAV-shScramble cells of the same treatment. B, C, D. PC12 cells were transiently transfected with empty pAAV control, pAAV-shScramble, pAAV-shRNF11, or pAAV-V5-RNF11. Cells were stimulated with saline (vehicle), 20 µM Bay11-7085, or 10 ng/ml TNF-α for 1 hour followed by saline or 100 µM 6-OHDA for 0, 30, or 120 minutes. Cells were immunostained for p65 and nuclear DNA (Hoechst 333258). Cells were analyzed for overlap of p65-positive and Hoechst 333258-positive pixels. The fold change in percentage overlap of p65- and Hoechst 333258-positive pixels was calculated relative to unstimulated conditions. Results were analyzed by two-way repeated measures ANOVA with Bonferroni post-tests. a, P < 0.001 compared to pAAV-shScramble cells at 30 minutes, b, P < 0.01 compared to pAAV-shScramble cells at 60 minutes, c, P < 0.001 compared to pAAV-shScramble cells at 60 minutes, d, P < 0.001 compared to pAAV-shScramble cells at 120 minutes.
A. Total RNA was extracted from murine primary neurons that were untransduced or transduced with shScramble, shRNF11, or V5-RNF11 AAV2 constructs, and examined by qRT-PCR for expression of RNF11 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GAPDH levels as an internal control. Results were analyzed by one-way ANOVA with Dunnett’s multiple comparisons post-tests. ***, P < 0.001. B. Murine primary neurons were transduced with shScramble, shRNF11, or V5-RNF11 AAV2 constructs and stimulated for 0, 30, or 120 min with TNF-α before being immunostained for p65 and Hoechst 333258. Transduced cells were analyzed for overlap of p65- and Hoechst 333258-positive pixels. The fold change in percentage overlap of p65- and Hoechst 333258-positive pixels was calculated relative to unstimulated conditions. Values represent means ± SEM of at least 50 cells in triplicate experiments. Results were analyzed by two-way ANOVA with Bonferroni selected multiple comparisons post-tests. ***, P < 0.001, **, P < 0.01 compared to AAV2-shScramble cells.
Animals were stereotaxically injected with AAV2 (tagged with GFP or V5) constructs in the nigra. Representative images of TH (red) and GFP (green) staining at the level of the substantia nigra (low magnification in A and high magnification in B) and striatum (C) in the uninjected and AAV2-injected hemispheres showing spread of AAV2 infection throughout the nigrostriatal pathway. Scale bar for A: 200 µM. Scale bar for B and C: 50 µM. D. Representative images of GFP- (green) and MAP2-positive (red) cells at the level of the substantia nigra in AAV2-injected hemisphere. Note the extensive co-localization of GFP with MAP2. Scale bar: 20 µM. E, Representative images of GFP- (green) and Iba1-positive (red) cells at the level of the substantia nigra in AAV2-injected hemisphere. Note the absence of co-localization. Scale bar: 20 µM.
A. Total RNA was extracted from neocortical tissue of control, Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and Parkinson’s disease (PD) cases and examined by real-time PCR for expression of A20 mRNA. Values expressed are mean comparative cycle threshold ± SEM with GUSB levels as an internal control. B. Frontal cortex from control and PD cases was analyzed by immunohistochemistry using an antibody against Itch. Scale bar: 50 µM.