Abstract
Objective
Nogo-B, also known as reticulon 4B, promotes liver fibrosis and cirrhosis by facilitating the transforming growth factor beta (TGFβ) signaling pathway in activated hepatic stellate cells. The aim of this study was to determine the role of Nogo-B in hepatocyte proliferation and liver regeneration.
Design
Partial hepatectomy (PHx, 70% resection) was performed in male wild-type (WT) and Nogo-A/B knockout mice (referred to as Nogo-B KO). Remnant livers were isolated 2 hours, 5 hours, 1, 2, 3, 7 and 14 days after PHx. Hepatocyte proliferation was assessed by Ki67 labeling index. Quantitative RT-PCR was performed for genes known to be involved in liver regeneration. Hepatocytes isolated from WT and Nogo-B KO mice were used to examine the role of Nogo-B in IL6, HGF, EGF and TGFβ signaling.
Results
Nogo-B protein levels increased in the regenerating livers in a time-dependent manner after PHx. Specifically, Nogo-B expression in hepatocytes gradually spread from the periportal toward the central areas by 7 days after PHx, but receded notably by 14 days. Nogo-B facilitated IL6/STAT3 signaling, increased HGF-, but not EGF-, induced hepatocyte proliferation and tended to reduce TGFβ1-induced suppression of hepatocyte proliferation in cultured hepatocytes. Lack of Nogo-B significantly induced TGFβ1 and Id1 expression one day after PHx and IL6 and EGF expression two days after PHx. Lack of Nogo-B delayed hepatocyte proliferation, but did not affect the liver to body ratio in the regenerative process.
Conclusion
Nogo-B expression in hepatocytes facilitates hepatocyte proliferation and liver regeneration.
Keywords: hepatocyte, IL6, HGF, TGFβ, liver regeneration
INTRODUCTION
Liver has a remarkable ability to regenerate, achieving this through a wide range of molecules and redundant signaling pathways in all adult liver cells(1–3). The mechanisms governing the process of liver regeneration are complex and remain to be fully elucidated.
Nogo-B, also known as reticulon 4B, belongs to the reticulon family of proteins that primarily reside in the endoplasmic reticulum (ER)(4, 5) and regulate ER structure(6–8), protein trafficking(9) and cell signaling(10). There are four groups of reticulons, 1, 2, 3 and 4 with each having multiple isoforms. Reticulon 4 has three isoforms, Nogo-A, B and C. Nogo-A (200 kDa), the most recognized isoform, is a potent neural outgrowth inhibitor(11–13) and mainly expressed in the nervous system(14). Nogo-C (25 kDa) is expressed in differentiated muscle fibers and in the brain(4, 5, 14). Its function is unknown. Nogo-B (55 kDa in human and 45 kDa in rats/mice), a splice variant of Nogo-A, is expressed in most tissues and has been implicated in regulation of endothelial and smooth muscle cellular responses following injury in blood vessels(15, 16), lung(17, 18), kidney(19), and liver(10).
In the liver, Nogo-B contributes to the progression of fibrosis and cirrhosis by facilitating transforming growth factor beta (TGFβ) signaling in activated hepatic stellate cells(10). Liver cirrhosis is characterized by impaired hepatocyte proliferation. In addition, TGFβ signaling plays a crucial role in liver regeneration(20, 21). These observations suggest Nogo-B’s link to the regenerative process. Thus, the aim of this study was to determine the role of Nogo-B in liver regeneration.
Hepatocyte growth factor (HGF) and epidermal growth factor (EGF) are potent mitogens for hepatocytes(22–24), and interleukin-6 (IL6) is an important cytokine for hepatocyte proliferation(25). The lack of these proteins or inactivation of their corresponding signaling pathways in mice results in delayed liver regeneration(26–29), although it does not block liver regeneration. However, delayed liver regeneration could cause life-threatening consequences in the setting of acute liver failure(1). In contrast to IL6, HGF and EGF, TGFβ1 is one of the most well-known hepatocyte anti-proliferative factors(1–3), thereby regulating the termination of liver regeneration and keeping hepatocytes quiescent under normal conditions(30).
The role of Nogo-B in the liver physiology and pathophysiology is still largely unknown. We thus explored Nogo-B’s role in liver regeneration with a focus on these proteins using the partial hepatectomy model in mice.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Institutional Animal Care and Use Committees of Yale University and Veterans Affairs Connecticut Healthcare System, and performed in adherence with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Nogo-A/B knockout (Nogo-B KO) mice were a gift from Stephen Strittmatter(31) and Mark Tessier-Lavigne(32). Nogo-B KO mice were backcrossed x7 generations to C57BL/6(16).
Partial hepatectomy
Two month-old male Nogo-B KO (Nogo-A/B −/−) and age-matched wild-type (WT, Nogo-A/B +/+) mice were subjected to resection of two-thirds of the liver as described(33) with minor modifications. The mice were sacrificed at 2 hours, 5 hours, 1, 2, 3, 7 and 14 days after surgery. The regenerating liver was isolated from each mouse and aliquots were fixed for histology or snap-frozen in liquid nitrogen for molecular and biochemical analyses.
Mouse tissue histology and immunohistochemistry
Formalin-fixed liver samples, embedded in paraffin, were cut to six-µm-thick sections. The sections were stained with Hematoxylin and Eosin (H&E) for structural evaluation. For immunohistochemistry, the sections were rehydrated and antigen retrieval was performed. Primary antibodies used were rabbit anti-Nogo serum (1761A, 1:5000, a kind gift from Dr. William C. Sessa, Yale University) and anti-Ki67 (1:100; Abcam, Cambridge, MA). The sections were incubated with secondary antibodies conjugated with horse radish peroxidase (HRP) (1:500; MILLIPORE, Billerica, MA) at room temperature for 1 hour. Counterstaining was performed using Mayer’s hematoxylin. For immunofluoresence, fluorochrome-conjugated secondary antibodies (Alexafluor anti-rat 488, anti-rabbit 568, 1:500; Invitrogen, Carlsbad, CA) were used. The sections were mounted with Prolong Gold antifade reagent with DAPI (Invitrogen, Grand Island, NY). The sections incubated only with secondary antibodies served as negative controls. Images were taken with a light microscope (Eclipse 80i; Nikon) or fluorescence microscope (Eclipse E800; Nikon). Image J1.41o (Wayne Rasband, NIH) was utilized for image analysis of the entire liver section.
Western blot analysis
Liver samples were homogenized in lysis buffer containing 50mM Tris HCl, 0.1mM EGTA, 0.1mM EDTA, 0.1% SDS, 0.1% deoxycholic acid, 1% (vol/vol) Nonidet P-40, 5mM sodium fluoride, 1mM sodium pyrophosphate, 1mM activated sodium vanadate, 0.32% protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), and 0.027% Pefabloc (Roche Diagnostics). Cultured hepatocytes (see below) were collected in the same lysis buffer. The lysates were centrifuged at 14,000 rpm, 4°C for 10 min. The protein concentration in the supernatants was quantified using the Lowry assay. An equal amount of protein (50–80µg) from each sample was loaded onto SDS-PAGE gels and transferred to nitrocellulose membranes. After blocking, membranes were probed with antibodies that recognize rabbit anti-Nogo serum (1761A, 1:10,000), Hsp90 (1:1000; BD Biosciences, San Jose, CA), phospho-STAT3 (Tyr705) (1:1000; Cell Signaling, Boston, MA), STAT3 (1:1000; Cell Signaling), proliferating cell nuclear antigen (PCNA, 1:1000; Cell Signaling), cyclin D3 (1:1000; Cell Signaling) and β-actin (1:5000; Sigma-Aldrich, St. Louis, MO). Membranes were then incubated with fluorophore-conjugated secondary antibodies (either 680nm or 800nm emission). Detection and quantification of bands were performed using the Odyssey Infrared Imaging System (Li-Cor Biotechnology, Lincoln, NE). Hsp90 was used for a loading control.
Hepatocyte isolation
Hepatocytes were isolated from WT and Nogo-B KO mice by collagenase perfusion and maintained in a collagen sandwich culture system as described(34) with slight modifications. Briefly, cells were cultured on collagen-coated cell culture dishes or glass coverslips in Hepatocyte Maintenance Medium (HMM; Clonetics/Lonza, Basel, Switzerland) supplemented with HMM SingleQuots (Clonetics/Lonza) and 3%-Matrigel (BD Biosciences, San Jose, CA). Matrigel was included only in the initial 6-hour attachment period. The initial plating density was 0.4×106/ml. Six hours later, cells were replaced with medium without any supplementation and incubated for 24 hours, except for the time-course experiment (Figure 2), in which cells were incubated with HMM and HMM SingleQuots throughout the experiment. Cells were then subjected to experiments for IL6, TGFβ, HGF and EGF signaling.
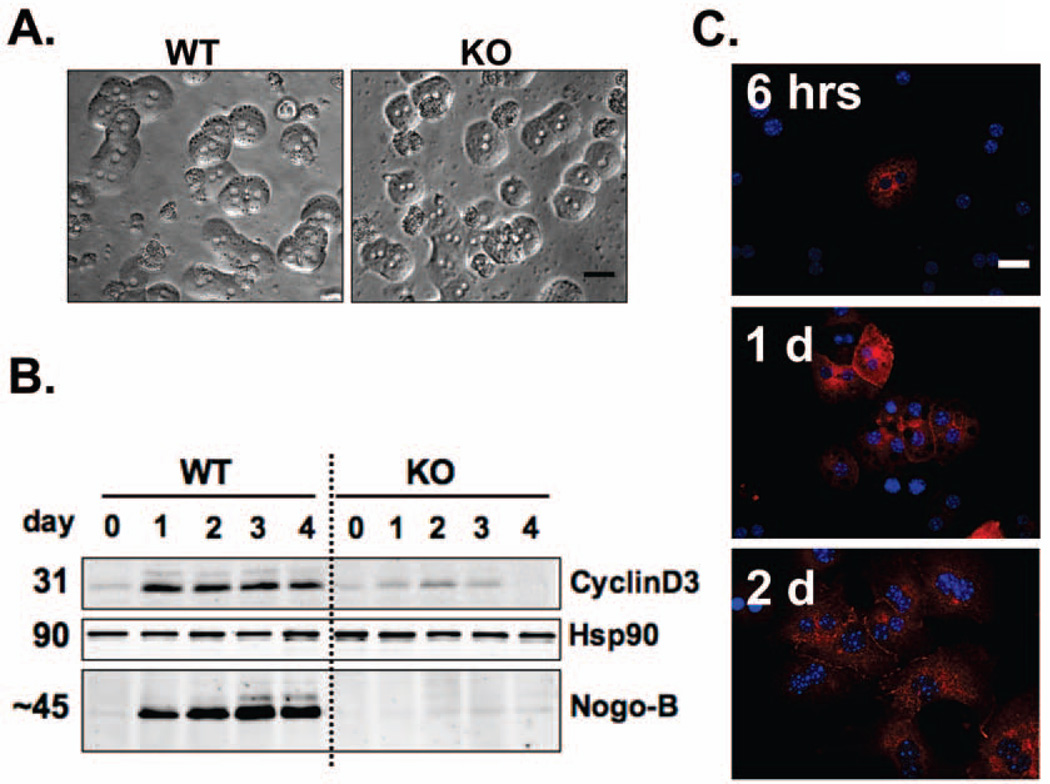
Figure 2. Nogo-B levels increase in cultured hepatocytes in association with up-regulation of an indicator of cell cycle progression.
A. Hepatocytes isolated from wild-type (WT) and Nogo-A/B knockout (Nogo-B KO) mice. Six hours after plating, hepatocytes started to show typical cord formation. Morphological features were similar between WT and Nogo-B KO hepatocytes. Scale bar; 30µm. B. Western blot of Nogo-B in hepatocytes collected after the indicated duration of cell culture. Cell culture medium (hepatocyte maintenance medium; HMM, Clonetics/Lonza) contained supplements (HMM SingleQuots, Clonetics/Lonza) necessary for hepatocyte growth. Nogo-B levels increased in a time-dependent manner in cell culture, in association with up-regulation of cyclin D3. The level of cyclin D3 was markedly lower in Nogo-B KO hepatocytes. Heat shock protein 90 (Hsp90) was used as a loading control. C. Nogo-B positive hepatocytes increased with time in cell culture. Nogo-B (red) was immuno-labeled with rabbit anti-Nogo-B serum (1761A) in cultured hepatocytes 6, 24 and 48 hours after plating. Nuclei were labeled in blue (DAPI). Scale bars; 30µm.
Immunofluorescence of PCNA, pSTAT3 and Nogo
Hepatocytes grown on coverslips were washed in cold PBS, fixed with 4%-paraformaldehyde (PFA), and washed in PBS. Next, cells were fixed with cold methanol at −20°C followed by PBS wash. Nonspecific antibody binding was blocked using 5% donkey serum and 0.3% TritonX100 in PBS. After aspirating the blocking buffer, cells were incubated with mouse anti-PCNA (1:3000; Cell Signaling), rabbit anti-pSTAT3 (1:50, Santa Cruz) or rabbit anti-Nogo (1:5000, 1761A) antibodies diluted in 1% bovine serum albumin (BSA) and 0.3% TritonX-100 in PBS at room temperature for 2 hours for anti-PCNA, 3 hours for anti-pSTAT3 and at 4°C for overnight for anti-Nogo. After washing in PBS, cells were incubated with anti-rabbit or anti mouse secondary antibodies conjugated to Alexa 488 (1:500) or Alexa 568 (1:500) at room temperature for 1 hour. DAPI was used to visualize nuclei. Images were acquired with a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss MicroImaging, Thornwood, NY) and recorded using Openlab3 software (Improvision, Lexington, MA). The number of PCNA positive or pSTAT3 nuclei was divided by the total number of nuclei and expressed as the percentage of total nuclei.
IL6, TGFβ1, HGF and EGF signaling study
Hepatocytes isolated from WT and Nogo-B KO mice were seeded at a density of 3×105/well in 6-well tissue culture plates or 1.5×105/well in 12-well tissue culture plates with or without coverslips. Cells were allowed to attach for 6 hours in the medium described above. Cells were then replaced with the medium without HMM SingleQuots supplement and Matrigel, incubated for 24 hours and stimulated with either of IL6, TGFβ1, HGF or EGF. For the IL6 time-course experiment, cells were incubated with 100ng/ml of recombinant human IL6 (R&D Systems, Minneapolis, MN) for 0, 5, 10, 15, 30 and 60 min. For the IL6 dose-response experiment, cells were incubated for 30 min with 0, 10, 50 and 100ng/ml of IL6. For TGFβ1, HGF and EGF experiments, cells were incubated with 0, 5 or 10ng/ml of recombinant human TGFβ1 (R&D Systems), with 100ng/ml of HGF (R&D Systems) or with 20ng/ml of EGF (Cell Signaling) for 24 or 48 hours. At the end of the experiments, cell lysates were collected and used for western blot analysis.
Quantitative real-time PCR analysis
Total RNA was extracted from approximately 50mg of frozen mouse liver using TRIZOL (Sigma-Aldrich, St. Louis, MO). RNA concentrations were measured using NANO DROP 2000 spectrophotometer (Thermo SCIENTIFIC, Wilmington, DE). Four µg of total RNA was used as a template to synthesize cDNA using SupertScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) and 10mM dNTP Mix (Invitrogen). The initial cDNA was diluted 5-fold prior to use as a real-time quantitative PCR template. Real-time PCR was performed using TaqMan gene expression assays (Applied Biosystems, Foster City, CA) and ABI 7500 SDS software (Applied Biosystems). The TaqMan gene expression assays used were glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Mm99999915_g1), transforming growth factor-β1 (Tgfβ1, Mm01178820_m1), transforming growth factor β receptor 1 (Tgfbr1, Mm00436971_m1), interleukin-6 (IL-6, Mm00446190_m1), vascular endothelial growth factor (VEGF, Mm01281449_m1), kinase insert domain receptor (kdr, Mm01222421_m1), hepatocyte growth factor (HGF, Mm01135193_m1), inhibitor of DNA binding 1(Id1, Mm00775963_g1), epidermal growth factor (EGF, Mm00438696_m1), EGF receptor (EGFR, Mm00433023_m1) and MET (Mm01156972_m1) gene expression.
Statistical analysis
Results are expressed as mean ± SE. Results were assessed using one-way ANOVA followed by Student’s t-test. P values less than 0.05 were considered statistically significant.
RESULTS
Nogo-B levels increase in periportal hepatocytes in regenerating liver after PHx
Nogo-B protein levels increased in the liver in a time-dependent manner until 7 days after PHx and decreased by 14 days (Figure 1A). While strong bands of Nogo-B (~45 kDa) were observed, Nogo-A (200 kDa) was not detected (Figures 1A and S1). In normal adult mouse liver, Nogo-B expression is minimal or absent in hepatocytes, and primarily seen in cholangiocytes and non-parenchymal cells (Figure 1B), as reported in our previous study in rat and human livers(10). However, Nogo-B expression became visible in periportal hepatocytes in WT livers by 2 days after PHx and gradually spread from the periportal to the central areas by 7 days post PHx (Figures 1C and S2). Hepatic Nogo-B declined notably by 14 days (Figure 1C).
Figure 1. Nogo-B expression increases in periportal hepatocytes in regenerating liver after PHx.
A. Nogo-B levels in liver homogenates prepared from wild-type (WT) mouse livers isolated at 0 (at PHx), 1, 2, 3, 7 and 14 days after PHx. β-actin was used as a loading control. B. Immunohistochemistry of Nogo-B in normal WT mouse liver. An enlarged image of the periportal area is presented in the inset. Nogo-B is not expressed in hepatocytes, but in cholangiocytes and non-parenchymal cells. Scale bar; 100µm. Scale bar in the inset; 30µm. C. Immunohistochemistry of Nogo-B in WT mouse livers isolated at 1, 2, 3, 7 and 14 days after PHx. An enlarged image of the periportal area is presented in the inset. Nogo-A/B knockout (Nogo-B KO) liver was used as a negative control. Scale bar; 100µm. Scale bar in the inset; 30µm.
Nogo-B levels increase in cultured hepatocytes in a time-dependent manner
Hepatocytes were isolated from WT and Nogo-B KO mice and cultured in the medium described above. These hepatocytes formed typical cord-like structures and showed no appreciable morphological differences between the two groups (Figure 2A). Nogo-B was not detected in freshly isolated WT hepatocytes prior to cell culture (Day 0 in Figure 2B). However, Nogo-B levels increased in a time-dependent manner in culture (Figure 2B). Further, a proliferation marker, cyclin D3, was up-regulated in WT hepatocytes 1 day after plating, in association with up-regulation of Nogo-B from Day 0 to Day 1, and kept elevated through Day 4 (Figure 2B). In contrast, the level of cyclin D3 was markedly decreased in Nogo-B KO hepatocytes at all time points. Immunofluorescence of Nogo-B in WT hepatocytes also showed that hepatocytes, as judged by their typical arrangement of the nucleus (two nuclei), expressed Nogo-B in a time dependent manner (Figure 2C).
The expression of Nogo-B in cultured hepatocytes may thus be analogous to up-regulation of Nogo-B expression in the periportal areas of regenerating liver in vivo. Therefore, we investigated the effects of Nogo-B induction in hepatocytes on signaling pathways important for liver regeneration.
Nogo-B facilitates the IL6/STAT3 signaling pathway in hepatocytes in response to IL6 treatment
To determine whether the presence of Nogo-B influences downstream signaling cascades of IL6, we examined phosphorylation (i.e., activation) of STAT3 in response to IL6. In both WT and Nogo-B KO hepatocytes, IL6 treatment (100ng/ml) increased STAT3 phosphorylation (pSTAT3) in a time-dependent manner up to 30 min, while IL6 treatment for 60 min returned phosphorylation to the basal level (Figure 3A). However, the level of pSTAT3 in response to IL6 was higher in WT hepatocytes than in KO hepatocytes.
Figure 3. Nogo-B increases STAT3 phosphorylation in hepatocytes in response to Interleukin-6 (IL6) treatment.
A. Time course experiment of hepatocytes isolated from wild-type (WT) and Nogo-A/B knockout (Nogo-B KO) mice, treated with 100ng/ml of IL6 for the indicated duration. Freshly isolated hepatocytes were incubated in hepatocyte maintenance medium (HMM, Clonetics/Lonza) containing supplements (HMM SingleQuots, Clonetics/Lonza) for 6 hours. The medium was replaced with HMM without supplements for 24 hours and stimulated with IL6 for the indicated durations. In both WT and KO hepatocytes, IL6 treatment increased STAT3 phosphorylation in a time-dependent manner up to 30 min and returned to baseline by 60 min. The level of pSTAT3 was higher in WT hepatocytes than in KO hepatocytes. B. Dose-dependence experiment of WT and Nogo-B KO hepatocytes treated for 30 min with the indicated concentrations of IL6. After isolation, hepatocytes were incubated in HMM containing supplements for 6 hours. The medium was replaced with HMM without supplements for 24 hours and stimulated with indicated concentrations of IL6 (0, 10, 50 and 100ng/ml) for 30 min. The level of pSTAT3 increased in a dose-dependent manner and was significantly higher (p<0.01) in WT hepatocytes than in KO hepatocytes at all the doses tested. * p<0.05, ** p<0.01. C. Immunofluorescence of pSTAT3 in hepatocytes treated with IL6. Nuclear pSTAT3 was significantly greater in WT hepatocytes compared with KO hepatocytes in response to IL6. WT and Nogo-B KO hepatocytes were cultured in HMM with supplements for 6 hours. Cells were then incubated in HMM without supplements for 24 hours, followed by IL6 treatment (100ng/ml) for 30 min. The number of pSTAT3 positive nuclei (pink) was divided by the total number of nuclei (DAPI positive cells). Scale bar; 100µm. D. Nogo-B facilitates the IL6/STAT3 signaling pathway in hepatocytes. IL6 binds to its receptor, soluble IL6 receptor (sIL-6r), and induces a signaling pathway through activation of gp130 receptor and Janus kinase (JAK). This leads to STAT3 activation by tyrosine phosphorylation, which causes dimerization of STAT3 and the subsequent translocation into the nucleus and activation of gene transcription.
We also investigated whether Nogo-B influenced the IL6/STAT3 signaling pathway in a dose-dependent manner in hepatocytes. Hepatocytes isolated from WT and Nogo-B KO mice were incubated with indicated concentrations of IL6 for 30 min. The level of pSTAT3 increased in a dose-dependent manner and was significantly higher (p<0.01) in WT hepatocytes than in KO hepatocytes at all the doses tested (0. 10, 50 and 100ng/ml) (Figure 3B).
We further performed immunofluorescence of pSTAT3 in hepatocytes treated with or without IL6 (100ng/ml). Consistently, the level of pSTAT3 significantly increased in WT hepatocytes in response to IL6, compared with KO hepatocytes (Figure 3C). The shape of the nucleus that is positive with pSTAT3 is typical of hepatocytes, indicating that hepatocytes, not other liver cells, respond to IL6 and induce pSTAT3. These observations indicate that the presence of Nogo-B enhances STAT3 phosphorylation in hepatocytes in response to IL6.
In addition, we examined pSTAT3 in liver tissue prepared from WT and Nogo-B KO livers collected 5 hours after PHx. Absence of Nogo-B in the liver significantly decreased pSTAT3 positive nuclei compared with WT liver (Figure S4). This result indicates that loss of Nogo-B impairs IL6/STAT3 signaling in regenerating livers.
Loss of Nogo-B may sensitize hepatocytes to the inhibitory effect of TGFβ1 on proliferation and decreases HGF-, but not EGF-, induced hepatocyte proliferation
We examined proliferation of cultured hepatocytes isolated from WT and Nogo-B KO mice in the presence or absence of TGFβ1 (Figure 4). TGFβ1 treatment for 48 hours with 5 or 10ng/ml significantly decreased PCNA levels in both WT and KO hepatocytes (p<0.01) (Figures 4A middle and 4B bottom). However, there was a trend toward lower PCNA levels in KO hepatocytes than in WT hepatocytes in response to TGFβ1 (p=0.17, 80% vs. 60% reduction, respectively) (Figure 4A middle).
Figure 4. Loss of Nogo-B may sensitize hepatocytes to the inhibitory effect of TGFβ1 on proliferation.
Hepatocytes isolated from wild-type (WT) and Nogo-A/B knockout (Nogo-B KO) mice were cultured in HMM with supplements for 6 hours. Cells were then incubated in HMM without supplements for 24 hours, followed by TGFβ1 treatment for 48 hours. A. Western blot analysis of cell cycle indices, proliferating cell nuclear antigen (PCNA) and cyclin D3, in hepatocytes treated with TGFβ1 for 48 hours. β-actin was used as a loading control. * p<0.05, ** p<0.01. B. PCNA immunolabeling in hepatocytes. Hepatocytes were treated with TGFβ1 (10ng/ml) for 48 hours, followed by immunolabeling of PCNA (green). The number of PCNA positive nuclei was divided by the total number of nuclei labeled with DAPI (blue) and expressed as the percent PCNA positive nuclei. At least 6–8 images were taken for each treatment. Representative images are shown from 3 independent experiments. ** p<0.01. Scale bar; 60µm.
Similarly, TGFβ1 treatment significantly reduced cyclin D3 levels in both groups of hepatocytes (p<0.01), (Figure 4A bottom). The magnitude of reduction in cyclin D3 levels was more pronounced in KO hepatocytes than in WT hepatocytes (p<0.05, 60% vs. 40% reduction, respectively). BrdU incorporation in hepatocytes in response to TGFβ1 tended to be lower in KO hepatocytes than in WT hepatocytes (p=0.067) (Figure S5).
HGF treatment for 24 and 48 hours with 100ng/ml increased PCNA positive nuclei more than 2-fold in WT hepatocytes, while there was no increase in PCNA positive nuclei in KO hepatocytes (Figures 5A bottom and 5B). Consistently, phosphorylation of cMET, a receptor of HGF, was increased 2-fold (p<0.01) in WT hepatocytes in response to HGF treatment (100ng/ml for 24 hour), while only a modest increase was observed in KO hepatocytes (Figure 5C). In contrast, EGF treatment (20ng/ml for 48 hours) increased PCNA levels similarly in both WT and KO hepatocytes (Figure 6). Collectively, these data suggest that Nogo-B may reduce TGFβ1-induced suppression of hepatocyte proliferation and increases HGF-, but not EGF-, induced hepatocyte proliferation.
Figure 5. Nogo-B increases HGF-induced hepatocyte proliferation.
Hepatocytes isolated from wild-type (WT) and Nogo-A/B knockout (Nogo-B KO) mice were cultured in HMM with supplements for 6 hours. Cells were then incubated in HMM without supplements for 24 hours, followed by HGF treatment for 24 and 48 hours. A. Proliferating cell nuclear antigen (PCNA) immunolabeling in hepatocytes. Hepatocytes were treated with HGF (100ng/ml) for 48 hours, followed by immunolabeling of PCNA (green). The number of PCNA positive nuclei was divided by the total number of nuclei labeled with DAPI (blue) and expressed as the percent PCNA positive nuclei. At least 6–8 images were taken for each treatment. Representative images are shown from 3 independent experiments. * p<0.05. Scale bar; 60µm. B. HGF treatment (100ng/ml) for 24 hours significantly increased PCNA levels (** p<0.01) in WT hepatocytes, but not in Nogo-B KO hepatocytes. Heat shock protein 90 (Hsp90) was used as a loading control. A representative blot from 3 independent experiments is presented. C. WT, but not Nogo-B KO, hepatocytes significantly increased phosphorylation of cMET (** p<0.01) in response to HGF treatment (100ng/ml) for 24 hours. A representative blot from 3 independent experiments is presented.
Figure 6. Nogo-B does not affect the mitogenic effect of epidermal growth factor (EGF) in vitro.
Twenty-four hours after the incubation with supplement-free HMM, WT and Nogo-B KO hepatocytes were treated with EGF (20ng/ml) for 48 hours. The number of proliferating cell nuclear antigen (PCNA; 1:3000) positive nuclei (green) was counted and expressed as % PCNA positive nuclei over the total nuclei determined by DAPI staining (blue). * p<0.05 vs control. N=3.
Loss of Nogo-B significantly induces TGFβ1 and Id1 expression one day after PHx and IL6 and EGF expression two days after PHx
Using quantitative real-time PCR analysis, we screened expression of several genes known to be important for liver regeneration, including TGFβ1, Id1, IL6, EGF, EGFR, TGFβRI, HGF, cMET, Kdr and VEGF (Figure 7). Overall, Nogo-B KO livers tended to have higher levels of expression of these genes compared with WT livers. In particular, KO livers showed a significant 1.4-fold increase (p=0.04) in TGFβ1 expression and a 3-fold increase (p=0.03) in Id1 expression one day after PHx. Moreover, KO livers had significantly greater IL6 (3-fold, p=0.03) and EGF (1.7-fold, p=0.05) expression than WT livers two days after PHx. There were no significant differences in EGFR, TGFβRI, HGF, cMET, Kdr or VEGF expression between WT and KO livers.
Figure 7. TGFβ1 and Id1 expression are greater in Nogo-B KO mice than in WT mice one day after PHx. IL-6 and EGF expression are greater in Nogo-B KO mice two days after PHx.
Quantitative PCR analysis was performed in livers collected from wild-type (WT) and Nogo-A/B knockout (Nogo-B KO) mice at each time point of post-PHx. N=6–8 per group at each time point.
Loss of Nogo-B delays the peak of liver regeneration, but liver mass increases similarly between WT and KO mice after PHx
There was no significant difference in the liver to body ratio between WT and Nogo-B KO mice during the regenerative process (Figure 8A). However, the measurement of Ki67 positive nuclei in livers isolated from WT and KO mice at different time points after PHx differed in the time to peak proliferation. Ki67 positive nuclei peaked two days after PHx in WT livers, but three days after PHx in KO livers (Figure 8B). BrdU incorporation in livers isolated from one and two days after PHx also showed its significantly higher levels in WT livers than KO livers two days post-PHx (Figure S3A). In total, these results suggest that hepatocytes of Nogo-B KO livers proliferate more slowly after PHx than those of WT livers.
Figure 8. Lack of Nogo-B delays the peak of hepatocyte proliferation, but does not influence the liver to body weight ratio during liver regeneration after PHx.
A. Liver to body weight ratio. N=7–8 per group at each time point. B. Liver sections were stained with antibody to Ki67 in livers isolated at the indicated time points after PHx. The total number of Ki67 positive nuclei (pink) was divided by the total number of nuclei labeled with DAPI (blue). Green color is due to autofluorescence of the liver. N=7–8 per group at each time point. At least 10 images per liver section were analyzed. ** p<0.01. Scale bar; 100µm.
DISCUSSION
Liver regeneration is a complex process with full participation of all types of mature hepatic cells and involves numerous molecules and signaling pathways(1–3). Hepatocytes are the primary structural and functional cells in the liver. Therefore, their proliferation is critical to the regenerative process. In this study, we found that Nogo-B facilitates hepatocyte proliferation and liver regeneration. Nogo-B is not normally observed in quiescent adult hepatocytes(10). As the liver regenerates after PHx, however, Nogo-B appears and its expression increases in periportal hepatocytes.
Nogo-B facilitates the IL6/STAT3 signaling pathway known to contribute to hepatocyte proliferation and tends to reduce TGFβ1-induced suppression of hepatocyte proliferation in cultured hepatocytes. Nogo-B also increases hepatocyte proliferation in response to HGF. Lack of Nogo-B decreases the Ki67 labeling index and delays its peak. In addition, it is known that proliferation of hepatocytes in response to PHx progresses from the periportal to pericentral areas of the lobule(35). Collectively, these observations suggest that unique appearance of Nogo-B in the periportal areas of regenerating liver supports liver regeneration by facilitating proliferation of hepatocytes. Partial hepatectomy rapidly induces more than 100 genes not expressed in normal liver(1). Given that hepatocyte proliferation starts from the periportal areas after PHx, gene induction is thought to be very active in these areas and Nogo-B is likely one of such induced genes. How Nogo-B is induced in hepatocytes after PHx is an important area to be explored in the future.
Despite the delay in hepatocyte proliferation in Nogo-B KO livers, there was no significant difference in the liver to body weight ratio between WT and Nogo-B KO mice throughout the course of liver regeneration. We speculate that the lack of Nogo-B stimulated compensatory pathways, which offset diminished hepatocyte proliferation, thus maintaining the liver to body weight ratio similar to WT mice.
Examination of the changes in expression of genes known to be involved in liver regeneration indicates that such compensatory pathways may have taken place. The lack of Nogo-B significantly increased TGFβ1 and Id1 expression in livers 1 day after PHx and increased IL6 and EGF expression 2 days after PHx. Given that administration of TGFβ1 delays or partially suppresses the peak of DNA synthesis at 1 day after PHx(36), decreased Ki67-positive cells observed in Nogo-B KO livers might be attributable to the increase in TGFβ1 expression observed 1 day after PHx. In contrast, Id1, IL6 and EGF contribute to hepatocyte proliferation during liver regeneration(37). Id1 is a transcription factor up-regulated in liver sinusoidal endothelial cells (LSECs) in the regenerative process(38). Increased Id1 levels in LSECs are essential for liver regeneration(39). Thus, the increase in Id1 expression could counterbalance the up-regulation of TGFβ1 observed in regenerating KO liver. In addition to Id1, the increases in IL6 and EGF expression may contribute to an increase in proliferating cells in KO livers by 3 days after PHx and help offset the initial inhibition of liver regeneration by increased TGFβ1 expression. Collectively, up-regulation of these genes in KO livers in response to PHx could be a compensatory mechanism to offset the opposing effects of each gene on liver regeneration.
Immediately after isolation, hepatocytes do not express Nogo-B, but Nogo-B expression is induced in response to cell culture, in association with cyclin D3. The increase in Nogo-B in cultured hepatocytes might resemble the condition of those hepatocytes around the periportal areas, where Nogo-B expression is induced by PHx. Interestingly, the increase in Nogo-B levels is transient both in vivo and vitro. In regenerating liver, Nogo-B expression declines notably by two weeks after PHx, while in cultured hepatocytes, Nogo-B levels plateau after six days in culture (data not shown).
One key finding of this study is that Nogo-B facilitates the IL6/STAT3 signaling pathway in hepatocytes. WT cultured hepatocytes expressing Nogo-B significantly increased pSTAT3 levels in response to IL6, compared with Nogo-B KO cultured hepatocytes. Immunofluorescence of livers isolated 5 hours after PHx also showed a significantly higher pSTAT3 positive hepatocyte level in WT livers than in Nogo-B KO livers. These findings may suggest that an increase in Nogo-B expression in periportal hepatocytes in regenerating liver primes a regenerative response to IL6, thereby facilitating hepatocyte proliferation. IL6 binds to its soluble IL6 receptor and induces signals through gp130 and Janus kinase-2 (JAK2), leading to activation of the STAT3 transcription factor(40–42) (Figure 3D). We observed that WT hepatocytes tended to have higher JAK2 phosphorylation than KO hepatocytes, although it was not statistically significant (data not shown). How Nogo-B facilitates the IL6/JAK2/STAT3 signaling pathway in hepatocytes is an interesting question, which requires further scrutiny.
Nogo-B also increases HGF-induced hepatocyte proliferation. This effect of Nogo-B on hepatocyte proliferation could be specific for HGF, since absence or presence of Nogo-B did not influence hepatocyte proliferation in response to EGF. Furthermore, Nogo-B may make hepatocytes less susceptible to TGFβ1-induced suppression of proliferation. Overall, the presence of Nogo-B helps to facilitate hepatocyte proliferation by influencing IL6, HGF and possibly TGFβ1 signaling pathways.
IL6, HGF and TGFβ1 signaling pathways are usually thought to be initiated at the plasma membrane where their respective receptors are situated. How could Nogo-B, an ER-resident protein, influence these signaling pathways in hepatocytes? Given that reticulons 2 and 3 are known to regulate cellular trafficking(9, 43), one possible answer to this question may be Nogo-B’s involvement in trafficking of these receptors to the plasma membrane. The differential effect of Nogo-B on hepatocyte proliferation between HGF and EGF may thus be explained by Nogo-B’s possible differential involvement in trafficking of their receptors, MET and EGFR. Given that ERp57, another ER-resident protein, significantly decreases STAT3 phosphorylation and expression by an unknown mechanism(44), understanding the role of ER proteins such as Nogo-B in important signaling pathways is a very interesting area of future inquiry, providing new insights into the regulation of cell signaling.
In conclusion, Nogo-B promotes hepatocyte proliferation by facilitating the IL6/STAT3 signaling pathway, increasing HGF-induced proliferation and possibly reducing TGFβ1-induced proliferation suppression. Lack of Nogo-B delays the peak proliferation of regenerating liver, although it does not abolish overall liver regeneration. The increase in Nogo-B expression in hepatocytes around the periportal areas of regenerating liver may play a role in the regenerative response to PHx, by involving the above-mentioned signaling pathways.
Supplementary Material
ACKNOWLEDGEMENT
We would like to thank Drs. Shi-Ying Cai, Man Li, and Emma A Kruglov for technical help, Ms. Kathy M. Harry for hepatocyte isolation.
FUNDING
This work was supported by grant RO1DK082600 and a Yale Liver Center Pilot Project Award (P30-34989) from the National Institutes of Health (NIH) (Iwakiri) and KO8DK073404 from NIH (Swenson).
Abbreviations
- TGFβ
transforming growth factor beta
- PHx
partial hepatectomy
- RT-PCR
reverse transcriptional-PCR
- IL6
interleukin 6
- HGF
hepatocyte growth factor
- EGF
epidermal growth factor
- STAT3
signal transducer and activator of transcription 3
- ER
endoplasmic reticulum
- EGTA
ethylene glycol tetraacetic acid
- EDTA
ethylene diaminete tetraacetic acid
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PFA
paraformaldehyde
- PCNA
proliferating cell nuclear antigen
- EGFR
epidermal growth factor (EGF) receptor
- PBS
phosphate buffered saline
- WT
wild-type
- KO
knockout
- LSECs
liver sinusoidal endothelial cells
Footnotes
CONFLICT OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: YI, TU. Performed the experiments: YI, LG, TU, KT, BL, DZ. Analyzed the data: YI, LG, TU, KT, BL. Contributed reagents/materials/analysis tools: YI, ESS, BL. Wrote the paper: YI, TU, ESS, LG.
REFERENCES
- 1.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 3.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 4.Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 5.Teng FY, Tang BL. Cell autonomous function of Nogo and reticulons: The emerging story at the endoplasmic reticulum. J Cell Physiol. 2008;216:303–308. doi: 10.1002/jcp.21434. [DOI] [PubMed] [Google Scholar]
- 6.Yang YS, Strittmatter SM. The reticulons: a family of proteins with diverse functions. Genome Biol. 2007;8:234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Shibata Y, Voss C, Shemesh T, Li Z, Coughlin M, Kozlov MM, et al. Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science. 2008;319:1247–1250. doi: 10.1126/science.1153634. [DOI] [PubMed] [Google Scholar]
- 9.Wakana Y, Koyama S, Nakajima K, Hatsuzawa K, Nagahama M, Tani K, Hauri HP, et al. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem Biophys Res Commun. 2005;334:1198–1205. doi: 10.1016/j.bbrc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D, Utsumi T, Huang HC, Gao L, Sangwung P, Chung C, Shibao K, et al. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–1315. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 12.GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 13.Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 14.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Fernandez-Hernando C, Suarez Y, Schleicher M, Hao Z, Wright PL, DiLorenzo A, et al. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc Natl Acad Sci U S A. 2009;106:17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 17.Wright PL, Yu J, Di YP, Homer RJ, Chupp G, Elias JA, Cohn L, et al. Epithelial reticulon 4B (Nogo-B) is an endogenous regulator of Th2-driven lung inflammation. J Exp Med. 2010;207:2595–2607. doi: 10.1084/jem.20100786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, et al. The role of Nogo and the mitochondria-endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002194. 88ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin EP, Moeckel G, Al-Lamki R, Bradley J, Yan Q, Wang T, Wright PL, et al. Identification and regulation of reticulon 4B (Nogo-B) in renal tubular epithelial cells. Am J Pathol. 2010;177:2765–2773. doi: 10.2353/ajpath.2010.100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Gallo J, Sozmen EG, Chytil A, Russell WE, Whitehead R, Parks WT, Holdren MS, et al. Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24:3028–3041. doi: 10.1038/sj.onc.1208475. [DOI] [PubMed] [Google Scholar]
- 21.Thenappan A, Li Y, Kitisin K, Rashid A, Shetty K, Johnson L, Mishra L. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology. 2010;51:1373–1382. doi: 10.1002/hep.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 23.Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, et al. Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest. 1991;87:1853–1857. doi: 10.1172/JCI115207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fausto N, Riehle KJ. Mechanisms of liver regeneration and their clinical implications. J Hepatobiliary Pancreat Surg. 2005;12:181–189. doi: 10.1007/s00534-005-0979-y. [DOI] [PubMed] [Google Scholar]
- 26.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 27.Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, et al. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281–11288. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, Demetris AJ. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- 29.Natarajan A, Wagner B, Sibilia M. The EGF receptor is required for efficient liver regeneration. Proc Natl Acad Sci U S A. 2007;104:17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houck KA, Cruise JL, Michalopoulos G. Norepinephrine modulates the growth-inhibitory effect of transforming growth factor-beta in primary rat hepatocyte cultures. J Cell Physiol. 1988;135:551–555. doi: 10.1002/jcp.1041350327. [DOI] [PubMed] [Google Scholar]
- 31.Kim JE, Li S, GrandPre T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 32.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabes HM. Kinetics of hepatocellular proliferation as a function of the microvascular structure and functional state of the liver; Ciba Found Symp; 1977. pp. 31–53. [DOI] [PubMed] [Google Scholar]
- 36.Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Jossic C, Ilyin GP, Loyer P, Glaise D, Cariou S, Guguen-Guillouzo C. Expression of helix-loop-helix factor Id-1 is dependent on the hepatocyte proliferation and differentiation status in rat liver and in primary culture. Cancer Res. 1994;54:6065–6068. [PubMed] [Google Scholar]
- 38.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–677. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 39.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 41.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 42.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Vidensky S, Ruggiero AM, Maier S, Sitte HH, Rothstein JD. Reticulon RTN2B regulates trafficking and function of neuronal glutamate transporter EAAC1. J Biol Chem. 2008;283:6561–6571. doi: 10.1074/jbc.M708096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Date M, Matsuzaki K, Matsushita M, Tahashi Y, Furukawa F, Inoue K. Modulation of transforming growth factor beta function in hepatocytes and hepatic stellate cells in rat liver injury. Gut. 2000;46:719–724. doi: 10.1136/gut.46.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.