Abstract
Warfare has long been associated with traumatic brain injury (TBI) in militarized zones. Common forms of TBI can be caused by a physical insult to the head-brain or by the effects of a high velocity blast shock wave generated by the detonation of an explosive device. While both forms of trauma are distinctly different regarding the mechanism of trauma induction, there are striking similarities in the cognitive and emotional status of survivors. Presently, proven effective therapeutics for the treatment of either form of TBI are unavailable. To be able to develop efficacious therapies, studies involving animal models of physical- and blast-TBI are required to identify possible novel or existing medicines that may be of value in the management of clinical events. We examined indices of cognition and anxiety-like behavior and the hippocampal gene transcriptome of mice subjected to both forms of TBI. We identified common behavioral deficits and gene expression regulations, in addition to unique injury-specific forms of gene regulation. Molecular pathways presented a pattern similar to that seen in gene expression. Interestingly, pathways connected to Alzheimer’s disease displayed a markedly different form of regulation depending on the type of TBI. While these data highlight similarities in behavioral outcomes after trauma, the divergence in hippocampal transcriptome observed between models suggests that, at the molecular level, the TBIs are quite different. These models may provide tools to help define therapeutic approaches for the treatment of physical- and blast-TBIs. Based upon observations of increasing numbers of personnel displaying TBI related emotional and behavioral changes in militarized zones, the development of efficacious therapies will become a national if not a global priority.
Keywords: Physical-traumatic brain injury, Blast-traumatic brain injury, Cognitive dysfunction, Gene expression, Molecular pathway(s), Neurodegeneration, Stem cells, Alzheimer’s disease
Introduction
Traumatic brain injury (TBI) covers a range of central nervous system (CNS) injuries; such injuries can be caused by a diffuse or a focused form of tissue damage in brain. TBIs are exemplified by blunt force trauma that can occur in a car accident, a fall or in a sporting or warfare related event. TBIs can vary from mild, moderate to severe, the greater the force involved in the injury the more severe is the TBI (DeKosky et al., 2010; Hoge et al., 2008; Mendez et al., 2005). Quite often TBIs can result in the development of diffuse axonal injury (DAI), as originally described by Strich (1956) and more lately by Adams and co-workers (Adams et al., 1982, 1984). Subsequent to an injury, a series of molecular and cellular events may occur that result in changes that often proceed to neuronal cell dysfunction and death. This, in turn, manifests as changes in neurological status, exemplified by issues of irritability; headache; fatigue; memory problems and, in some cases, an increase in antisocial behavior that may lead to criminal activity (Falk 2012; Luukkainen et al., 2012; Yang et al., 2012). Typically in mild TBI, no gross tissue damage occurs at the time of trauma. However, clinical neurological consequences of the trauma develop over time, and, for the most part (~85% of cases), regress within a few months after injury (see Alexander 1995 for a clinical perspective on diagnosis of mild traumatic brain injury).
A more recently recognized insidious form of TBI originating from warfare has become a significant medical problem; this form of brain injury has drawn considerable attention due to its prevalence in active combat zones. Primarily through the use of high explosives, and more specifically improvised explosive devices, explosive detonations are causing an increased incidence of complex TBIs. These explosive devices can cause primarily shock wave dependent damage (blast damage) to multiple organ systems, including the brain. Blast-induced brain damage and the ensuing neurological disorders caused by blast injuries are proving to be difficult to clearly diagnose without the use of extensive neurological and psychological assessments, and are an increasing problem for military and civilian healthcare providers. The consequences of these ‘lesion-less’ injuries often result in deficits in military personnel reaction times, spatial memory function, and an increased occurrence of headache, dizziness, sleep disturbances, and other emotional and behavioral changes (Bryan and Hernandez, 2012; Shively and Perl, 2012).
The origins of the cognitive and psychological disorders induced by blast injuries in combat zones are most likely due to undefined changes in the molecular and cellular homeostasis of different regions of the brain, and, as such, highly warrant further study. Non-blast (concussive) injuries are most likely mediated by shearing force damage to neurons causing a DAI (Strich, 1956; Adams et al., 1982, 1984; Messé et al., 2012; see Johnson et al., 2012 for review), while in a primary blast injury, the role of DAI appears to be more complex (Garman et al., 2011; Risling et al., 2011; Sajja et al., 2012; Wang et al., 2011). However, it is feasible that the final end stages of neuronal damage induced by different mechanisms are mediated by the activation of shared pathways that could be identified by sensitive screening approaches. We have utilized a mild, closed head weight drop model of physical head trauma (physical-TBI) in mouse developed and extensively characterized by our laboratories (Baratz et al., 2011; Milman et al., 2005; Rachmany et al., 2012; Tashlykov et al., 2007, 2009; Tweedie et al., 2007; Zohar et al., 2003, 2011) and we have compared physical-TBI with a novel mouse model of a mild blast-TBI (Rubovitch et al., 2011). Our purpose was to compare the effects of the two different forms of mild brain injury on mouse cognitive and emotional paradigms and on hippocampal gene expression. The hippocampus was selected due to its role in memory and cognition. Behavioral changes and the hippocampal transcriptome were examined at a time point after injury where TBI related impairments are known to occur in mild physical-TBI (Baratz et al., 2011; Rachmany et al., 2012; Zohar et al., 2011).
Cognitive and emotional behavior was assessed by use of novel object recognition (NOR), Y-maze, passive avoidance (PA) and elevated plus maze (EPM) initiated 7 days after exposure to either physical- or blast-TBI. All TBI animals demonstrated deficits in novel object recognition compared to control mice, yet there were no marked differences between control and TBI mice for Y-maze, passive avoidance or elevated plus maze, thus indicating that these TBI models were mild in nature. In contrast to mouse behavioral measures, we observed marked changes in gene expression in RNA from intact TBI hippocampal tissue. There was a degree of overlap in genes co-regulated by the two mechanisms of injury. However there were a larger number of genes uniquely expressed in an injury specific manner. Consequently, molecular pathways displayed similar patterns of injury dependent regulation. While these data suggest a potentially shared injury behavioral outcome, there is a divergence in gene expression and pathway regulation that would suggest that the two types of injury are different at the molecular level. This divergence may aid in the identification of novel drug targets that may be of clinical benefit in the setting of complex human TBIs.
Methods
Animal studies
Male ICR mice weighing 30–40 g were kept five per cage under a constant 12-h light/dark cycle, at room temperature (22±2°C). Food (Purina rodent chow) and water were available ad libitum. Each mouse was used for one experiment. The Ethics Committee of the Sackler Faculty of Medicine approved the experimental protocols (M-09-055; M-07-055), in compliance with the guidelines for animal experimentation of the National Institutes of Health (DHEW publication 85–23, revised, 1995). All experimental manipulations were conducted during the light phase of the cycle.
Induction of a mild physical- and blast-TBI
A mild physical-TBI was induced using a concussive head trauma device described previously (Milman et al., 2005; Zohar et al., 2003). Briefly, mice were lightly anesthetized (Isoflurane) and placed under the weight-drop concussive head trauma instrument. The device consisted of a metal tube (inner diameter 13 mm), placed vertically over the mouse head. A metal weight (30 g) was dropped from the top of the tube (80 cm) and struck the skull at the right side temporal area between the corner of the eye and the ear. A sponge supported the head, allowing some antero-posterior motion without any rotational head movement at the moment of the impact.
Experimental conditions used to create a mild low-level blast-TBI and the subsequent model characterization, have been described in detail elsewhere (Rubovitch et al., 2011). In brief, mice were anaesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg). Once the animals were fully anaesthetized they were placed at a defined distance from a detonation source, in this case 7 meters. Pressure sensors were used to measure the explosion shock wave pressure (PSI) generated by the detonation (Free-Field ICP® Blast Pressure Sensor; PCB Piezoelectronics, Depew, NY, USA, Model 137). At 7 meters from the source of the detonation, the animals were exposed to a maximum of a 2.5 PSI (17.2 kPa) pressure shock wave. Immediately after the induction of the injury, mice were placed back in their cages. Once the animals had recovered from the anesthesia, basic neurological assessments were undertaken to identify any acute neurological dysfunction. Only animals exhibiting no evidence of acute neurological damage post injury were subsequently used in further experiments. Sham treated mouse groups were treated identically; however, they were not exposed to physical- or blast-TBI.
Cognitive and emotional behaviors
Behavioral assessments were initiated 7 days after the animals were exposed to TBI. This time point was selected based upon prior studies with these models where behavioral deficits were observed to occur from this time point. Mouse cognition and emotional behavior was assessed using the NOR; Y maze; PA and EPM paradigms. For a diagram of the experimental time course see Figure. 1 A. The NOR and the Y maze behavioral paradigms have been described in detail elsewhere (Baratz et al., 2010, 2011; Edut et al., 2011; Rachmany et al., 2012; Rubovitch et al., 2010). Mouse treatment groups utilized were as follows: sham, n= 27–42; physical-TBI, n = 22–34; blast-TBI, n = 23–30. All equipment used for behavioral testing was cleaned with a 70% ethanol solution between testing sessions and animals to minimize any olfactory dependent cognitive influences on mouse behavior.
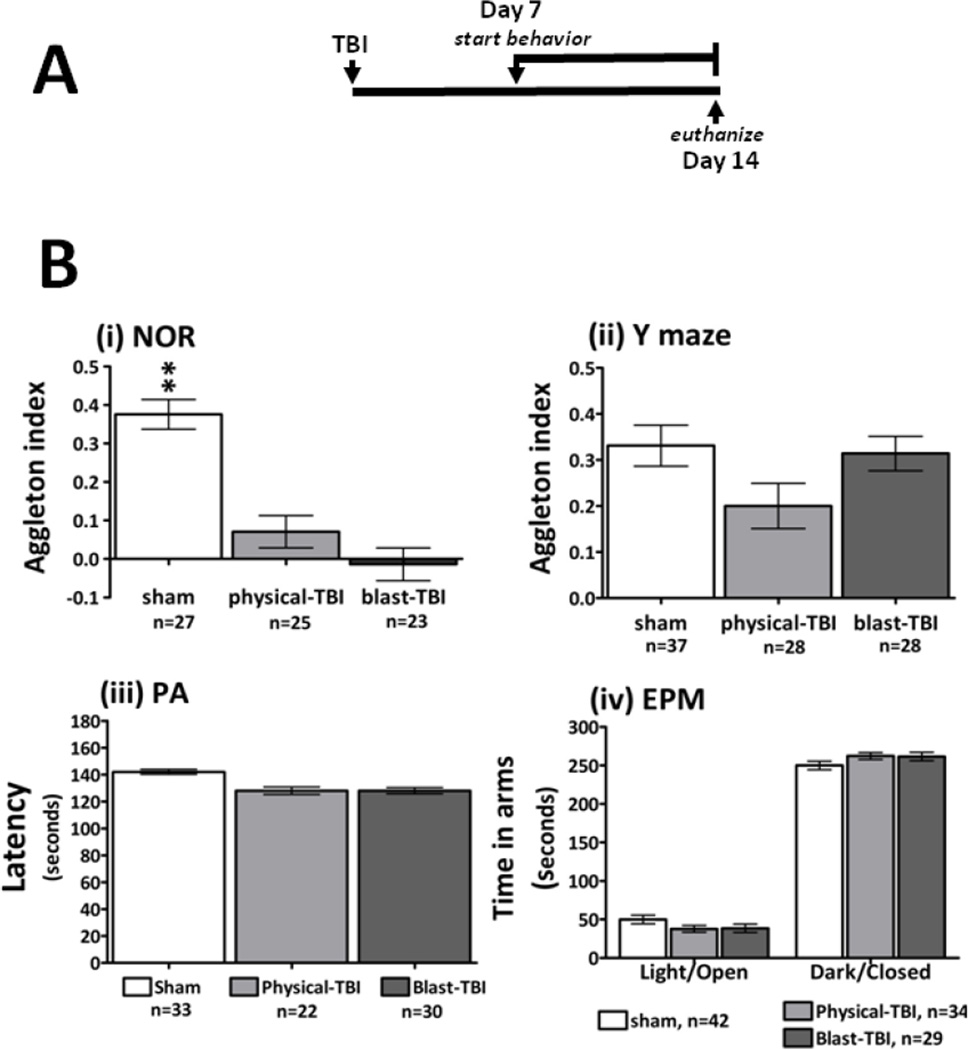
Figure 1.
A the time line for the induction of TBI and the evaluation of behavioral paradigms with the subsequent euthanasia day is shown: 7 days after the induction of either form of TBI a series of behavioral assessments was undertaken. This time point was selected based upon prior studies with these models where behavioral deficits were observed to occur from 7 days post injury. At 14 days after TBI the animals were euthanized and hippocampal tissue was harvested for use in gene expression studies. B: The effects of TBI on measures of mouse cognition and emotional wellbeing are shown. For the novel object recognition (NOR) behavioral paradigm, deficits were observed in both sets of TBI mice compared to sham animals, deficits were observed by comparing the Aggleton index for the sham and injured animal groups (i). For the Y maze paradigm there were no marked differences between sham and TBI animals (ii). The methods for calculating the Aggleton index for the NOR and Y maze are described in the methods section. The latency times of the animals to enter the dark, foot shock chamber are presented for the passive avoidance paradigm (PA, iii); comparing the latency times to enter the dark chamber indicated no significant differences between sham or either TBI group. The levels of anxiety-like behavior were assessed by the elevated plus maze paradigm (EPM, iv). All test groups responded similarly when comparing the total time spent in the dark-enclosed and light-open arms of the maze.
Novel object recognition paradigm
The NOR task was used to evaluate recognition memory; typically rodents display a natural inquisitiveness to explore new objects in their environment. This characteristic can be used to evaluate visual recognition memory function in rodents, and more interestingly it can illustrate how various interventions influence this natural tendency. Animals were allowed to explore a set of two identical objects for a 5 minute period, afterwards the mice were returned to their cages. Twenty four hours later the animals were presented with a similar set of objects in the same environment, where one object was novel to them; they were allowed to freely explore the objects again for a 5 minute period. A discrimination preference, Aggleton index for a novel over a familiar object was calculated as follows: time near a new object less the time near the old object, divided by time near the new object plus the time near the old object. Methodologies used were adapted from those used by Dix and Aggleton (1999), and have been described elsewhere in detail (Baratz et al., 2010, 2011; Edut et al. 2011; Messier 1997; Rachmany et al., 2012).
Y maze paradigm
The Y maze task was used to evaluate spontaneous exploration, responsiveness to novel environments and spatial memory function. The apparatus used for the Y maze study was constructed out of black Plexiglass where the three arms of the maze were separated by a 120° angle. Each arm was identical (8 × 30 × 15 cm); however different spatial cues were placed in each arm (i.e. a triangle, a square, or a circle). The start arm for each experiment was chosen randomly; each mouse was placed into the Y maze environment on two occasions separated by a 2 minute interval. During the first 5 minute trial, one of the two arms was randomly blocked. On the second 2 minute trial all arms were open for exploration; the total amount of time the mouse explored in each arm was recorded. During the inter-trial interval the animal was returned to its home cage and the maze was cleaned. A discrimination preference, Aggleton index similar to what was utilized in the NOR, was employed herein. An index of the time spent in the new, previously unexplored arm over the familiar explored arm was used to assess for any behavioral differences between each animal treatment group. It was calculated as follows: time in the new arm less the time in the old arm, divided by the time in new arm plus the time in the old arm.
Passive avoidance paradigm
The PA task was used to evaluate non-spatial memory function; it is an assessment of whether an animal can learn to associate an aversive stimulus, in this case a mild foot shock, with a specific environment. The passive avoidance apparatus (San Diego Instruments, San Diego, CA, USA) was created from black Plexiglass (48 × 22 × 22 cm) and consisted of two separate chambers, one dark (preferable to rodents) the other light-illuminated (less preferable to rodents) of equal dimensions. The chambers were joined by a door, which was closed at the start of each trial that could be opened by the experimenter. The test involved two sessions separated by a 24 hour interval. Animals were positioned within the illuminated chamber and after 30 seconds the connecting door was opened. On crossing into the dark compartment, the door was closed, and the animal received a mild electric foot shock (1 mA for 3 seconds). After the mild electric shock the animals were kept in the dark chamber for 5 seconds and were then returned to their home cage. After a 24 hour interval, animals were evaluated for their ability to remember the aversive foot shock in relation to the darkened chamber, again by placing them in the illuminated compartment prior to opening the door between chambers. The latency time to enter the dark compartment within a 3 minute period was measured and used to indicate learning deficits induced by TBI (Milman et al., 2005). No shock was delivered during the second day; this paradigm was conducted last due to its aversive nature.
Elevated plus maze paradigm
The EPM apparatus was used to assess the levels of fear or anxiety-like behavior in rodents, this assessment is based upon the natural tendency of rodents to fear to explore open, illuminated elevated environments over a preferred exploration of an enclosed darkened environment. The maze consisted of four arms in a ‘plus’ shape formation. Two of the arms have low walls (30 × 5 × 1 cm) and the other two arms have high walls (30 × 5 × 15 cm), with an open top; the similar arms faced one another. The maze was elevated 50 cm above floor level. The high and low wall arms of the maze were constructed of black and white acrylic glass, respectively. On the test day each mouse was placed in the center of the elevated plus maze, facing one of the open arms. During the 5 minute observation period the following variable was measured: the total time spent in the two open and the two closed arms.
Mouse behavior data analysis
All results are given as mean ± SEM and were analyzed using SPSS 17 software (Genius Systems, Petah Tikva, Israel). One way ANOVA was used to analyze the behavioral data, for novel object recognition; p values of post hoc tests were adjusted using the Bonferoni test and a nominal significance level of 0.05 was used. When a comparison was made between the familiar and novel objects within a specific treatment group, to show the level of memory retention within the group, a two tailed t-test was used. In some behavioral assessments mice failed to respond to a behavioral test and were excluded from analysis; also where measurements were found to be more than two standard deviations from the group mean those animals were excluded from analysis. As such there are differences in n numbers shown for the specific behavioral assessments observed in Figure 1B.
Hippocampus RNA extraction, cDNA microarray hybridizations and bioinformatic array analysis
After the battery of behavioral assessments, animals were euthanized and the right hippocampus was dissected and used to prepare total RNA. Due to the significance of the hippocampus in learning and memory and its vulnerability to mTBI (Tweedie et al., 2007), we chose to study hippocampal gene expressions over other brain regions. Methods to extract total RNA for use with Illumina's SentrixMouse Ref-8, v2 Expression BeadChips (Illumina, San Diego, CA) have been previously described (Tweedie et al., 2012). Arrays were scanned at a resolution of 0.8 um using the Beadstation 500 X from Illumina, and data were extracted from the image using Illumina BeadStudio software, V3. Mouse tissues were randomly selected from the larger library of samples generated from the behavioral experiments and the numbers utilized in the gene expression study were as follows: sham, n = 5: physical-TBI, n = 4; blast-TBI, n = 7. Bioinformatic methods used were as have been described previously (Tweedie et al., 2012). In brief, raw array chip hybridization image signals were filtered and processed to generate normalized data that was then transformed to create Z-scores for each gene. The Z-score transformed data was then utilized to generate a Z-ratio measurement, which allowed for the statistical analysis of the gene expression data sets. We selected significant genes by the following criteria: 1) gene expression changes had a z-test value of ≤ 0.05 vs. sham; 2) the absolute value of Z-ratio was calculated to be ≥ 1.5 vs. sham; 3) the False Discover Rate for the genes was ≤ 0.30; 4) the average Z-score over all sample comparisons were not negative and lastly; 5) an one way independent ANOVA test p value cut off was ≤ 0.05. Thus only genes that displayed consistent significant expression changes in all samples from a given TBI group were considered for further statistical analysis. Hippocampus gene expression profile comparisons were made between the following mouse data sets: physical-TBI vs. sham mice and blast-TBI vs. sham mice. Detailed lists of significantly regulated genes are provided in the Supplemental Tables, the Tables are composed of the following: Accession number, gene symbol, Z-score and fold change; all data were sorted based on descending fold change. Data sets underwent parametric analysis of gene set enrichment analysis (PAGE) which enabled the identification of injury effects on pathway and function mapping. We used a Z-test for the pathway/functional group gene Z-ratio with a cut off p value ≤ 0.05, and more than three genes for each pathway/functional group were required to be significantly regulated for the pathway to be considered for analysis. The raw data file and the filtered, normalized results are available online in the Gene Expression Omnibus, Accession Number XXXX, (this information will be provided by authors at a later time).
Array validation by quantitative (Q)-RT-PCR
To validate our cDNA array, gene expression levels were characterized by Q-RT-PCR; gene expression comparisons were made between the array and Q-RT-PCR methods described herein for the following genes of interest (GOI): Acot1 (acyl-CoA thioesterase 1); Fgf10 (fibroblast growth factor 10) and Mag (myelin associated glycoprotein). Total RNA from the same samples used for gene array studies was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using SuperScript II reverse transcriptase with random hexamers (Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed on two occasions once in duplicate and the other in single well format, using the iTaq Sybr Green supermix with ROX (Bio-Rad, Hercules, CA). The final reaction volume of 20 µl consisted of 10 µl of the premade reaction mix, 1 µl primer pair and 9 µl cDNA (20 ng) in water. Reaction conditions for Q-RT-PCR for the Step 1 plus qPCR System (Applied Biosystems, Foster City, CA) were as follows: 95°C for 10 minutes and 45 cycles of 95°C for 30 seconds (denaturation step) and 60°C for 45 seconds (combined annealing and extension steps). A melting curve analysis was performed by denaturation at 95°C for 15 seconds, annealing at 60°C for 1 minute and at a melting rate of 0.3°C/s to 95°C. Primers for amplification were as followed: Acot1 forward 5’- GCAGCGATGAGGCTTTGGA-3’ and reverse 5’-TGATGGATGTGTAAGGGTTCACT-3’ (Primer Bank 12331400a1, GenBank accession no. NM_022816.1, 104 bp). Fgf10 forward 5’-CAATGGCAGGCAAATGTATG-3’ and reverse 5’-GGAGGAAGTGAGCAGAGGTG-3’ (GenBank accession no. NM_008002.4, 94 bp). Mag forward 5’-CTGCCGCTGTTTTGGATAATGA-3’ and reverse 5’-CATCGGGGAAGTCGAAACGG-3’ (GenBank accession no. NM_010758.2, 127 bp). Tuba1c forward 5’- TAGCAGAGATCACCAATGCC-3’ and reverse 5’-GGCAGCAAGCCATGTATTTA-3’ (GenBank accession no. NM_011654.2, 86 bp). The relative levels of transcript were quantified using the standard curve method. Levels of mRNA expression are reported as a geometrical average of the expression of the GIO relative to α-tubulin mRNA levels. The Q-RT-PCR gene expression data are expressed as mean ± SEM. The calculated fold change in gene expression was (1) utilized to represent the overall treatment-induced change and: (2) used for statistical analysis between the appropriate groups (Unpaired two tailed students t-test).
Results
Behavioral measures 14 days after TBI
TBI in mice induced by either a physical or a blast injury, led to the development of marked deficits in NOR compared to sham control mice (Figure 1B (i)). Observation of mouse behavior during the Y maze indicated that there were no deficits detected in blast-TBI mice, however; there was a trend towards a deficit observed in physical-TBI mice (Figure 1B (ii)). Interestingly in the PA assessment TBI animals displayed a slightly shorter latency time to enter the dark shock chamber, but the difference was not statistically significant to that of the sham animals (Figure 1B (iii)). All mice subjected to the EPM spent more time in the preferred, darkened-closed arm(s) of the maze over the illuminated-open arm(s). Comparing the time spent in each environment across groups indicated that there was no effect of TBI on mouse EPM behavioral outcomes (Figure 1B (iv)).
Hippocampal tissue gene expression measures 14 days after TBI
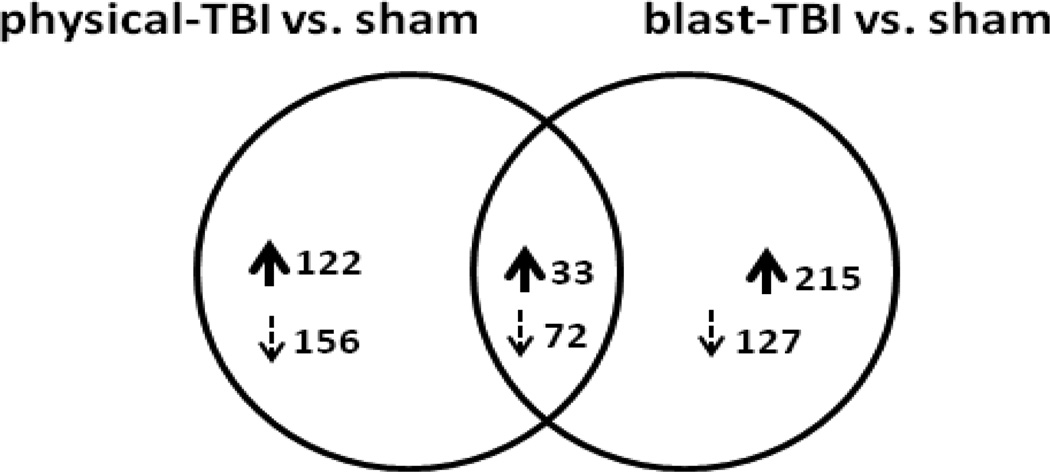
Gene expression levels were examined in right hemisphere hippocampal tissues dissected from sham and both forms of TBI treated mouse brains. As the RNA was generated from intact tissue, the extracted RNA will originate from all cell types present in the hippocampus at the time of dissection. Accordingly, observations of gene transcript regulations and pathway mapping will not necessarily be exclusive to hippocampal-neuronal RNAs, but a mixture of neuronal and non-neuronal tissues. Employing stringent bioinformatical analysis we were able to identify significantly regulated genes in hippocampal tissues obtained from TBI animals. To assess for any possible overlaps in significant genes identified in the two forms of injury a Venn diagram was constructed comparing the two data sets (Figure 2). As indicated in Figure 2, there were both unique and common regulated genes observed in hippocampal tissues from the two forms of head injury. In physical-TBI vs. sham, 122 and 156 genes were exclusively up and down regulated, respectively. In blast-TBI, 215 and 127 genes were exclusively up and down regulated, respectively. Interestingly, 33 and 72 genes were commonly up and down regulated, respectively. As the numbers of significantly regulated genes are too large to describe here, the gene identities are listed in Supplemental Tables: exclusively regulated genes are presented in Supplemental Table 1 (physical-TBI vs. sham) and 2 (blast-TBI vs. sham). Supplemental Table 3 lists regulated genes common to both forms of injury. The fold changes in gene expressions observed 14 days after both forms of trauma were small; the maximum up regulated, exclusive gene observed in physical-TBI was Hist1h2bm (+1.59 fold) and the maximum down regulated gene was Ccdc53 (−2.42 fold), Supplemental Table 1. The maximum up regulated exclusive gene observed in blast-TBI was Stmn1 (+2.27 fold) and the maximum down regulated gene was Tigd2 (−1.50 fold), Supplemental Table 2. Of the commonly regulated genes, the gene presenting with the largest up regulation for physical-TBI was 2610524H06Rik (+1.53 fold), and for the blast-TBI injury the gene was Ndufb9 (+1.96 fold). The gene Megf9 was the maximally down regulated gene for both forms of injury, −1.62 and −1.52 fold for physical- and blast-TBI, respectively Supplemental Table 3.
Figure 2.
presented is a Venn diagram illustration of the number of up and down regulated genes observed in hippocampal tissue from physical-TBI vs. sham and blast-TBI vs. sham mice 14 days after injury. For physical-TBI mice, 122 and 156 unique genes were significantly up and down regulated, respectively. Similarly for blast-TBI mice, 215 and 127 unique genes were significantly up and down regulated, respectively. Comparing the two different forms of injury indicated that there were 33 and 72 commonly regulated genes that were significantly changed compared to the common sham animal samples. The gene symbols, Z-ratio and fold changes of these genes are provided in Supplemental Tables 1, 2 and 3. The criteria used to determine significantly regulated genes are described in detail in the methods section.
Hippocampal tissue functional pathway measures 14 days after TBI
The significantly altered genes were then organized into functional pathways and are described in Tables 1 and 2 and Figure 3. The present study is aimed at elucidating relationships between mouse behavior and gene expressions, thus we have focused our data description upon CNS relevant pathway data sets observed 14 days after injury. In order illustrate the wider picture of trauma regulated pathways we have included data relevant to CNS and non-CNS pathways in Tables 1 and 2 and Figure 3. Physical-TBI regulated pathways are described in Table 1 and Figure 3; 29 and 19 pathways were exclusively up and down regulated, respectively. Several pathways were related to the CNS; such up regulated pathways are ‘Chesler Brain Neural High Genes’, which had a Z-score of +2.34. ‘Hippocampus Developmental Postnatal’ and the ‘NOS1 Pathway’ were regulated with Z-scores of +3.29 and +2.88, respectively. Interestingly, ‘Stem Cell Neural Up’; ‘Stemcell Embryonic Up’ and ‘Stemcell Common Up’ were found to be down regulated with Z-scores of −11.36, −10.98 and − 3.89, respectively. Gliomas are tumors of CNS glial cell types, and several pathways related to Gliomas were significantly regulated by physical-TBI 14 days post injury; ‘IFN Beta Glioma Dn’, the Z-score was +3.82; for ‘BCNU Glioma Mgmt 48hrs Dn’, and ‘BCNU Glioma Nomgmt 48hrs Dn’, the Z-scores were +3.05 and +2.73, respectively.
Table 1.
exclusive pathway regulation observed for physical-TBI animals vs. sham
| Pathway name | Z-score |
|---|---|
| TARTE MATURE PC | 5.272910 |
| IFN BETA GLIOMA DN | 3.827570 |
| FALT BCLL UP | 3.784676 |
| REOVIRUS HEK293 DN | 3.617474 |
| DAC PANC UP | 3.498043 |
| SANSOM APC 5 DN | 3.387190 |
| MOREAUX TACI HI VS LOW UP | 3.285589 |
| PROSTAGLANDIN AND LEUKOTRIENE METABOLISM | 3.268012 |
| BCNU GLIOMA MGMT 48HRS DN | 3.052330 |
| SHEPARD GENES COMMON BW CB MO | 2.912913 |
| STRESS IONIZING SPECIFIC UP | 2.912775 |
| UCALPAIN PATHWAY | 2.909392 |
| NADLER OBESITY UP | 2.852387 |
| ATRIA UP | 2.844928 |
| INOS ALL DN | 2.837245 |
| ICHIBA GVHD | 2.827359 |
| BREAST CANCER ESTROGEN SIGNALING | 2.764218 |
| BCNU GLIOMA NOMGMT 48HRS DN | 2.736289 |
| GUO HEX DN | 2.725759 |
| AKAPCENTROSOME PATHWAY | 2.720747 |
| AGUIRRE PANCREAS CHR19 | 2.684420 |
| HPV31 DN | 2.619904 |
| IL6 FIBRO UP | 2.544787 |
| FETAL LIVER VS ADULT LIVER GNF2 | 2.503900 |
| TGFBETA C4 UP | 2.460696 |
| MPR PATHWAY | 2.370174 |
| ST GAQ PATHWAY | 2.344003 |
| CHESLER BRAIN NEURAL HIGH GENES | 2.343172 |
| LIAN MYELOID DIFF TF | 2.322706 |
| Pathway name | Z-score |
| UVB SCC UP | −2.302706 |
| STOSSI ER UP | −2.374485 |
| UVC TTD-XPCS COMMON DN | −2.558764 |
| IGF VS PDGF DN | −2.588686 |
| CROMER HYPOPHARYNGEAL MET VS NON UP | −2.603047 |
| UVB NHEK3 ALL | −2.643358 |
| GH GHRHR KO 24HRS UP | −2.664048 |
| UVC TTD ALL DN | −2.788376 |
| VALINE LEUCINE AND ISOLEUCINE BIOSYNTHESIS | −2.818138 |
| CHEN HOXA5 TARGETS UP | −2.867378 |
| DIAB NEPH DN | −2.877445 |
| BLEO MOUSE LYMPH LOW 24HRS DN | −2.920743 |
| YAGI AML PROG FAB | −3.055590 |
| BRCA BRCA1 POS | −3.084896 |
| ET743 SARCOMA 24HRS DN | −3.108203 |
| VHL NORMAL UP | −3.112808 |
| SANA TNFA ENDOTHELIAL DN | −3.115437 |
| LEE TCELLS1 UP | −3.337326 |
| LEE TCELLS10 UP | −3.337326 |
| LEE TCELLS8 UP | −3.337326 |
| ZHAN MM CD1 VS CD2 UP | −3.340927 |
| UVC TTD 4HR DN | −3.352074 |
| BRCA1KO MEF DN | −3.418057 |
| REOVIRUS HEK293 UP | −3.450305 |
| TARTE PLASMA BLASTIC | −3.494061 |
| BRCA PROGNOSIS NEG | −3.728247 |
| UVC HIGH ALL DN | −3.810951 |
| RCC NL UP | −3.822973 |
| ET743 SARCOMA 72HRS DN | −3.845168 |
| STEMCELL COMMON UP | −3.892492 |
| LEE TCELLS2 UP | −3.954467 |
| GH GHRHR KO 24HRS DN | −4.025383 |
| KREBS TCA CYCLE | −4.129869 |
| FLECHNER KIDNEY TRANSPLANT REJECTION DN | −4.155822 |
| ET743 SARCOMA 48HRS DN | −4.160649 |
| UVC XPCS 8HR DN | −4.477127 |
| ET743 SARCOMA DN | −4.968812 |
| UVC XPCS ALL DN | −5.401954 |
| FLECHNER KIDNEY TRANSPLANT WELL UP | −6.678798 |
Table 2.
exclusive pathway regulation observed for blast-TBI animals vs. sham
| Pathway name | Z-score |
|---|---|
| AGED MOUSE HYPOTH UP | 5.058069 |
| MITOCHONDRIA | 4.887640 |
| HUMAN MITODB 6 2002 | 4.767919 |
| ASTON OLIGODENDROGLIA MYELINATION SUBSET | 4.073755 |
| ROME INSULIN 2F UP | 3.561892 |
| PGC | 3.541123 |
| SMOOTH MUSCLE CONTRACTION | 3.367659 |
| PGC1A PATHWAY | 2.049962 |
| CSK PATHWAY | 1.648778 |
| AMI PATHWAY | 1.648778 |
| GATA3 PATHWAY | 1.591920 |
| T CYTOTOXIC PATHWAY | 1.479758 |
| T HELPER PATHWAY | 1.479758 |
| ADIPOGENESIS HMSC CLASS 1 UP | 1.323011 |
| CROONQUIST IL6 RAS DN | 1.181635 |
| FLUMAZENIL PATHWAY | 1.106139 |
| UV ESR WS UNREG | 0.961717 |
| GAMMA HEXACHLOROCYCLOHEXANE DEGRADATION | 0.765936 |
| BRENTANI_HORMONAL_FUNCTION | 0.7011706 |
| Pathway name | Z-score |
| SULFUR METABOLISM | −0.8954174 |
| IFN ALL UP | −1.100068 |
| PTC1 PATHWAY | −1.172143 |
| CELL CYCLE CHECKPOINT | −1.391008 |
| IL1R PATHWAY | −1.499458 |
| CDC25 PATHWAY | −1.522204 |
| VEGF MMMEC 6HRS UP | −1.610168 |
| PARKIN PATHWAY | −1.616718 |
| RELA PATHWAY | −1.665030 |
| MATRIX METALLOPROTEINASES | −1.689397 |
| RECK PATHWAY | −1.697159 |
| CHEOK HDMTX DN | −1.832808 |
| HDACI COLON TSA48HRS DN | −2.100699 |
| BENNETT SLE UP | −2.237192 |
| NOUZOVA CPG METHLTD | −2.280571 |
| BIOSYNTHESIS OF STEROIDS | −2.381442 |
| HUMAN TISSUE TESTIS | −2.421096 |
| POD1 KO UP | −2.444630 |
| JNK DN | −2.469562 |
| CHEOK HDMTX UP | −2.513970 |
| BAF57 BT549 UP | −2.629853 |
| SLRP PATHWAY | −2.769880 |
| CPR NULL LIVER UP | −2.823547 |
| POD1 KO MOST UP | −2.851374 |
| CPR LOW LIVER UP | −2.921130 |
| PANTOTHENATE AND COA BIOSYNTHESIS | −3.483098 |
| IRITANI ADPROX LYMPH | −3.678670 |
| BOQUEST CD31PLUS VS CD31MINUS DN | −3.691753 |
Figure 3.
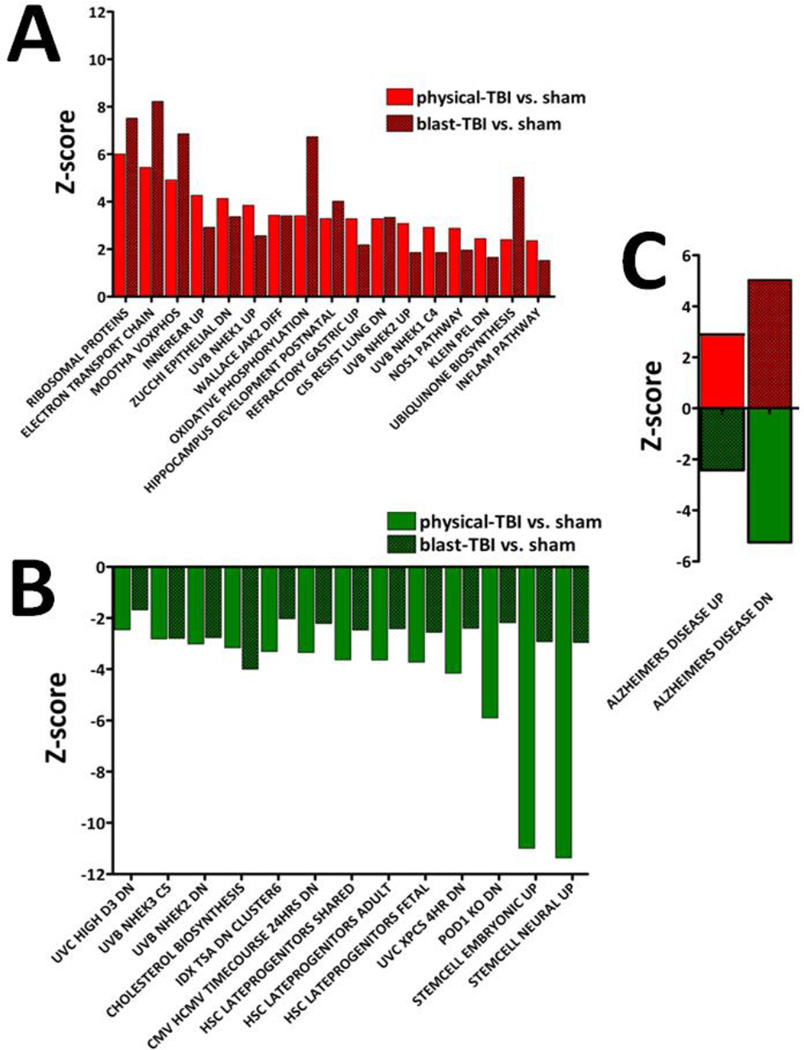
Based on significantly regulated genes observed 14 days after TBI, the identities of co-regulated and oppositely regulated functional pathways for both forms of TBI compared to the same sham animals are illustrated. For A, B, C the functional pathway name is indicated on the x-abscissa and the Z-score value for the pathway is indicated on the y-abscissa. A: 17 pathways were co-up regulated by physical- and blast-TBI. B: 13 pathways were co-down regulated. For several pathways there appeared to be an injury dependent difference in Z-scores values; this was most notable for ‘Stemcell Embryonic up’ and ‘Stemcell Neural up’. C: there was a marked injury dependent difference in the regulation of the ‘Alzheimer’s Disease Up/Dn’ pathways observed for physical- and blast-TBI. The regulations of these pathways shifted in an opposite direction depending on the mode of injury.
The identities of pathways regulated in blast-TBI at 14 days after injury are provided in Table 2 and Figure 3; 19 and 28 pathways were exclusively up and down regulated, respectively. ‘Aged Mouse Hypoth Up’, was the most up regulated with a Z-score of +5.05 (Table 2). At 14 days post blast-TBI, the pathway ‘Aston Oligodendroglia Myelination Subset’ was found to be up regulated, the Z-score was +4.07, while the ‘Parkin Pathway’, was down regulated, the Z-score was −1.61 (both Table 2). Changes were observed for the pathway ‘Matrix Metalloproteinases’, substrates of this family of proteins play important roles in synaptogenesis, synaptic plasticity, and long-term potentiation (LTP) and regulation of the blood-brain barrier (BBB). In the present study, 14 days post injury the Z-score for this pathway was −1.68 (Table 2). The following pathways were up regulated in blast-TBI in common with physical-TBI: ‘Hippocampus Developmental Postnatal’ and the ‘NOS1 Pathway’ with Z-scores of +4.01 and +1.96, respectively; and Stem Cell Neural Up’; ‘Stemcell Embryonic Up’, were down regulated with Z-scores of −2.95 and −2.92, respectively (Figure 3A and B).
Only two significantly altered pathways presented with a more complicated form of injury dependent regulation; they were the ‘Alzheimer’s Disease Up’ and ‘Alzheimer’s Disease Dn’ pathways. ‘Alzheimer’s Disease Up’ was up regulated by physical-TBI and down regulated by blast-TBI; the corresponding Z-scores were +2.89 and −2.42, respectively. ‘Alzheimer’s Disease Dn’ was down regulated in physical-TBI and up regulated by blast-TBI; the corresponding Z-scores are −5.25 and +5.03, respectively Figure 3 C.
Gene array validation by Q-RT-PCR
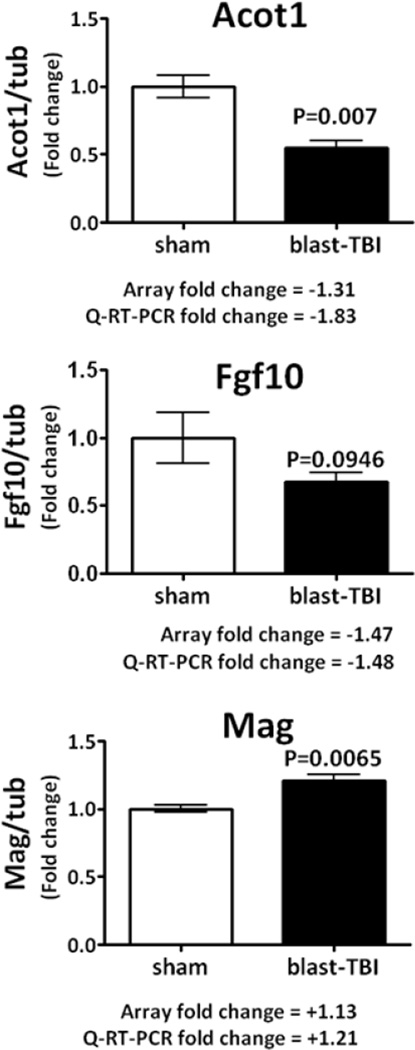
Aliquots of the same mRNA used in the gene array study were used to validate a small number of genes by Q-RT-PCR methods; the genes were Acot1; Fgf10 and Mag. The validated genes belong to the following Gene Ontology classes: metabolic process - Acot1 (acyl-CoA thioesterase 1, an enzyme involved in lipid metabolism, Hunt et al., 2012). Developmental, multicellular organismal and cellular process - Fgf10 (fibroblast growth factor 10, a member of the FGF family with neuron restricted expression in brain, Hattori et al., 1997). Cellular process - Mag (myelin associated glycoprotein, an adhesion molecule in postnatal neural development that has a role in interactions between neuronal and myelinating cells) Figure 4. Trauma-induced changes in the mRNA transcript levels of the selected goi were determined to be well matched between the array data and the Q-RT-PCR data in terms of direction and magnitude of changes (Figure 4).
Figure 4.
Q-RT-PCR data obtained for hippocampus genes measured in the same RNA samples used in the gene array studies are presented; the relative levels of gene transcripts are expressed as a fold change normalized to the house keeping gene alpha-tubulin. For the selected genes, although the fold changes in expression were small, less than a 2 fold change, for the three genes examined there was a strong agreement between the gene array and Q-RT-PCR fold change in gene expression. Data are expressed as mean ± SEM, of n, observations; sham, n=5; blast-TBI, n=7. The appropriate statistical p values are presented.
Discussion
The closed head model of mild physical-TBI has been extensively used by our laboratories and long lasting ensuing behavioral deficits have been found to occur from 7 days up to at least 90 days after injury (Baratz et al., 2011; Milman et al., 2005; Tweedie et al., 2007; Zohar et al., 2003, 2011). Assessments of cellular histological and anatomical changes in the physical-TBI closed head model described in this study have been undertaken at time points earlier than 14 days post injury, as used here. Measures of BBB integrity; Magnetic Resonance Imaging (MRI); silver impregnation staining; TUNEL; triphenyltetrazolium chloride (TTC) and hematoxylin and eosin staining have been performed on whole brain sections from control and injured mice. Mice were subject to varying degrees of TBI by the utilization of weights ranging from 5 g up to and including 50 g. Assessments were performed from 1 hour up to 5 days post injury. Zohar et al., 2003 provide evidence that illustrates no changes in BBB integrity, no trauma induced edema and no gross changes in neuroanatomy assessed by MRI (1 hour to 5 days post injury). Tweedie et al., 2007 illustrate by use of TTC staining (an indicator for metabolically active mitochondria) that there are, likewise, no gross differences between control and TBI mouse brain tissues up to 5 days after injury, even when a 50 g weight was used to induce the injury. However, in contrast to these studies Tashlykov and co-workers (2007; 2009) illustrate significant changes in neurodegeneration and apoptosis in injured cortical (anterior cingulate, frontal, senso-motor and temporal cortex) and hippocampal tissues (hippocampus and dentate gyrus) by 3 days post injury. These studies carefully illustrate that weights of 20 g and above are required to allow the detection of neurodegeneration by silver staining; TUNEL positive staining cells and cells with nuclear chromatin condensation. In light of the above published work, the observed changes in behavior and hippocampal genes observed in our current study, at 14 days post injury with a 30 g weight will be as a direct consequence of time-dependent pathological changes occurring in the mouse medial temporal lobe brain regions.
The behavioral disturbances observed with our closed head model compare favorably with other models of mild to moderate open head trauma, namely fluid percussion injury (FPI) and controlled cortical impact (CCI) injury models. These models employ stereotaxic instruments to induce a highly reproducible damage to a defined region of the brain dura (Abdel Baki et al., 2009; Li et al., 2006; McNamara et al., 2010; Sanders et al., 2001; Washington et al., 2012; Yu et al., 2009). While our model may lack injury reproducibility, compared to the FPI and CCI injury models, a main advantage of our model is that it is mild in nature and similar to the greatest injury prevalence clinically (De Kruijke et al., 2001). Our explosive detonation driven blast-TBI model has, likewise, been characterized (Rubovitch et al., 2011). Behavioral deficits and changes in diffusion tensor MRI imaging occurred at 7 and 30 days post injury, alterations in BBB integrity were noted 30 days after injury. Behavioral deficits from blast-TBI were in line with other small animal models of blast injury, as has been discussed in detail elsewhere (Rubovitch et al., 2011). Based upon basic acute neurological and histological measures, this model was similarly determined to be mild in nature.
Our described behavioral deficits resemble those previously described for blast-TBI (2.5 PSI; Rubovitch et al., 2011) and the physical-TBI animals (Baratz et al., 2011; Milman et al., 2005; Rachmany et al., 2012; Tashlykov et al., 2007, 2009; Tweedie et al., 2007; Zohar et al., 2003, 2011). The most striking alterations were seen in the NOR assessment (Figure 1 B (i)). The involvement of the hippocampus in the NOR test appears to depend on the species utilized (Baxter and Murry, 2001; Beason-Held et al., 1999; Cave and Squire, 1991; Gilbert and Kesner, 2003; Murry et al., 2000; Reed and Squire, 1997; Squire and Zola, 1996; Zola and Squire. 2001), and the time interval between object memory acquisition and object memory retention in a given experiment (Clark et al., 2000; Hammond et al., 2004; Mumby et al., 1999; Mumby 2001). In our NOR study there was a 24 hour interval between memory acquisition and memory recall, and we observed significant deficits that complement the studies of Clark (2000) and Hammond (2004). This would suggest that the neural circuitry involved in object memory recall after a 24 hour interval was impaired by both forms of TBI. The Y maze and passive avoidance tests, both hippocampal dependent assessments of short term spatial and non-spatial memory (Ambrogi Lorenzini et al., 1997; Conrad et al., 1996; Deacon et al., 2002; Pittenger at al., 2002) and elevated plus maze, a test of anxiety-like behavior in rodents (Cole and Rodgers, 1993, 1995; Pellow et al., 1985) failed to display any significant injury dependent behavioral differences, indicating that both models were mild in nature, as are most human TBIs (De Kruijk et al. 2001).
A potential caveat of our gene array study may be related to the random selection of a smaller subset of animal tissues from the larger set of tissues obtained from the behavioral assessments. However, based upon the small level of variability observed in our animal behavioral measures in a given treatment group and between the treatment groups, and the strict inclusion criteria for the bioinformatic analysis of the array data, we believe it unlikely that any confounding bias was thereby introduced into the identification of significantly altered TBI relevant gene expressions and pathways. Thus we have confidence that our observations of changes in gene expressions and animal behaviors are well matched. Despite the mildness of the TBI models, as illustrated by the behavioral studies, large numbers of gene expressions and molecular pathways were altered in whole hippocampus RNA samples. Unique CNS related pathways observed in physical-TBI, included the ‘BCNU Glioma MGMT 48hrs Down’, ‘BCNU Glioma NOMGMT 48 hrs Down’ and ‘IFN Beta Glioma Dn’ pathways, that were up regulated 14 days after injury, likely as a consequence of the activation of highly abundant glial cells within the injured brain region (Azevedo et al., 2009; Israelsson et al., 2009; Bandres et al., 2005; Saijo and Glass, 2011). Genes with strong expression in brain occurring in the pathway ‘Chesler Brain Neural High Genes’ were also up regulated (Chesler et al., 2005), while there is sparse literature relating to the functional role of this pathway in CNS disease, its regulation observed here will be due to the source of the hippocampal RNA used for array studies.
CNS Pathways of note regulated in blast-TBI tissue include the ‘Aston Oligodendroglia Myelination Subset’ pathway and ‘Parkin Pathway’. ‘Aston Oligodendroglia Myelination Subset’ genes are associated with oligodendroglia and myelination. Genes in this data set have been found to be reduced in major depressive disorders and schizophrenia, (Aston et al., 2005; Takahashi et al., 2011). In our study where the animals were euthanized at 14 days post injury, the majority of genes were up regulated, as was verified by the Q-RT-PCR data for the myelin structural encoding component; myelin associated glycoprotein (Mag, Figure 2). The ‘Parkin Pathway’ involves genes associated with Parkinson's Disease (PD), which in the present study were all slightly down regulated 14 days after injury. To determine a possible role of the alterations of these pathways on mouse CNS function, longer term experiments with normal and mice predisposed to PD and depressive like disease will be required. Only then will one be able to make any reasonable hypothesis relating to the effects of blast-TBI on the susceptibility to develop PD and depressive disorders in humans. Other pathways of note are, ‘Aged Mouse Hypothalamus Up’, sets of genes that are up regulated in the hypothalamus of aged (22 months) BALB/c mice, compared to young (2 months) controls (Jiang et al., 2001). This may suggest that the hippocampus of mice exposed to blast-TBI more closely resembled that of an aged mouse. The pathway ‘Matrix Metalloproteinases’ was found to be modestly down regulated in blast-TBI animals 14 days post injury. Gene products of this pathway encompass a family of proteinases, and their inhibitors, with roles in neuronal developmental and acute and chronic disease states, and maintenance of the BBB, (Gong et al., 2012; Fernandez-Lopez et al., 2012; Lee et al., 2010; Lin et al., 2012; Seo et al., 2012; Van Hove et al., 2012). In a more severe open head FPI model in rat, Lin and co-workers (2012) describe a time-dependent increase of the gelatin zymographic activity of MMP-9 measured in brain extracts, the activity peaked by 12 hours and declined by 24 hours post injury. In agreement with this time-dependent finding, Lee and co-workers (2010) describe an irradiation based rodent model where the mRNA levels of the gelatinase, MMP-2, increased and then declined over a 24 hour period. Furthermore, in human plasma MMP-9 protein levels were shown to increase and dissipate in a time-dependent manner following a 21 day period after a severe TBI (Gong et al., 2012). Presently, there are no published data examining the effects of blast-TBI on MMP regulation. Whereas Rubovitch et al., 2011 provide evidence of a leaky BBB observed at 30 days after blast injury in the same model used herein, we have no data relating to MMP levels at this time point. If both forms of TBI-induced MMP regulations are time-dependent, at 14 days post blast injury our data may illustrate an intermediate time point where MMPs involved in the regulation of the BBB may be more active at later times. Subsequent studies would need to be performed on tissues from 30 days post injury to address this question.
The pathway ‘Hippocampus Development Postnatal’ was up regulated in both types of TBI that we evaluated. Genes involved in these pathways are related to neuronal proliferation, differentiation and synapse formation (Mody et al., 2001). Studies describing the up regulations of neuronal NOS (NOS1) after TBI have been documented, while the precise role of NOS1 is under debate, some have attributed increased nitric oxide reactive species with a role in TBI-induced neurodegeneration (Rao et al., 1999; Wada et al., 1999), while others suggest more of a protective role (Khaldi et al., 2002). The identification of up regulations of the ‘NOS1 Pathway’ in both forms of TBI here, may have contributed to the observed NOR deficit. Trauma-induced activation of brain glial cells is a common finding in rodent models of TBI (Acosta et al., 2013; Saijo and Glass, 2011). The induction of inflammation in brain is likely due to the high abundance of glial cells in brain tissues (Azevedo et al., 2009). Unfortunately, without detailed immunohistochemical analysis it is difficult to be definitive regarding the cellular source of the changes in observed genes related to non-CNS specific pathways such as cellular metabolism. With this caveat in mind the following metabolic pathways were found to be significantly up regulated: ‘Mootha Voxphos’; ‘Electron Transport Chain’; ‘Oxidative Phosphorylation’; ‘Ubiquinone Biosynthesis’. These pathways are involved in cellular energy generation, suggesting that both injuries induce changes in the regulation of energy metabolism (Mootha et al., 2003). As injury-induced dysregulation in energy metabolism is commonly seen in TBI (Marino et al., 2007; Scafidi et al., 2009; Verweij et al., 2007), data observed here from pathways measured at 14 days after induction of injury may represent changes in the metabolic states of glial cells. Detailed immunohistochemical analysis of physical- and blast-TBI brain tissues over an expanded time course would be required to provide a clearer understanding of any changes in glial and neuronal inflammatory and metabolic states.
The pathways ‘Stemcell Neural Up’, ‘Stemcell Embryonic Up’, Stemcell Common Up’, ‘HSC Late progenitors Shared, Adult and Fetal’ and ‘IDX TSA Down Clusters’ are related to genes associated with the differentiation of various cell types and stem cells (Burton et al., 2004; Ivanova et al., 2002; Ramalho-Santos et al., 2002). CNS insults such as TBI have been documented to activate neural progenitor cells to differentiate into immature neurons (Bye et al., 2011 and Thau-Zuchman et al., 2010). However, there is also a significant body of evidence that indicates a low level of survival of newly generated neurons following stroke and TBI (Arvidsson et al., 2002; Parent et al., 2002; Ng et al., 2012; Acosta et al., 2013). While the precise mechanisms responsible for the low survival rate of neurons are under investigation, inroads have been made that point to a detrimental role of inflammatory factors on newly generated neuron cell survival (Das and Basu, 2008; Ekdahl et al., 2003; Monje et al., 2003; Russo et al., 2012; Acosta et al., 2013). Israelsson and co-workers (2009) illustrated marked time-dependent changes in neocortex and hippocampal gene expressions of proteins involved in inflammation in this model of physical-TBI. Similarly, work by Baratz and co-workers (2011) described beneficial effects of a small molecule synthesis inhibitor of TNF-α protein, a potent pro-inflammatory protein, on behavioral measures evaluated in the same weight drop model used herein. These studies, together, provide strong supporting evidence of a time-dependent development of inflammation in this closed head weight drop model that, importantly, is confirmed by the significant detection of the ‘Inflammation Pathway’ shown in Figure 3. The observed down regulation of genes associated with stem cell pathways presented here represent TBI-induced changes in the cellular microenvironment that are detrimental towards the survival of neurogenesis derived neurons in the setting of TBI observed at this later time point.
The implications of the changes in regulation of ‘Alzheimer’s Disease Up’ and ‘Alzheimer’s Disease Down’ likely will only be understood after studying behavioral and pathological changes in mice predisposed to dementia like disease states after TBI over longer time periods than that used herein. From these studies, novel targets for pharmacological treatments with relevance to human disease may be identified. Due to the evidence that points towards a relationship between TBI and the subsequent development of dementias exemplified by Alzheimer’s disease (Campdelacreu, 2012; Shively et al., 2012; Wang et al., 2012), such studies warrant immediate attention.
In summary we describe a body of work where cognitive and emotional behavioral outcomes induced by two distinctly different forms of TBI appear, on the surface, to be identical. To a certain extent this also has been seen in human TBI, Belanger and co-workers (Belanger et al., 2009) describe findings in which they assessed and compared human neuropsychological measures in patients exposed to blast- and non blast-TBI. Our behavioral data are inline with these findings. However, based upon our molecular screening of gene transcripts regulated by the two forms of TBI, there are many common and unique changes in gene expression. Likely the most significant findings and advancements presented lie in the observations of reductions in a series of stem cell pathways (e.g. ‘Stemcell Neural Up’), and the identification of altered ‘Aston Oligodendroglia Myelination Subset’ and the ‘Alzheimer’s Disease Up/Dn’ pathways. The array data was in part validated by Q-RT-PCR, and the identification of these pathways provides a foundation to allow for more focused exploration of genes and families of genes activated by TBI that should be investigated and pursued for drug discovery.
Supplementary Material
Highlights.
Physical-TBI and blast-TBI induce similar behavioral deficits.
Physical-TBI and blast-TBI induce common and unique gene expressions.
Physical-TBI and blast-TBI induce common and unique molecular pathways.
Identified pathways will provide a basis for novel drug discovery.
Acknowledgements
The authors wish to acknowledge Dr. Henriette van Pragg as well as William Wood III and Elin Lehrmann for their help in the preparation of this manuscript. This research was supported in part by the Intramural Research Programs of both the National Institute on Aging and National Institute on Drug Abuse, National Institutes of Health, and by the Sackler School of Medicine, Tel-Aviv University, and in part by a grant from the Israeli science foundation, grant no. 108/09.
Abbreviations
- TBI
traumatic brain injury
- physical-TBI
physical-traumatic brain injury
- blast-TBI
blast-traumatic brain injury
- DAI
diffuse axonal injury
- NOR
novel object recognition
- EPM
elevated plus maze
- PA
passive avoidance
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- CNS
central nervous system
- BBB
blood-brain barrier
- cDNA
complementary DNA
- cRNA
complementary RNA
- PAGE
Parametric Analysis of Gene Set Enrichment
- Q-RT-PCR
quantitative reverse transcriptase PCR
- GO term
gene ontology term
- GOI
genes of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Tweedie, Email: tweedieda@grc.nia.nih.gov.
Lital Rachmany, Email: litalrac@post.tau.ac.il.
Vardit Rubovitch, Email: rubovitc@post.tau.ac.il.
Yongqing Zhang, Email: zhangyon@grc.nia.nih.gov.
Kevin G. Becker, Email: BeckerK@grc.nia.nih.gov.
Evelyn Perez, Email: perezev@grc.nia.nih.gov.
Barry J. Hoffer, Email: bjh82@case.edu.
Chaim G. Pick, Email: pickc@post.tau.ac.il.
Nigel H. Greig, Email: greign@grc.nia.nih.gov.
References
- Abdel Baki SG, Kao HY, Kelemen E, Fenton AA, Bergold PJ. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res. 2009;1280:98–106. doi: 10.1016/j.brainres.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond D, et al. Long-Term Upregulation of Inflammation and Suppression of Cell Proliferation in the Brain of Adult Rats Exposed to Traumatic Brain Injury Using the Controlled Cortical Impact Model. PLoS One. 2013;8(1):e53376. doi: 10.1371/journal.pone.0053376. Published online 2013 January 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR. Diffuse axonal injury in head injuries caused by a fall. Lancet. 1984;324:1420–1422. doi: 10.1016/s0140-6736(84)91620-9. [DOI] [PubMed] [Google Scholar]
- Alexander MP. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- Ambrogi Lorenzini CG, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of ventral hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response memory trace. Brain Res. 1997;768:242–248. doi: 10.1016/s0006-8993(97)00651-3. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurology. 2009;513:532–541. doi: 10.1002/cne.21974. 2009. [DOI] [PubMed] [Google Scholar]
- Bandres E, Andion E, Escalada A, Honorato B, Catalan V, Cubedo E, et al. Gene expression profile induced by BCNU in human glioma cell lines with differential MGMT expression. J Neuro-Oncol. 2005;73:189–198. doi: 10.1007/s11060-004-5174-5. [DOI] [PubMed] [Google Scholar]
- Baratz R, Rubovitch V, Frenk H, Pick CG. The influence of alcohol on behavioral recovery after mTBI in mice. J Neurotrauma. 2010;27:555–563. doi: 10.1089/neu.2009.0891. [DOI] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, et al. Tumor necrosis factor-α synthesis inhibitor, 3,6'-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem. 2011;118:1032–1042. doi: 10.1111/j.1471-4159.2011.07377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching to-sample deficits in monkeys. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9:562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Kretzmer T, Yoash-Gantz R, Pickett T, Tupler LA. Cognitive sequelae of blast-related versus other mechanisms of brain trauma. J Int Neuropsychol Soc. 2009;15:1–8. doi: 10.1017/S1355617708090036. [DOI] [PubMed] [Google Scholar]
- Bryan C, Hernandez AM. Magnitudes of decline on Automated Neuropsychological Assessment Metrics subtest scores relative to predeployment baseline performance among service members evaluated for traumatic brain injury in Iraq. J Head Trauma Rehabil. 2012;27:45–54. doi: 10.1097/HTR.0b013e318238f146. [DOI] [PubMed] [Google Scholar]
- Burton GR, Nagarajan R, Peterson CA, McGehee RE., Jr Microarray analysis of differentiation-specific gene expression during 3T3-L1 adipogenesis. Gene. 2004;329:167–185. doi: 10.1016/j.gene.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Campdelacreu J. Parkinson disease and Alzheimer disease: environmental risk factors. Neurologia. 2012 doi: 10.1016/j.nrl.2012.04.001. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bye N, Carron S, Han X, Agyapomaa D, Ng SY, Yan E, Rosenfeld JV, Morganti-Kossmann MC. Neurogenesis and glial proliferation are stimulated following diffuse traumatic brain injury in adult rats. J Neurosci Res. 2011;89:986–1000. doi: 10.1002/jnr.22635. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus. 1991;1:329–340. doi: 10.1002/hipo.450010323. [DOI] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, Qu Y, Gu J, Wang J, et al. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neuroscience. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. An ethological analysis of the effects of chlordiazepoxide and bretazenil (Ro 16-6028) in the murine elevated plus-maze. Behav Pharmacol. 1993;4:573–580. [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological comparison of the effects of diazepam and acute/chronic imipramine on the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav. 1995 Nov;52:473–478. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury-football, warfare, and longterm effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- De Kruijk JR, Twijnstra A, Meerhoff S, Leffers P. Management of mild traumatic brain injury: lack of consensus in Europe. Brain Inj. 2001;15:117–123. doi: 10.1080/026990501458353. [DOI] [PubMed] [Google Scholar]
- Dix S, Aggleton JP. Extending the spontaneous preference of recognition: evidence of object-location and object-context recognition. Behav Brain Res. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Edut S, Rubovitch V, Schreiber S, Pick CG. The intriguing effects of ecstasy (MDMA) on cognitive function in mice subjected to a minimal traumatic brain injury (mTBI) Psychopharmacology. 2011;214:877–889. doi: 10.1007/s00213-010-2098-y. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk AC. A nurse-led paediatric head injury follow-up service. Scand J Caring Sci. 2012 doi: 10.1111/j.1471-6712.2012.00999.x. [DOI] [PubMed] [Google Scholar]
- Fernández-López D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 2012;32:9588–9600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman RH, Jenkins LW, Switzer RC, 3rd, Bauman RA, Tong LC, Swauger PV, et al. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learning & Memory. 2003;10:525–530. doi: 10.1101/lm.64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Hao M, Liu L, Liu C, Dong J, Cui Z, et al. Prognostic relevance of circulating endothelial progenitor cells for severe traumatic brain injury. Brain Inj. 2012;26:291–297. doi: 10.3109/02699052.2011.648710. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Yamasaki M, Konishi M, Itoh N. Spatially restricted expression of fibroblast growth factor-10 mRNA in the rat brain. Brain Res Mol Brain Res. 1997;47:139–146. doi: 10.1016/s0169-328x(97)00044-2. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Hunt MC, Siponen MI, Alexson SE. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta. 2012;1822:1397–1410. doi: 10.1016/j.bbadis.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Israelsson C, Wang Y, Kylberg A, Pick CG, Hoffer BJ, Ebendal T. Closed head injury in a mouse model results in molecular changes indicating inflammatory responses. J Neurotrauma. 2009;26:1307–1314. doi: 10.1089/neu.2008.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proc Natl Acad Sci U S A. 2001;98:1930–1934. doi: 10.1073/pnas.98.4.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldi A, Chiueh CC, Bullock MR, Woodward JJ. The significance of nitric oxide production in the brain after injury. Ann N Y Acad Sci. 2002;962:53–59. doi: 10.1111/j.1749-6632.2002.tb04055.x. [DOI] [PubMed] [Google Scholar]
- Lee WH, Warrington JP, Sonntag WE, Lee YW. Irradiation alters MMP-2/TIMP-2 system and collagen type IV degradation in brain. Int J Radiat Oncol Biol Phys. 2012;82:1559–1566. doi: 10.1016/j.ijrobp.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kuroiwa T, Katsumata N, Ishibashi S, Sun LY, Endo S, et al. Transient versus prolonged hyperlocomotion following lateral fluid percussion injury in mongolian gerbils. J Neurosci Res. 2006;83:292–300. doi: 10.1002/jnr.20720. [DOI] [PubMed] [Google Scholar]
- Lin Y, Pan Y, Wang M, Huang X, Yin Y, Wang Y, Jia F, Xiong W, Zhang N, Jiang JY. Blood-brain barrier permeability is positively correlated with cerebral microvascular perfusion in the early fluid percussion-injured brain of the rat. Lab Invest. 2012;92:1623–1634. doi: 10.1038/labinvest.2012.118. [DOI] [PubMed] [Google Scholar]
- Luukkainen S, Riala K, Laukkanen M, Hakko H, Räsänen P. Association of traumatic brain injury with criminality in adolescent psychiatric inpatients from Northern Finland. Psychiatry Res. 2012 doi: 10.1016/j.psychres.2012.04.018. http://dx.doi.org/10.1016/j.psychres.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, et al. Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2007;78:501–507. doi: 10.1136/jnnp.2006.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara KC, Lisembee AM, Lifshitz J. The whisker nuisance task identifies a late-onset, persistent sensory sensitivity in diffuse brain-injured rats. J Neurotrauma. 2010;27:695–706. doi: 10.1089/neu.2009.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez CV, Hurley RA, Lassonde M, Zhang L, Taber KH. Mild traumatic brain injury: neuroimaging of sports-related concussion. J Neuropsychiatry Clin Neurosci. 2005;17:297–303. doi: 10.1176/jnp.17.3.297. [DOI] [PubMed] [Google Scholar]
- Messé A, Caplain S, Pélégrini-Issac M, Blancho S, Montreuil M, Lévy R, et al. Structural integrity and postconcussion syndrome in mild traumatic brain injury patients. Brain Imaging Behav. 2012;6:283–292. doi: 10.1007/s11682-012-9159-2. [DOI] [PubMed] [Google Scholar]
- Messier C. Object recognition in mice: improvement of memory by glucose. Neurobiol Learn Mem. 1997;67:172–175. doi: 10.1006/nlme.1996.3755. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: Lessons from studies in rats. Behavioural Brain Research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Astur RS, Weisend MP, Sutherland RJ. Retrograde amnesia and selective damage to the hippocampal formation: Memory for places and object discriminations. Behavioural Brain Research. 1999;106:97–107. doi: 10.1016/s0166-4328(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Ann New York Acad Sci. 2000;911:166–174. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- Ng SY, Semple BD, Morganti-Kossmann MC, Bye N. Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. J Neurotrauma. 2012;29:1410–1425. doi: 10.1089/neu.2011.2188. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampusdependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Rachmany L, Tweedie D, Li Y, Rubovitch V, Holloway HW, Miller J, et al. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr) 2012 doi: 10.1007/s11357-012-9464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. "Stemness": transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Bowen KK, Dempsey RJ. Traumatic injury to rat brain upregulates neuronal nitric oxide synthase expression and L-[3H]nitroarginine binding. J Neurotrauma. 1999;16:865–877. doi: 10.1089/neu.1999.16.865. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behavioural Neuroscience. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, et al. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54(Suppl 1):S89–S97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG. The intricate involvement of the Insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis. 2010;38:299–303. doi: 10.1016/j.nbd.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, et al. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011;232:280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Caracciolo L, Tweedie D, Choi SH, Greig NH, Barlati S, et al. 3,6'-Dithiothalidomide, a new TNF-α synthesis inhibitor, attenuates the effect of Aβ1–42 intracerebroventricular injection on hippocampal neurogenesis and memory deficit. J Neurochem. 2012;122:1181–1192. doi: 10.1111/j.1471-4159.2012.07846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sajja VS, Galloway MP, Ghoddoussi F, Thiruthalinathan D, Kepsel A, Hay K. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. NMR Biomed. 2012 doi: 10.1002/nbm.2805. [DOI] [PubMed] [Google Scholar]
- Sanders MJ, Dietrich WD, Green EJ. Behavioral, electrophysiological, and histopathological consequences of mild fluid-percussion injury in the rat. Brain Res. 2001;904:141–144. doi: 10.1016/s0006-8993(01)02424-6. [DOI] [PubMed] [Google Scholar]
- Scafidi S, O'Brien J, Hopkins I, Robertson C, Fiskum G, McKenna M. Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J Neurochem. 2009;109 Suppl 1:189–197. doi: 10.1111/j.1471-4159.2009.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JH, Guo S, Lok J, Navaratna D, Whalen MJ, Kim KW. Neurovascular matrix metalloproteinases and the blood-brain barrier. Curr Pharm Des. 2012;18:3645–3648. doi: 10.2174/138161212802002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Perl DP. Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military-past, present, and future. J Head Trauma Rehabil. 2012;27:234–239. doi: 10.1097/HTR.0b013e318250e9dd. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, Pick CG. Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res. 2007;1130:197–205. doi: 10.1016/j.brainres.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, et al. Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci. 2009;37:16–24. doi: 10.1007/s12031-008-9094-2. [DOI] [PubMed] [Google Scholar]
- Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:1008–1016. doi: 10.1038/jcbfm.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, et al. Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res. 2007;85:805–815. doi: 10.1002/jnr.21160. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Rachmany L, Rubovitch V, Lehrmann E, Zhang Y, Becker KG, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exper Neurol. 2012 doi: 10.1016/j.expneurol.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove I, Verslegers M, Buyens T, Delorme N, Lemmens K, Stroobants S, et al. An aberrant cerebellar development in mice lacking matrix metalloproteinase-3. Mol Neurobiol. 2012;45:17–29. doi: 10.1007/s12035-011-8215-z. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Amelink GJ, Muizelaar JP. Current concepts of cerebral oxygen transport and energy metabolism after severe traumatic brain injury. Prog Brain Res. 2007;161:111–124. doi: 10.1016/S0079-6123(06)61008-X. http://dx.doi.org/10.1016/S0079-6123(06)61008-X. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain injury in the rat. J Neurotrauma. 1999;16:203–212. doi: 10.1089/neu.1999.16.203. [DOI] [PubMed] [Google Scholar]
- Wang HK, Lin SH, Sung PS, Wu MH, Hung KW, Wang LC, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry. 2012;83:1080–1085. doi: 10.1136/jnnp-2012-302633. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei Y, Oguntayo S, Wilkins W, Arun P, Valiyaveettil M, et al. Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. J Neurotrauma. 2011;28:2171–2183. doi: 10.1089/neu.2011.1990. [DOI] [PubMed] [Google Scholar]
- Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP. The Effect of Injury Severity on Behavior: A Phenotypic Study of Cognitive and Emotional Deficits after Mild, Moderate, and Severe Controlled Cortical Impact Injury in Mice. J Neurotrauma. 2012;29:2283–2296. doi: 10.1089/neu.2012.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Hua MS, Lin WC, Tsai YH, Huang SJ. Irritability following traumatic brain injury: Divergent manifestations of annoyance and verbal aggression. Brain Inj. 2012;26:1185–1191. doi: 10.3109/02699052.2012.666374. [DOI] [PubMed] [Google Scholar]
- Yu S, Kaneko Y, Bae E, Stahl CE, Wang Y, van Loveren H, et al. Severity of controlled cortical impact traumatic brain injury in rats and mice dictates degree of behavioral deficits. Brain Res. 2009;1287:157–163. doi: 10.1016/j.brainres.2009.06.067. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]
- Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG. Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp. 2011;71:36–45. doi: 10.55782/ane-2011-1821. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR. Relationship between magnitude of damage to the hippocampus and impaired recognition memory in monkeys. Hippocampus. 2001;11:92–98. doi: 10.1002/hipo.1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.