Abstract
Application of isoflurane, a volatile anesthetic, after brain ischemia can reduce ischemic brain injury in rodents (isoflurane postconditioning). This study is designed to determine whether isoflurane postconditioning improves long-term neurological outcome after focal brain ischemia and whether this protection is mediated by attenuating neuroinflammation. Adult male Sprague–Dawley rats were subjected to a 90-min middle cerebral arterial occlusion (MCAO). Isoflurane postconditioning was performed by exposing rats to 2% isoflurane for 60 min immediately after the MCAO. Isoflurane postconditioning reduced brain infarct volumes, apoptotic cells in the ischemic penumbral brain tissues and neurological deficits of rats at 4 weeks after the MCAO. Isoflurane postconditioning reduced brain ischemia/reperfusion-induced nuclear transcription factor (NF)-κB (NF-κB) activation as well as interleukin 1β (IL-1β) and interleukin-6 production in the ischemic penumbral brain tissues at 24 h after the MCAO. IL-1β deficient mice had smaller brain infarct volumes and better neurological functions than wild-type mice at 24 h after a 90-min focal brain ischemia. Isoflurane posttreatment failed to induce neuroprotection in the IL-1β deficient mice. Our results suggest that isoflurane postconditioning improved long-term neurological outcome after transient focal brain ischemia. This protection may be mediated by inhibiting NF-κB activation and the production of the proinflammatory cytokine IL-1β.
Keywords: interleukin 1β, isoflurane, neuroprotection, nuclear factor-κB, postconditioning
Introduction
Ischemic brain injury is the underlying pathophysiology for common human diseases, such as stroke and brain trauma. However, up till now, clinically effective and safe methods for reducing ischemic brain injury have not been established.
Postconditioning is a promising approach to reduce ischemic cell injury. The concept “ischemic postconditioning” was introduced in the literature in 2003 to describe a phenomenon in which short episodes of ischemia during the early phase of reperfusion after a prolonged ischemia in the heart reduced cardiac infarct size (Zhao et al., 2003). Ischemic postconditioning-induced neuroprotection was shown for the first time in 2006 (Zhao et al., 2006; Burda et al., 2006; Danielisova et al., 2006). However, it may be difficult to apply ischemic postconditioning in clinical practice because well-controlled, short episodes of brain ischemia are almost impossible to perform. We have shown that isoflurane application during reperfusion also reduces ischemic brain injury in rats (Lee et al., 2008). Isoflurane is a volatile anesthetic and has been used in clinical practice for 3 decades. The isoflurane postconditioning-induced neuroprotection requires the application of isoflurane within 2 h after the onset of simulated reperfusion in human neuron-like cell cultures (Lin et al., 2011). If this neuroprotection is confirmed in humans, it may be particularly applicable to patients during cardiovascular surgery and use of tissue plasminogen activator for ischemic stroke because the time of brain reperfusion can be predicated accurately in these patients.
Very little is known about the mechanisms for isoflurane postconditioning effects in the brain. It is known that brain ischemia and reperfusion induce neuroinflammation that can activate intracellular signaling pathways to cause cell death or injury (Lipton, 1999; Iadecola and Anrather, 2011; Huang et al., 2006; Lakhan et al., 2009). Isoflurane has been shown to inhibit the activation of nuclear transcription factor (NF)-κB (NF-κB) by proinflammatory initiators, such as lipopolysaccharide (de Rossi et al., 2004). NF-κB activation is a critical step to induce cytokine production (Liu et al., 1999; Giacomini et al., 2011). Thus, we hypothesize that isoflurane postconditioning-induced neuroprotection involves attenuation of ischemia/reperfusion-induced NF-κB activation and proinflammatory cytokine production. To test this hypothesis, we used a transient focal brain ischemia model in rats and mice. Since testing whether a protective strategy can improve the long-term neurological outcome after brain ischemia in pre-clinical studies is recommended by experts in the field (Fisher et al., 2009), we also determined whether isoflurane postconditioning improved the long-term neurological outcome after focal brain ischemia in this study.
Materials and methods
Animals
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80–23, revised in 1996). Efforts were made to minimize the number of animals used and their suffering.
Study groups of rats
Two-month old male Sprague–Dawley rats weighing 280 to 300 g (Charles River Laboratories Inc, Wilmington, MA) were randomly assigned to be postconditioned or not postconditioned with 2.0% isoflurane for 60 min immediately after a 90-min right middle cerebral arterial occlusion (MCAO). Their motor coordination and neurological deficit scores were evaluated 7, 14 and 28 days after the MCAO. The infarct volumes were evaluated 28 days after the MCAO. A control group was used in the study determining the density of terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labeling (TUNEL) positive cells in the ischemic penumbral cerebral cortex. These control rats were not subjected to MCAO or isoflurane exposure.
In the second set of experiments, male Sprague–Dawley rats weighing 280 to 300 g were randomly assigned to five groups. In group A, rats were subjected to a 90-min right MCAO only. Rats in group B were postconditioned with 2% isoflurane for 60 min immediately after the MCAO. Rats in group C received intraperitoneal injection of 200 mg/kg pyrrolidine dithiocarbamate (PDTC) and 30 min later were subjected to the 90-min MCAO. Rats in group D received intraperitoneal injection of 200 mg/kg PDTC 30 min before the MCAO that was followed up immediately with the exposure to 2% isoflurane for 60 min. Rats in group E received sham operation.
Middle cerebral arterial occlusion and isoflurane postconditioning in rats
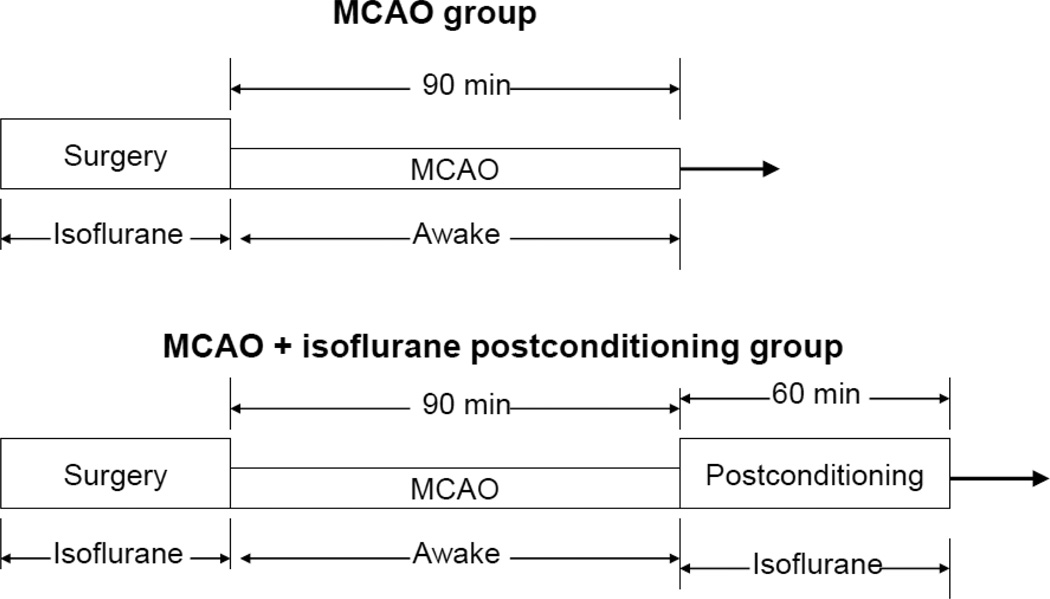
Right MCAO was created as we described before (Lee et al., 2008). Briefly, rats were induced with isoflurane, intubated and mechanically ventilated. Anesthesia was maintained with 2% isoflurane. Right MCAO was achieved by advancing a 3-0 monofilament nylon suture (Beijing Sunbio Biotech Co. Ltd., Beijing, China). Isoflurane anesthesia was stopped immediately for all groups of rats once the suture was in place (Fig. 1). After recovery from anesthesia, rats were placed back into their cages with ad libitum access to food and water. The nylon suture was removed at 90 min after the onset of MCAO. The rats in the MCAO plus isoflurane postconditioning group were maintained under anesthesia with 2% isoflurane via an endotracheal tube for 60 min after the removal of the nylon suture (Fig. 1). During MCAO and postconditioning period, temporalis muscle temperature was strictly maintained at 37 ± 0.2 °C by a warming blanket. The inhaled and exhaled gases were also monitored with a Datex infrared analyzer (Capnomac, Helsinki, Finland) and mechanical ventilation was used to maintain normal end-tidal carbon dioxide concentrations. Their blood pressures were monitored non-invasively by a CODA monitor (Kent Scientific Corporation, Torrington, CT). Their heart rates, breathing rates, and pulse oximeter oxygen saturation were monitored continuously and noninvasively using a MouseOX Murine Plus Oximeter System (Starr Life Sciences Corporation, Oakmont, PA, USA) as we did before (Li and Zuo, 2011). While the animals in the isoflurane postconditioning group were exposed to isoflurane, animals in the MCAO group or sham-operated group were placed in an air-tight chamber gassed with the carrier gases (85% O2) for 60 min.
Fig. 1. Diagrammatic presentation of the experimental protocol.
Evaluation of motor coordination, neurological deficit scores and infarct volumes of rats
Motor coordination was evaluated 24 h before the MCAO and 7, 14 and 28 days after MCAO (Li and Zuo, 2009; Zhao et al., 2007; Li et al., 2012). The rats were placed on an accelerating rotarod. The speed of the rotarod was increased from 4 rpm to 40 rpm in 5 min. The latency and the speed at which rats fell off the rotarod were recorded. Each rat was tested three times. The speed–latency index (latency in seconds × speed in rpm) of each of the three tests was calculated. The mean index of the three trials was used to reflect the motor coordination function of each rat before or after the MCAO. All rats were trained for three continuous days before the formal tests. They were placed on the rotarod three times each day, and this training occurred just before they were subjected to the MCAO protocol.
Neurological deficit scores were evaluated 7, 14 and 28 days after MCAO. Neurological deficit scores were evaluated based on an eight-point scale by a person blinded to the group assignment. Rats were scored as follows: zero, no apparent deficits; one, failure to extend left forepaw fully; two, decreased grip of the left forelimb; three, spontaneous movement in all directions, contralateral circling only if pulled by the tail; four, circling or walking to the left; five, walking only if stimulated; six, unresponsiveness to stimulation and with depressed level of consciousness; and seven, dead (Rogers et al., 1997).
The evaluation of infarct volumes at 4 weeks after the MCAO was performed after Nissl staining as described previously (Li and Zuo, 2009; Sakai et al., 2007). The assessments of infarct volumes were performed by a person blinded to the group assignment. Briefly, rats were euthanized by 5% isoflurane and transcardiacally perfused with saline and then 4% phosphate-buffered paraformaldehyde. Brains were removed and stored in the same fixative solution for 7 days. Eight-micrometer-thick paraffin coronal sections were taken by using a microtome at 2 mm intervals (usually total 7 slices) over the entire brain. The infarct areas in each brain section were quantified using the NIH Image 1.60. The infarct volumes were calculated as follows. The sum of the infarct areas in the rostral and caudal sides of each brain slice was divided by two to get the average infarct area of the brain slice. The infarct volume of the brain slice was calculated by multiplying the average infarct area of the slice by the thickness of the slice (2 mm). The total infarct volume in the brain was the sum of infarct volume of each brain slice. To account for differential shrinkage resulting from brain ischemia and tissue processing and to correct for the individual difference in brain volumes, the percentage of infarct volume in the ipsilateral hemisphere volume was calculated (Swanson et al., 1990).
TUNEL and TUNEL-positive cell density counting
To determine the density of TUNEL positive cells in the ischemic penumbral regions, sections at bregma +1.5 mm were stained using a TUNEL kit (catalog number 17–141; Millipore, Temecula, CA). The staining was performed according to the protocol provided by the manufacturer. Positive staining was revealed by fluorescein isothiocyanate–conjugated avidin and visualized under a fluorescent microscope. The density of TUNEL-positive cells was determined by a person blinded to the group assignment. A reticle (~0.585 mm2) was used to count cells in the same size area. Five determinations, each on different locations in the ischemic penumbral cerebral cortex that is immediately adjacent to the infarct areas under microscope, were performed and averaged to yield a single number (density of the TUNEL-positive cells) for the individual rat.
Brain tissue harvesting
Rats were killed by deep isoflurane anesthesia and transcardially perfused with normal saline at 24 h after the MCAO. The right frontal brain cortex area 1 (Fr1), an ischemic penumbral region in this model (Zheng and Zuo, 2004), between bregma +2 to 0 mm was harvested for Western analysis of NF-κB expression in the nuclear fraction. The left Fr1 and the bilateral hippocampi also were harvested for ELISA assay of cytokines.
Preparation of nuclear proteins
The nuclear proteins were prepared according to the protocol described by Abcam (Cambridge, MA). Briefly, brain tissues were incubated on ice for 10 min in a buffer containing 10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol and 0.05% NP40 (pH 7.9) as well as protease inhibitors (10 mg/ml aproteinin, 5 mg/ml peptastin, 5 mg/ml leupeptin, and 1 mM phenylmethanesulfonylfluoride). They were then centrifuged at 4°C at 3000 rpm for 10 min. The resulted pellet was suspended in a buffer containing 5 mM HEPES, 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol and 26% glycerol (v/v) (pH 7.9) as well as the protein inhibitor combination. The sample was homogenized with 20 full strokes in a glass homogenizer on ice. They were left on ice for 30 min and then centrifuged at 24,000 g for 20 min at 4°C. The supernatant was harvested as the fraction containing nuclear proteins.
Western analysis
Nuclear proteins prepared from right Fr1 (50 µg protein per lane) were subjected to Western analysis as we described before (Li and Zuo, 2009). The primary antibodies used were the rabbit monoclonal anti-NF-κB p65 antibody (1:1000 dilution; catalog number: 8242; Cell signaling Technology, Danvers, MA) and the rabbit polyclonal anti-histone H3 antibody (1:1000 dilution; catalog number: 9715; Cell signaling Technology). The protein bands were visualized with the enhanced chemiluminescence methods. Quantitative analysis of the protein bands was performed using an Image-Quant 5.0 GE Healthcare Densitometer (GE Healthcare, Sunnyvale, CA). The densities of NF-κB p65 protein bands were normalized to those of H3 proteins from the same sample to control for errors in protein sample loading and transferring during Western analysis. The results from various experimental conditions were normalized to the data of rats subjected to MCAO only on the same film to control for the error caused by different exposure times of films.
ELISA assay of cytokines in the brain tissues
The brain levels of interleukin (IL)-1β, IL-6 and IL-10 at 24 h after the MCAO was determined with Quantikine ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions and as we described before (Lin and Zuo, 2011). Briefly, brain tissues were homogenized on ice in 20 mM Tris-HCl buffer (pH 7.3) containing protease inhibitors (10 mg/ml aproteinin, 5 mg/ml peptastin, 5 mg/ml leupeptin, and 1 mM phenylmethanesulfonylfluoride). Homogenates were centrifuged at 10,000 g for 10 min at 4°C. The supernatant was then ultracentrifuged at 150,000 g for 2 h at 4°C. Bradford protein assay of the supernatant was performed for each sample. The supernatant was used in ELISA. The optical density was measured at 450 nm (correction wavelength was set at 570 nm) and the amount of cytokines was calculated using the assay standard curves. The quantity of IL-1β in each brain sample was standardized to its protein contents. The results from animals under various experimental conditions were then normalized by the mean values of the sham-operated animals in each ELISA assay.
Mouse middle cerebral arterial occlusion and neurological outcome assessment
Six-month old male C57BL/6J mice (stock number 000664) and IL-1β deficient mice (stock number 005576) were obtained from Jackson Laboratories (Bar Harbor, ME). These IL-1β deficient mice have a gene background similar to that of C57BL/6J. They were subjected to the right 90-min MCAO created by the suture technique as we described previously (Li and Zuo, 2011). Isoflurane post-treatment was performed by exposing them to 2% isoflurane for 60 min that was started at the end of the MCAO. The brain infarct volume, neurological deficit scores and performance on rotarod were assessed at 24 h after the MCAO as we described before (Li and Zuo, 2011) and in a similar way as described above for rats.
Mouse exposure to lipopolysaccharide and measurement of IL-1β in the mouse cerebral cortex
As we described before (Kim et al., 2009), eight-week old C57BL/6J male mice received intraperitoneal injection of 4 mg/kg lipopolysaccharide in saline. Two hours later, they were maintained for 30 min in an air-tight chamber that was gassed with oxygen containing 1.5% isoflurane. Mice were killed by deep isoflurane anesthesia and transcardially perfused with normal saline at 6 h after the lipopolysaccharide injection. Right cerebral cortex was harvested and homogenized in a buffer (pH 7.4) containing 200 mM Mannitol, 80 mM HEPES, 41 mM KOH, and protease inhibitor cocktail (Sigma Aldrich, St Louis, MO). The homogenates were centrifuged at 14,000 g for 10 min at 4°C. The supernatants were used for Western blotting with use of the following two primary antibodies: rabbit polyclonal anti-IL-1β antibody (1:200 dilution; Catalog number: SC-7884; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal antiglyceraldehydes 3-phosphate dehydrogenase (GAPDH) antibody (1:2000 dilution; Catalog number: G9545; Sigma-Aldrich).
Statistical analysis
Statistical analysis of the results of physiological parameters, speed–latency index ratio and infarct size between the MCAO group and isoflurane postconditioning plus MCAO group or between the wild-type mice and IL-1β deficient mice was performed by Student’s t-test or by Mann–Whitney rank sum test when the data are not normally distributed. TUNEL positive cell density, Western blotting results and cytokine levels were analyzed by one way analysis of variance followed by the Tukey test. These data are presented as means ± S.E.M. Neurological deficit scores were analyzed by Mann–Whitney rank sum test and are presented in a box plot format. A P ≤ 0.05 was accepted as significant. All statistical analyses were performed with the SigmaStat (SYSTAT Software Inc., Point Richmond, CA).
Results
No rats had an episode of pulse oximeter oxygen saturation (SpO2) less than 90% during the surgery to create MCAO or the postconditioning phase (Table 1). Total 25 rats were used in the MCAO group and 20 rats in the MCAO plus isoflurane postconditioning group. Eight rats in the MCAO group and 2 rats in the MCAO plus isoflurane postconditioning group died during the 4-week observation period. All of these deaths occurred from 6 h to 7 days after the MCAO and had severe brain infarction and edema. The mortality was 32% and 10%, respectively, for the MCAO group and MCAO plus isoflurane group (P = 0.161 by z-test for the comparison). Rats that died during the observation period were included in the analysis of the neurological deficit scores. Only those rats that survived till the end of the 4-week observation period were included in the analyses of brain infarct volumes, performance on rotarod and TUNEL-positive cell density.
Table 1.
Physiological data of rats during transient middle cerebral arterial occlusion (MCAO).
| MCAO group | MCAO + isoflurane group | |||||
|---|---|---|---|---|---|---|
| Before MCAO | During MCAO | after MCAO | Before MCAO | During MCAO | After MCAO | |
| MABP (mmHg) | 102 ± 8 | 105 ± 7 | 104 ± 7 | 101 ± 6 | 103 ± 6 | 103 ± 6 |
| Heart rate (beat/min) | 387 ± 15 | 391 ± 17 | 393 ± 18 | 393 ± 15 | 389 ± 16 | 395 ± 16 |
| Temperature (°C) | 37.0 ± 0.2 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.2 | |
| EtCO2 (mmHg) | 35 ± 4 | 36 ± 4 | 35 ± 4 | 36 ± 4 | 34 ± 5 | |
| SpO2 (%) | 97 ± 1 | 96 ± 1 | 97 ± 1 | 97 ± 1 | 96 ± 1 | 97 ± 1 |
Arterial samples were taken 10 min before and after the onset of MCAO as well as 20 min after MCAO. There were no temperature and EtCO2 data after MCAO for the MCAO group because those rats were awake. Data are means ± S.D. (n = 17 – 18). There were no statistical differences in these physiological data among the rats post-treated with or without 2% isoflurane for 60 min at the onset of reperfusion. MABP: mean arterial blood pressure. EtCO2: end-tidal CO2; SpO2: pulse oximeter oxygen saturation.
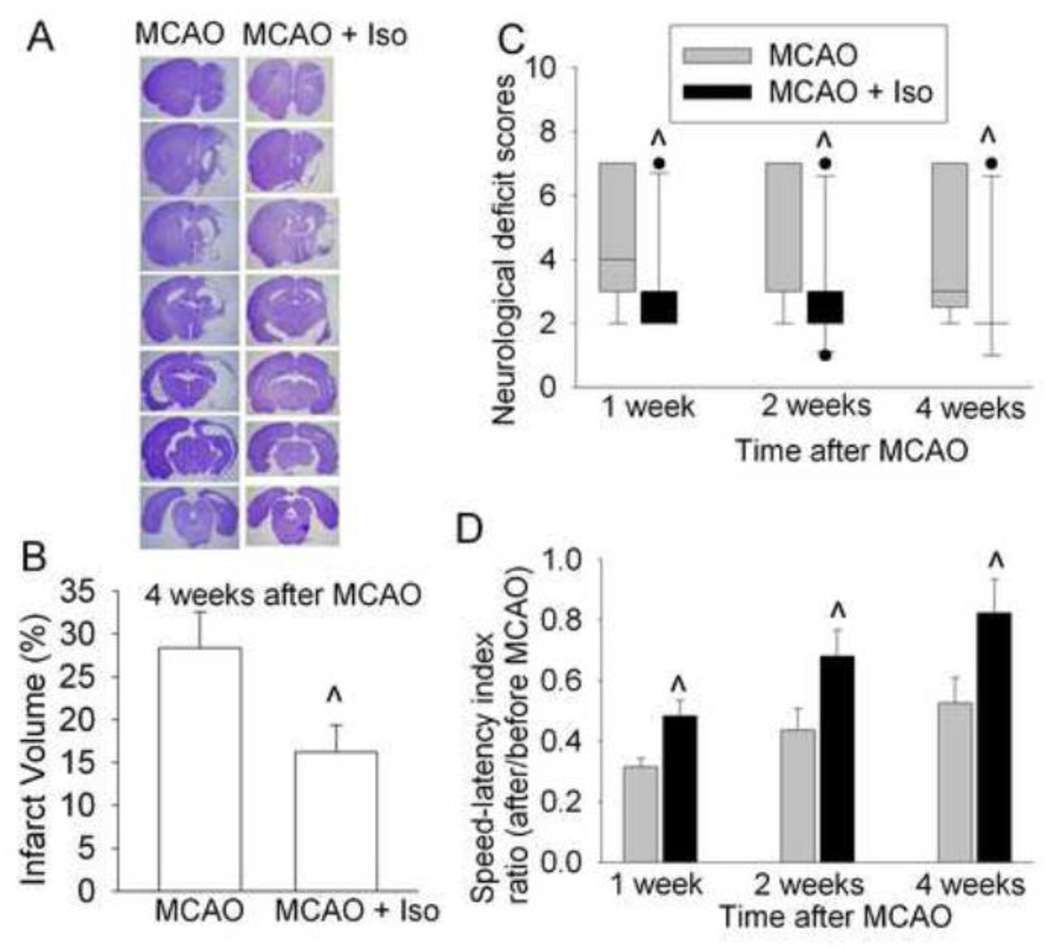
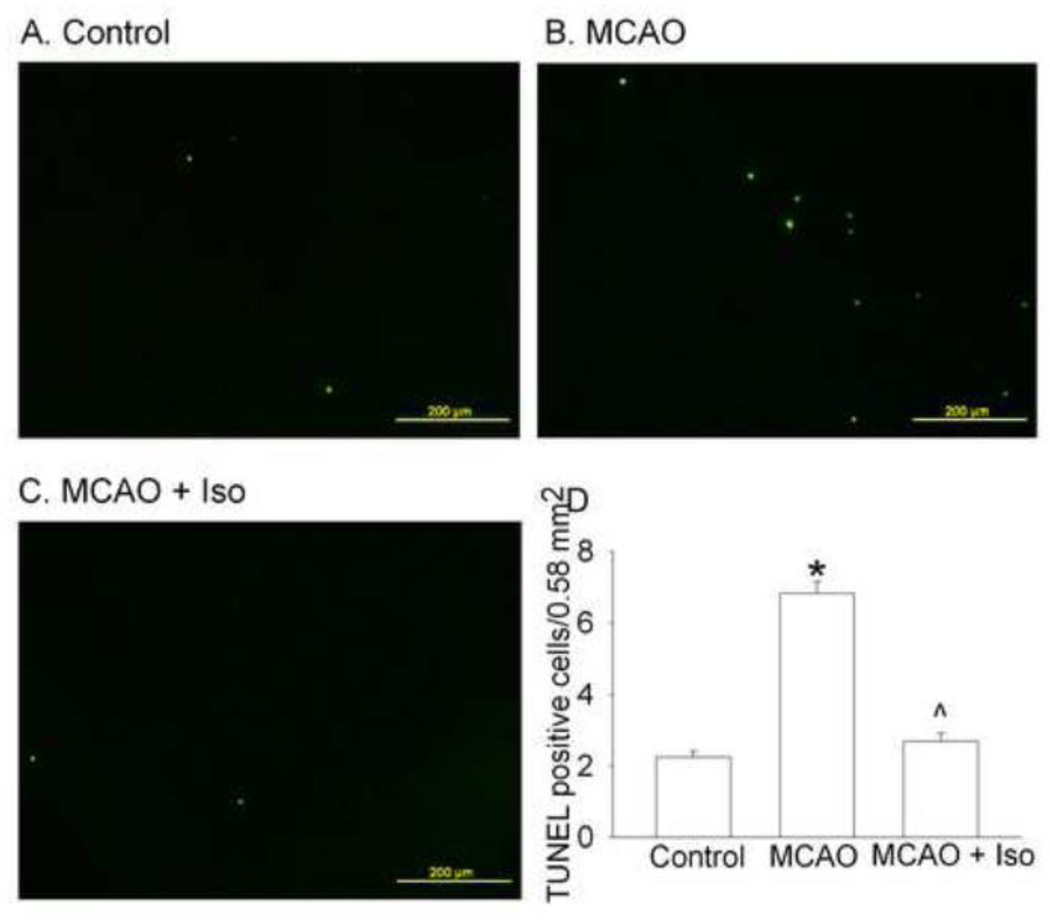
Isoflurane postconditioning significantly reduced the brain infarct volumes at 4 weeks after the transient focal brain ischemia. The neurological deficit scores and performance on rotarod in the rats of MCAO plus isoflurane postconditioning group were better than those of rats in the MCAO only group no matter whether the assessment was performed at 1, 2 or 4 weeks after the MCAO (Fig. 2). Consistent with our previous study (Li and Zuo, 2009), TUNEL-positive cell density in the ischemic penumbral cerebral cortex of rats with MCAO only at 4 weeks after the MCAO was higher than that in the corresponding area of control rats (Fig. 3). These results suggest increased cell death at this delayed time point after brain ischemia. This delayed cell death was blocked by isoflurane postconditioning.
Fig. 2. Isoflurane postconditioning improved histological and neurological function outcome assessed 4 weeks after focal brain ischemia in adult rats.
A: Brain sections after Nissl staining from representative rats subjected to MCAO only or MCAO plus isoflurane postconditioning. B: Percentage of brain infarct volume in ipsilateral hemisphere volume. Results are the means ± S.E.M. (n = 17 – 18). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panel B) or assigned 7 to the animals that died before this end time point for observation. Results are presented in a box plot format (n = 20 – 25). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25 – 75% interval including the median of the data. D: The performance on rotarod. Rats were tested before and 4 weeks after the MCAO and the speed-latency index ratio of these two tests are presented. Results are the means ± S.E.M. (n = 17 – 18). ^ P < 0.05 compared with the corresponding MCAO only group. Iso: isoflurane postconditioning.
Fig. 3. Isoflurane postconditioning reduced dying cells evaluated at 4 weeks after the 90-min right middle cerebral arterial occlusion (MCAO) in rats.
Cells in the penumbral cerebral cortex were examined after terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labeling (TUNEL). Panels A, B and C are representatives of the sections from control, MCAO only and MCAO plus isoflurane postconditioning. Panel D: quantitative data are presented as the means ± S.E.M. (n = 5 for the control group, = 17 for MCAO, and = 18 for iso + MCAO groups). * P < 0.05 compared with the control group. ^ P < 0.05 compared with the MCAO only group. Iso: isoflurane postconditioning.
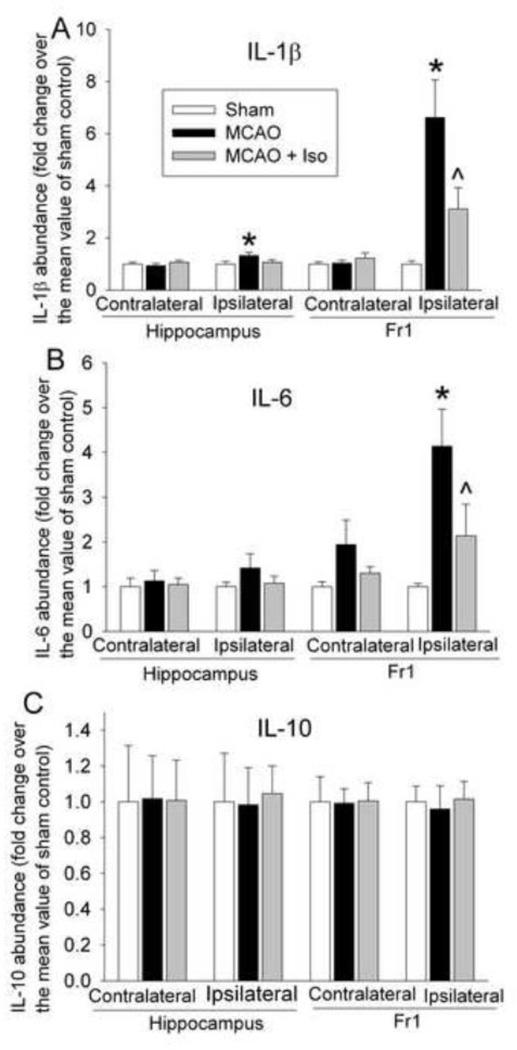
There was a significant increase of IL-1β and IL-6 in the ischemic penumbral cerebral cortex of rats with MCAO only. This increase was attenuated by isoflurane postconditioning. There was also a small but statistical significant increase of IL-1β in the hippocampus ipsilateral to the ischemia side in rats with MCAO only when compared with sham-operated rats. This increase appeared to be attenuated by isoflurane postconditioning. There was no difference in the IL-1β and IL-6 levels in the hippocampus or cerebral cortex contralateral to the brain ischemia side among the animals in the sham-operated, MCAO and MCAO plus isoflurane postconditioning groups. Also, IL-10 levels were not different among the three groups of rats in the hippocampus or cerebral cortex ipsilateral or contralateral to the ischemia side (Fig. 4).
Fig. 4. Attenuation of ischemia-reperfusion-induced interleukin (IL)-1β (panel A), IL-6 (panel B) and IL-10 (panel C) expression by isoflurane postconditioning in rats.
The frontal cerebral cortex area 1 (Fr1) and hippocampus ipisilateral or contralateral to the side of middle cerebral arterial occlusion (MCAO) were harvested at 24 h after the MCAO. Results are the means ± S.E.M. (n = 7 – 17). * P < 0.05 compared to sham-operated control. ^ P < 0.05 compared to MCAO only. Iso: isoflurane postconditioning.
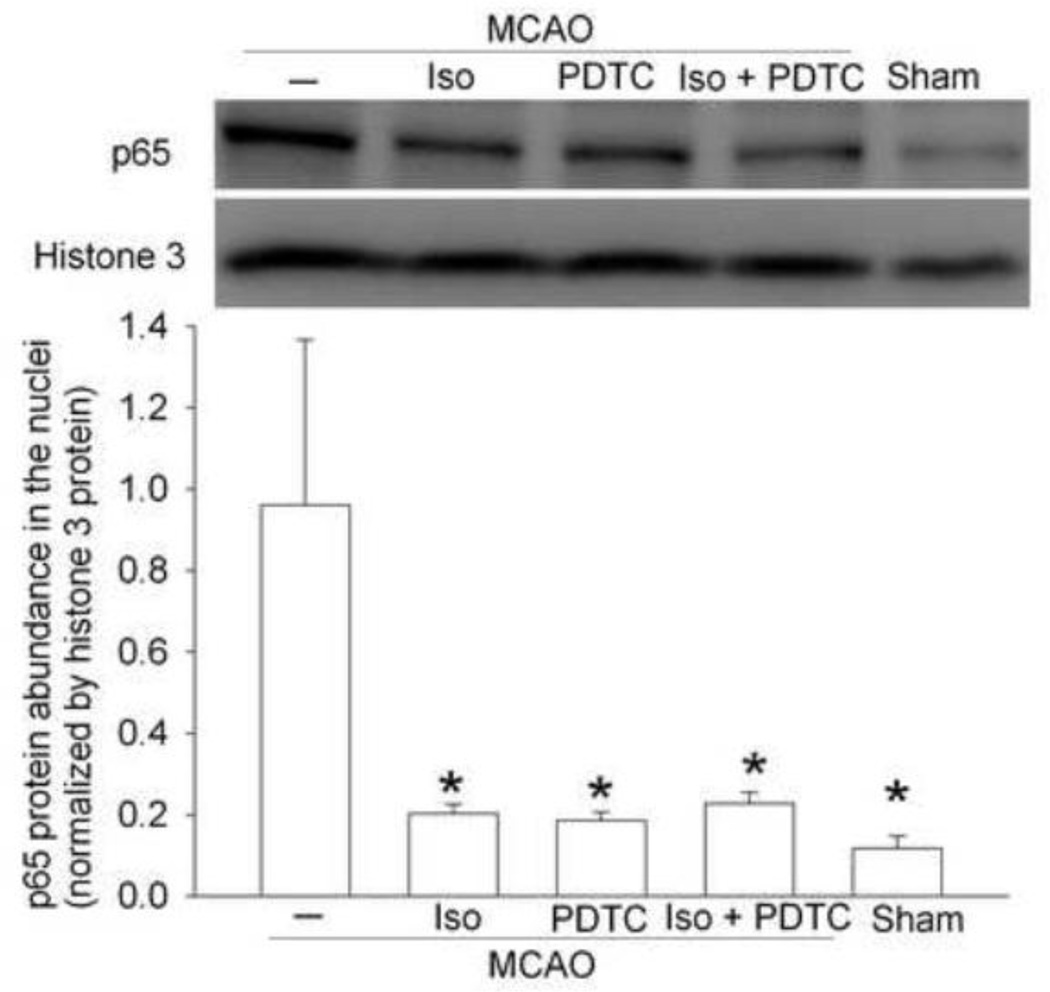
To determine the effects of isoflurane postconditioning on brain ischemia-induced activation of NF-κB, we measured p65 translocation to the nucleus. The NF-κB unit p65 in cell nucleus in the ischemic penumbral cerebral cortex was significantly increased in animals with MCAO only. Isoflurane postconditioning and the NF-κB inhibitor PDTC attenuated this increased nuclear translocation of p65 (Fig. 5). PDTC was used as a positive control in the study. These results suggest that isoflurane postconditioning and PDTC reduced brain ischemia-induced NF-κB activation.
Fig. 5. Isoflurane postconditioning reduced translocation of p65 to nuclei after the middle cerebral arterial occlusion (MCAO) in rats.
The frontal cerebral cortex area 1 ipsilateral to the ischemia side was harvested at 24 h after the MCAO. Nuclear proteins were prepared for Western blotting. A representative Western blot is shown on the top panel and the graphic presentation of the p65 protein abundance quantified by integrating the volume of autoradiograms from 4 rats for each experimental condition is shown in the bottom panel. Values in graphs are the means ± S.E.M. * P < 0.05 compared with MCAO only. Iso: isoflurane postconditioning, PDTC: pyrrolidine dithiocarbamate.
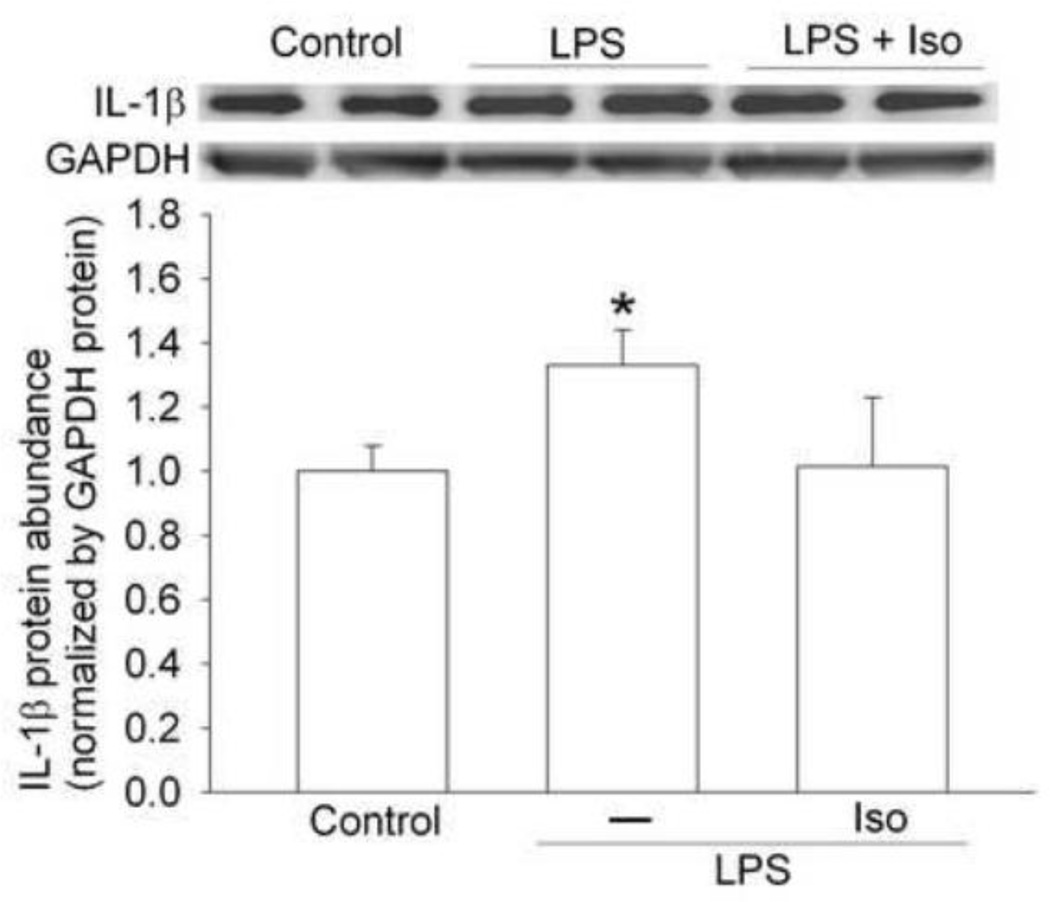
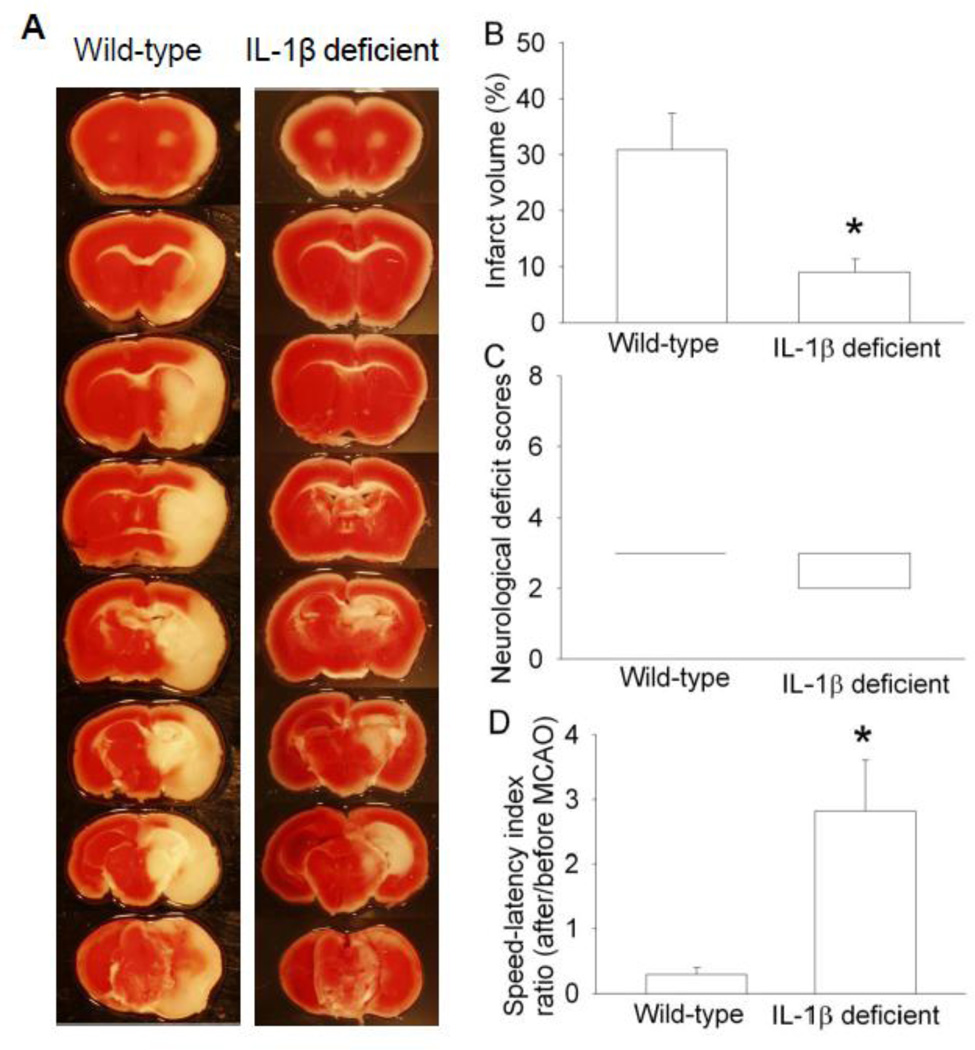
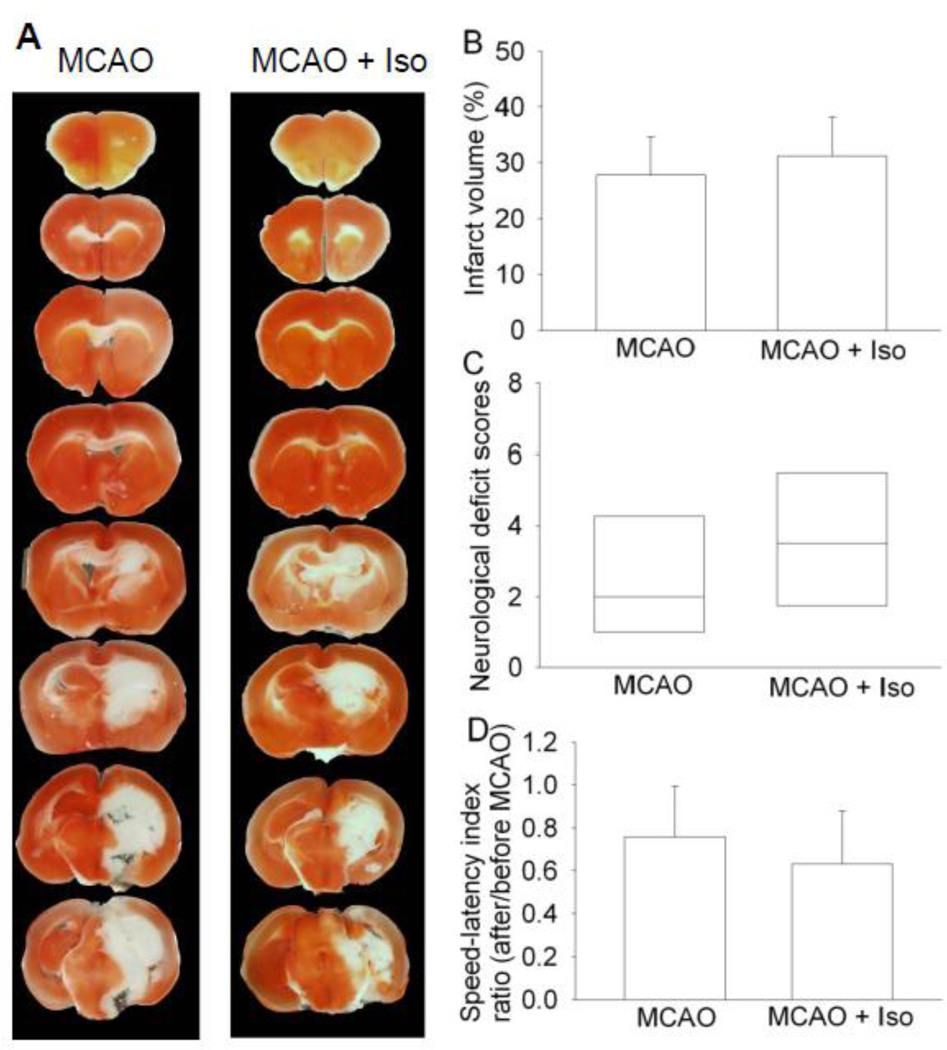
To determine whether isoflurane postconditioning-induced inhibition of IL-1β production after brain ischemia plays a role in the isoflurane postconditioning effects, we performed three experiments. In the first experiment, exposure of mice to 1.5% isoflurane for 30 min at 2 h after the lipopolysaccharide injection attenuated the lipopolysaccharide-induced IL-1β expression in the cerebral cortex (Fig. 6). Second experiment showed that the IL-1β deficient mice had smaller brain infarct volumes and better performance on rotarod than wild-type mice when assessed at 1 day after transient brain ischemia (Fig. 7). In the third experiment, isoflurane exposure at the end of the MCAO did not change the neurological outcome of the IL-1β deficient mice (Fig. 8). These results suggest that attenuation of brain ischemia-induced IL-1β increase by isoflurane postconditioning contributes to its neuroprotective effect.
Fig. 6. Isoflurane posttreatment reduced lipopolysaccharid (LPS)-induced interleukin (IL)-1β increase in mice.
The frontal cerebral cortex was harvested at 6 h after intraperitoneal LPS injection for Western blotting. A representative Western blot is shown on the top panel and the graphic presentation of the IL-1β protein abundance quantified by integrating the volume of autoradiograms from 4 – 5 mice for each experimental condition is shown in the bottom panel. Values in graphs are the means ± S.E.M. * P < 0.05 compared with control mice. Iso: isoflurane postconditioning.
Fig. 7. Interleukin (IL)-1β deficient mice had better histological and neurological function outcome than wild-type mice after middle cerebral arterial occlusion (MCAO).
The results were evaluated at 24 h after the MCAO. A: Brain slices stained with 2,3,5-triphenyltetrazolium chloride from representative mice. B: Percentage of infarct volume in ipsilateral hemisphere volume. Results are the means ± S.E.M. (n = 6 – 8). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panel B) or assigned 7 to the animals that died before the end time point for observation. Results are presented in a box plot format (n = 6 – 8). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25–75% interval including the median of the data. D: The performance on rotarod. Mice were tested before and 24 h after the MCAO and the speed-latency index ratio of these two tests are presented. Results are the means ± S.E.M. (n = 6 – 8). * P < 0.05 compared with the wild-type mice.
Fig. 8. Isoflurane post-treatment failed to induce neuroprotection in interleukin (IL)-1β deficient mice after middle cerebral arterial occlusion (MCAO).
The results were evaluated at 24 h after the MCAO. A: Brain slices stained with 2,3,5-triphenyltetrazolium chloride from representative mice. B: Percentage of infarct volume in ipsilateral hemisphere volume. Results are the means ± S.E.M. (n = 6). C: Neurological deficit scores evaluated immediately before the animals were euthanized for the assessment of infarct sizes (data are presented in panel B) or assigned 7 to the animals that died before the end time point for observation. Results are presented in a box plot format (n = 6). ●: lowest or highest score (the score will not show up if it falls in the 95% interval); between lines: 95% interval of the data; inside boxes: 25–75% interval including the median of the data. D: The performance on rotarod. Mice were tested before and 24 h after the MCAO and the speed-latency index ratio of these two tests are presented. Results are the means ± S.E.M. (n = 6). Iso: isoflurane postconditioning.
Discussion
Our previous studies have shown that isoflurane can induce a postconditioning effect against brain ischemia (Lee et al., 2008; Li and Zuo, 2011; Lin et al., 2011). However, neurological outcome in adult animals after brain ischemia is assessed within a few days after brain ischemia in the previous studies. It has been realized that cell death after brain ischemia is a dynamic process that can last for a few weeks in rodents (Li and Zuo, 2009; Li et al., 1995). Thus, testing the effectiveness of neuroprotective strategies in improving long-term neurological outcome in preclinical studies has been recommended by experts in the research field (Fisher et al., 2009). We showed here that application of isoflurane at the onset of reperfusion reduced brain infarct volumes and improved neurological deficit scores and performance on rotarod at 4 weeks after the MCAO. These results suggest that isoflurane postconditioning improves the long-term neurological outcome after a transient focal brain ischemia in rats.
Many studies have focused on determining how various postconditioning stimuli can activate intracellular protective mechanisms to provide the protection. All of the previous studies on volatile anesthetic postconditioning aim to determine this type of mechanisms (Lee et al., 2008; Li and Zuo, 2011; Lin et al., 2011; Zhou et al., 2010). However, postconditioning may also work on reducing severity of the secondary insults after brain ischemia and reperfusion to provide protection. Neuroinflammation is a recognized secondary insult contributing to cell injury after brain ischemia and reperfusion (Lipton, 1999; Iadecola and Anrather, 2011; Huang et al., 2006; Lakhan et al., 2009). Inflammation can cause cell injury or death through many mechanisms. Inflammatory cells release free radicals that damage cellular proteins, lipids and nucleic acids. The complement system is activated during inflammation. Activated complement system causes proteolytic reaction, which ultimately leads to cell lysis. Inflammation also activates apoptosis-inducing receptors, such as Fas (Iadecola and Anrather, 2011). These damaging mechanisms can result in brain-blood barrier breakdown, which increases the permeation of water, various toxic molecules and peripheral blood cells into brain tissues to further increase the injury. Our results showed that brain ischemia and reperfusion caused a significant increase of the proinflammatory cytokines IL-1β and IL-6 in the ischemic penumbral cerebral cortex. This increase was attenuated by isoflurane postconditioning. In addition, IL-1β deficient mice had smaller brain infarct volumes and better neurological functions after transient brain ischemia than wild-type mice. Isoflurane posttreatment failed to induce a protective effect in the IL-1β deficient mice. Finally, delayed isoflurane treatment attenuated IL-1β increase induced by lipopolysaccharide, indicating that isoflurane-induced attenuation of IL-1β increase in the ischemic penumbral brain tissues may not be secondary to the protective effect of isoflurane postconditioning. Together, these results strongly and for the first time suggest that inhibition of neuroinflammation, especially the production of IL-1β, plays a role in the isoflurane postconditioning-induced neuroprotection. A recent study showed that postconditioning with sevoflurane reduced cytokine production in the blood after focal brain ischemia in rats (Zhang et al., 2011). It is difficult to know from the study whether this decreased cytokines in the blood has any relevance to the sevoflurane postconditioning effects in the brain because the decreased cytokines can just represent the inhibition of sevoflurane on the peripheral inflammatory responses to the surgery of creating focal brain ischemia.
IL-1β is a major cytokine initiator for inflammatory response after brain ischemia and reperfusion (Iadecola and Anrather, 2011). It is increased within a few hours after the brain ischemia. This increase can last for 4 days after the brain ischemia (Lakhan et al., 2009). IL-1β also increases other cytokines, such as IL-6, to enhance the inflammatory response (Cahill and Rogers, 2008). Thus, IL-6 may be an event downstream of IL-1β. Also, IL-6 can work as a pro-inflammatory and anti-inflammatory cytokine. In addition, mice lacking IL-6 do not have worse neurological outcome (Clark et al., 2000). Finally, IL-10 is a major anti-inflammatory cytokine (Lakhan et al., 2009; Spera et al., 1998). Our data did not show that brain ischemia/reperfusion with or without isoflurane postconditioning had any effects on IL-10 levels in the ischemic penumbral brain cortex. Thus, we chose to use IL-1β deficient mice to determine whether reduction of brain ischemia/reperfusion-induced IL-1β increase was a major mechanism for the isoflurane postconditioning effect.
Interestingly, our results showed that brain ischemia/reperfusion-induced delayed cell death in the ischemic penumbral cerebral cortex was attenuated by isoflurane postconditioning. This finding is significant because it suggests that an exposure of isoflurane after brain ischemia can affect the ongoing cell death 4 weeks later. The mechanisms for this phenomenon are not known but may be related to the inhibition of neuroinflammation by isoflurane postconditioning. Neuroinflammation after brain ischemia can last for weeks (Dirnagl et al., 2003; Barone et al., 2002; Clark et al., 1993; Zhang et al., 1994). The inhibition of the initiation of neuroinflammation may attenuate the neuroinflammation for a long time and, therefore, inhibit the delayed cell death. Another possibility is that brain ischemia/reperfusion may have programmed some cells for apoptosis in a delayed phase. Attenuating this process during the acute phase after brain ischemia reduces this delayed cell death.
It has been well-documented that NF-κB is a major transcription factor that controls the expression of many proinflammatory mediators including cytokines (Liu et al., 1999; Giacomini et al., 2011). Our results showed that brain ischemia/reperfusion increased the translocation of p65, a NF-κB unit, to nuclei. Translocation of NF-κB to nuclei represents activation of NF-κB. This ischemia/reperfusion-induced NF-κB translocation was inhibited by isoflurane postconditioning. Similarly, PDTC, a known NF-κB inhibitor (Li et al., 2012; Liu et al., 1999), also inhibited this increase of translocation. In addition, our previous study showed that PDTC improved neurological outcome after brain ischemia (Li et al., 2012). Finally, delayed isoflurane exposure attenuated IL-1β increase induced by lipopolysaccharide, which is known to be NF-κB dependent (Liu et al., 1999). These results suggest that isoflurane postconditioning inhibits NF-κB activation to reduce proinflammatory cytokine production, which then improves neurological outcome after brain ischemia/reperfusion.
Our findings may have significant implications. If volatile anesthetic postconditioning effects in the brain are confirmed in humans, patients under many clinical situations, such as ischemic stroke on thrombolytic therapy, may benefit from this protective strategy. Also, our results clearly show that isoflurane postconditioning reduces proinflammatory cytokine production after brain ischemia. Many human diseases, such as brain trauma, have significant components of brain ischemia in their pathophysiology and, therefore, may benefit from this strategy.
One possible limitation of our study is that rats were anesthetized by isoflurane during surgery. This isoflurane application may have provided some protection to animals, which may have affected the overall protective effects observed for isoflurane postconditioning in this study. However, this situation is difficult to avoid because it is necessary to provide anesthesia during the surgery for creating focal brain ischemia. All general anesthetics by nature have significant effects on brain (Wilson and Gelb, 2002). We used a volatile anesthetic so that its use could be stopped immediately after the achievement of MCAO. This method allowed rats to be awake during most of the 90 min of brain ischemia to simulate clinical situation: most patients have brain ischemia without anesthesia. We used isoflurane during surgery in the study to investigate isoflurane postconditioning effects to avoid possible interaction between different anesthetics. This practice has been used by us and others in studies investigating anesthetic postconditioning (Lee et al., 2008; Zhou et al., 2010). Also, isoflurane postconditioning involves anesthetizing the animals immediately after brain ischemia. This procedure will affect the physiological parameters. This effect may contribute to the neuroprotection observed with isoflurane postconditioning. However, we mechanically ventilated the rats to maintain end-tidal CO2 and SpO2 during isoflurane exposure. Although isoflurane can reduce blood pressure, this level of reduction has not been found to induce neuroprotection (Kapinya et al., 2002; Sakai et al., 2007). Finally, isoflurane posttreatment failed to induce neuroprotection in the IL-1β deficient mice. Thus, the neuroprotection induced by isoflurane postconditioning may not be secondary to the physiological parameter changes caused by isoflurane.
In summary, we have shown that isoflurane postconditioning improved the long-term neurological outcome after brain ischemia in rats. This effect may be mediated by isoflurane postconditioning-induced inhibition of NF-κB and then IL-1β production in the ischemic penumbral brain tissues.
Highlights.
Isoflurane postconditioning improves long-term neurological outcome after focal brain ischemia in rats
Isoflurane postconditioning reduces inflammatory cytokine production in the ischemic brain tissues
Isoflurane postconditioning-induced neuroprotection may be mediated by reducing inflammatory cytokines
Acknowledgments
Grant support: This study was supported by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, OH, by grants (R01 GM065211 and R01 GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, MD, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, MD, and the Robert M. Epstein Professorship endowment, University of Virginia, Charlottesville, VA. The funding agencies have no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations
- Fr1
frontal brain cortex area 1
- IL-1β
interleukin 1β
- MCAO
middle cerebral arterial occlusion
- NF-κB
nuclear transcription factor-κB
- PDTC
pyrrolidine dithiocarbamate
- SpO2
pulse oximeter oxygen saturation
- TUNEL
terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick-end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no other financial supports for this study, except for those grants stated on the title page from funding agencies for nonprofit. The authors also declare no conflict of interest in the content of this study.
References
- Barone FC, et al. Brain inflammation, cytokines, and p38 MAP kinase signaling in stroke. Boca Raton (Florida): CRC Press LLC; 2002. [Google Scholar]
- Burda J, et al. Delayed postconditioning initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell. Mol. Neurobiol. 2006;26:1141–1151. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RK, et al. Development of tissue damage, inflammation and resolution following stroke: an immunohistochemical and quantitative planimetric study. Brain Res. Bull. 1993;31:565–572. doi: 10.1016/0361-9230(93)90124-t. [DOI] [PubMed] [Google Scholar]
- Clark WM, et al. Lack of interleukin-6 expression is not protective against focal central nervous system ischemia. Stroke. 2000;31:1715–1720. doi: 10.1161/01.str.31.7.1715. [DOI] [PubMed] [Google Scholar]
- Danielisova V, et al. The changes in endogenous antioxidant enzyme activity after postconditioning. Cell. Mol. Neurobiol. 2006;26:1181–1191. doi: 10.1007/s10571-006-9034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rossi LW, et al. Xenon and isoflurane differentially modulate lipopolysaccharide-induced activation of the nuclear transcription factor KB and production of tumor necrosis factor-alpha and interleukin-6 in monocytes. Anesth. Analg. 2004;98:1007–1012. doi: 10.1213/01.ANE.0000106860.27791.44. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, et al. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Fisher M, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacomini E, et al. Expression of proinflammatory and regulatory cytokines via NF-kappaB and MAPK-dependent and IFN regulatory factor-3-independent mechanisms in human primary monocytes infected by Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011 doi: 10.1155/2011/841346. 841346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, et al. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapinya KJ, et al. Tolerance Against Ischemic Neuronal Injury Can Be Induced by Volatile Anesthetics and Is Inducible NO Synthase Dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, et al. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon ?-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111:566–573. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakhan SE, et al. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JJ, et al. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, et al. Pyrrolidine dithiocarbamate attenuates brain Abeta increase and improves long-term neurological outcome in rats after transient focal brain ischemia. Neurobiol. Dis. 2012;45:564–572. doi: 10.1016/j.nbd.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, et al. Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1995;15:389–397. doi: 10.1038/jcbfm.1995.49. [DOI] [PubMed] [Google Scholar]
- 22.Lin D, et al. Volatile anesthetic post-treatment induces protection via inhibition of glycogen synthase kinase 3beta in human neuron-like cells. Neuroscience. 2011;179:73–79. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 25.Liu SF, et al. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents In vivo expression of proinflammatory genes. Circulation. 1999;100:1330–1337. doi: 10.1161/01.cir.100.12.1330. [DOI] [PubMed] [Google Scholar]
- 26.Rogers DC, et al. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–2065. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- 27.Sakai H, et al. Isoflurane Provides Long-term Protection against Focal Cerebral Ischemia in the Rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Spera PA, et al. IL-10 reduces rat brain injury following focal stroke. Neurosci. Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 29.Swanson RA, et al. A semiautomated method for measuring brain infarct volume. [see comments] J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 30.Wilson JX, Gelb AW. Free radicals, antioxidants, and neurologic injury: possible relationship to cerebral protection by anesthetics. J. Neurosurg. Anesthesiol. 2002;14:66–79. doi: 10.1097/00008506-200201000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Zhang RL, et al. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J. Neurol. Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. Inhibition of sevoflurane postconditioning against cerebral ischemia reperfusion-induced oxidative injury in rats. Molecules. 2011;17:341–354. doi: 10.3390/molecules17010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, et al. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- 34.Zhao P, et al. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 35.Zhao ZQ, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 36.Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of p38 mitogen-activated protein kinase. Mol. Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, et al. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]