Abstract
Delayed secondary biochemical and cellular changes after traumatic brain injury continue for months to years, and are associated with chronic neuroinflammation and progressive neurodegeneration. Physical activity can reduce inflammation and facilitate recovery after brain injury. Here, we investigated the time-dependent effects, and underlying mechanisms of post-traumatic exercise initiation on outcome after moderate traumatic brain injury using a well-characterized mouse controlled cortical impact model. Late exercise initiation beginning at 5 weeks after trauma, but not early initiation of exercise at 1 week, significantly reduced working and retention memory impairment at 3 months, and decreased lesion volume compared to non-exercise injury controls. Cognitive recovery was associated with attenuation of classical inflammatory pathways, activation of alternative inflammatory responses and enhancement of neurogenesis. In contrast, early initiation of exercise failed to alter behavioral recovery or lesion size, while increasing the neurotoxic pro-inflammatory responses. These data underscore the critical importance of timing of exercise initiation after trauma and its relation to neuroinflammation, and challenge the widely held view that effective neuroprotection requires early intervention.
Keywords: Traumatic brain injury, exercise, neurodegeneration, inflammation, neurogenesis
Introduction
Traumatic brain injury (TBI) induces a series of delayed secondary biochemical and cellular changes that contribute to irreversible tissue damage (Loane and Faden, 2010). The mechanisms underlying the secondary injury involve a complex cascade of biochemical, metabolic and gene expression changes; these events contribute to neuroinflammation and delayed cell death (Stoica and Faden, 2010). Although much of the research focus has been directed at elucidating relatively early cellular and molecular events, experimental evidence suggests that the pathobiological processes initiated by TBI may continue for as long as a year or more after trauma (Bramlett and Dietrich, 2002; Smith et al., 1997), contributing to progressive neurodegeneration and chronic functional deficits. Persistent neuroinflammation following central nervous system (CNS) trauma may provide a mechanistic link between acute injury and chronic neurodegeneration (Faden, 2011; Ramlackhansingh et al., 2011).
In parallel with these neurodegenerative and inflammatory/neurotoxic reactions, trauma also induces neuroplasticity responses (Adkins et al., 2006; Carmichael, 2006; Jones and Schallert, 1994). These include increased expression of growth and proliferation factors (Cramer and Chopp, 2000; Kelley and Steward, 1997). Thus, trauma initiates both neurodegenerative and neurorestorative responses, with the relative balance of these activities believed to determine the extent of subsequent neurological deficits.
Increasing evidence suggests that both pathophysiological changes and neurological recovery after CNS injuries can be modulated by physical exercise. The mechanisms involved may include up-regulation of neurotrophic factors leading to enhanced neuronal plasticity (Griesbach et al., 2004b; Kobilo et al., 2011; van Praag et al., 1999), as well as anti-apoptotic and anti-inflammatory effects (Itoh et al., 2011; Mota et al., 2012). An important variable appears to be the timing of initiation of exercise as a function of injury severity (Griesbach et al., 2004a; Griesbach et al., 2007), which can affect the neurotrophic factor response to injury. Early use-dependent neuronal injury and reduced neuroplasticity have been demonstrated following unilateral sensorimotor lesions (Kozlowski et al., 1996). In addition, early initiation of exercise failed to improve cognitive function and exacerbated neurological deficits after experimental TBI (Crane et al., 2012; Griesbach et al., 2004b). Clinical studies of the benefits of exercise interventions in TBI patients have led to conflicting results, in part due to lack of uniformity in neurological outcome measures and heterogeneity of clinical brain injury (Bland et al., 2011). Nonetheless, randomized controlled trials in adult TBI have shown that post-acute cognitive rehabilitation and physical rehabilitation approaches produce various beneficial treatment effects (Lu et al., 2012).
Recent studies have suggested that a persisting microglial-associated inflammatory response after TBI may contribute to progressive neurodegeneration (Byrnes et al., 2012; Faden, 2011; Kabadi et al., 2012; Ramlackhansingh et al., 2011). Exercise has been shown to modulate immune and inflammatory responses in animals and humans (Petersen and Pedersen, 2005). However, the time-dependent effects of post-traumatic exercise initiation on neuroinflammation and progressive neurodegeneration, or on neurogenesis, have not been addressed. We tested the hypothesis that exercise after TBI can attenuate chronic trauma-induced microglial activation and release of associated inflammatory factors, thereby improving recovery both by limiting progressive neurodegeneration and producing a more favorable environment for neuronal cell differentiation and survival after neurogenesis. Moreover, we propose that the effectiveness of exercise intervention on such outcomes depends upon its timing after injury, with delayed exercise providing superior benefits as compared to early post-traumatic exercise.
Materials and Methods
Mouse controlled cortical impact (CCI) model
All surgical procedures were complied with the Guide for the Care and Use of Laboratory Animals published by NIH (DHEW publication NIH 85-23-2985), and the protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. CCI was performed as previously described (Piao et al., 2012). Briefly, the custom-designed CCI injury device consists of a microprocessor-controlled pneumatic impactor with a 3.5 mm diameter tip. Ten week-old, male C57BL/6 (Taconic) mice (20-25g) were anesthetized with 2% isoflurane gas mixture containing 70% N2O and 30% O2 and administered through a nose mask. Respiration rate and pedal withdrawal reflexes were used to assess the depth of anesthesia. The head was mounted in a stereotaxic frame, and a 4-mm craniotomy was made on the central aspect of the left parietal bone. Moderate injury was induced using an impactor velocity of 6 m/s and deformation depth of 2 mm. The core body temperature was maintained at 37°C using heating pad with rectal probe. After injury, the animal was placed into a heated cage to maintain normal core temperature for 45 minutes post-injury. All animals were monitored for at least 4 hours after surgery and then daily. Uninjured naïve C57Bl/6 mice served as controls in these studies.
Voluntary wheel exercise
Mice were individually caged with ad libitum access to food and water, and were maintained on a 12 h light/dark cycle. Exercise group mice were placed in cages equipped with running wheel (30.3 × 20.6 × 26 cm by L × W × H; Mini Mitter) to permit spontaneous exercise; non-exercised animals were housed in cages without running wheels. The number of wheel revolutions per hour was recorded by an automated computer monitoring system and software (Vital View Application software, Mini Mitter). Physical activity was recorded continuously as wheel revolutions per 1-hour interval, converted to kilometers and calculated by week for analysis. TBI mice were randomly divided into three groups- early (acute) exercise beginning at 1 week for 4 weeks (acEX); late (delayed) exercise beginning at 5 weeks for 4 weeks (deEX); or no exercise (noEX), n=15 in each group. Body weights were measured at the end of study immediately prior to euthanasia and there were no significant differences between groups.
Morris Water Maze and Reversal Morris Water Maze tests
Spatial learning and memory were assessed using an acquisition phase of the standard Morris Water Maze (sMWM) and reversal MWM (rMWM) tests as previously described (Piao et al., 2012; Zhao et al., 2012). The Morris water maze protocol included 4 phases: (1) standard hidden platform training (acquisition); (2) standard probe test; (3) reversal hidden platform training (reversal acquisition); (4) reversal probe test. Spatial learning and working memory performance were assessed by determining the latency (seconds) to locate the sub-merged hidden platform (NE quadrant) with a 90 second limit per trial and 4 trials per day (post-injury day (PID) 72 - 75) by a computer-based Any-Maze automated video tracking system (Stoelting Co., Wood Dale, IL). Reference spatial memory was assessed by a probe trial carried out on PID 76, as the time spent (seconds) with a 60 second limit in the quadrant where the platform had been hidden during acquisition phase. For the rMWM training, the hidden platform was moved to the opposite quadrant (SW) without changing any visual cues. Mice were trained to find the new platform position for the following 4 days (PID 77 - 80; 4 trials/day). A reversal probe test was performed on PID 81, and the time spent in each quadrant was recorded. A visual cue test was subsequently performed on post-injury day 81 using a flagged platform placed on the platform in one of the quadrants (with a 90 second limit per trial) and latency (seconds) to locate the flagged platform was recorded.
Open Field and Novel Object Recognition Test
The open field test was used to measure locomotor activity (Zhang et al., 2008) on post-injury day 84 as previously described (Zhao et al., 2012). The apparatus consists of an open field (22.5 cm × 22.5 cm) with two adjacently located imaginary circular zones. Mice were individually placed in a corner facing the wall of the open-field chamber (22.5 cm × 22.5 cm) and allowed to freely explore the chamber for 5 minutes. The distance travelled was recorded by Any-Maze software. Novel object recognition (NOR), conducted as previously reported (Zhao et al., 2012), evaluated non-spatial hippocampal-mediated memory (Clark et al., 2000) on post-injury day 85. Mice were habituated to the open field and were allowed to freely explore the area for 5 minutes. After 24 hours, mice were placed into the chamber where two identical objects were placed near the left and right corners of the open field for training (sample phase) and allowed to freely explore until they spent a total of 15 s exploring the objects (exploration recorded when the front paws or nose contacted the object). After 1 hour, a familiar object was substituted with a novel object (novel object location counterbalanced across mice), and time spent with each object was recorded. A preference for the novel object, more time than chance (7.5 s) spent with the novel object compared to the familiar object indicates intact memory because mice inherently prefer to explore novel objects.
Tail-Suspension Test
The tail-suspension (TS) test assesses depression-like behavior in mice and is based on the observation that mice develop an immobile posture when placed in an inescapable hemodynamic stress of being hung by their tails (Cryan et al., 2005; Zhao et al., 2012). The TS was performed on post-injury day 86 as described previously (Zhang et al., 2008; Zhao et al., 2012). Each mouse was suspended at a height of 50 cm using adhesive tape placed approximately 1 cm from the tip of its tail. The immobility time was recorded during the 5-minute test period. The definition of immobility was passive hanging and lack of motion.
Quantitative real time-polymerase chain reaction (qRT-PCR)
Total RNA was isolated by using TRIzol reagent (Invitrogen) by following the manufacturer’s instructions. Verso™ cDNA Kit (Thermo Scientific) was used to synthesize cDNA from purified total RNA. RNA (1 μg) was heated to 70°C for 5 min and mixed with 5× cDNA-synthesis buffer, dNTP mix (0.5 nM final concentration) and Verso Enzyme Mix, and finally random hexamers (400 ng/μL) were added. Tubes were incubated at 42°C for 30 min, followed by 95°C for 2 min. Quantitative real-time PCR amplification was performed by using cDNA TaqMan® Universal Master Mix II (Applied Biosystems). In brief, reactions were performed in duplicate containing 2× TaqMan® Universal Master Mix II, 1 μL of cDNA (corresponding to 50 ng RNA/reaction) and TaqMan® Gene Expression Assay (Applied Biosystems), 20× in a final volume of 20 μL. TaqMan® Gene Expression assays for following genes were used: IL-1β (Mm01336189_m1), IL-6 (Mm00446190_m1), IL-10 (Mm00439614_m1), CREB (Mm00501607_m1), BDNF (Mm01334042_m1), IGF-1 (Mm00439560_m1). GAPDH (Mm99999915_g1) was used as control. Reactions were amplified and quantified by using a 7900HT Fast Real-Time PCR System and the manufacturer’s corresponding software (Applied Biosystems). The PCR profile consisted of one cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Gene expression was normalized to β-actin, and the relative quantity of mRNAs was calculated based on the comparative Ct method (Livak and Schmittgen, 2001).
Western blotting
At 90 days post-CCI, a 5 mm area ipsilateral cortical tissue was collected and used for Western blotting analysis as described previously (Piao et al., 2012). Briefly, twenty-five μg of protein was run on SDS polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane. The blot was then probed with the following antibodies: goat anti-C1qB (1:1000, Santa Cruz), mouse anti-galectin-3 (1:1000, Abcam), mouse anti-gp91phox (1:1000, BD), rabbit anti-p22phox (1:1000, Santa Cruz). Beta-actin (1:10000, Sigma-Aldrich) was used as an endogenous control. Immune complexes were detected with the appropriate HRP-conjugated secondary antibodies and visualized using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific). Chemiluminescence was captured on a Kodak Image Station 4000R station and protein bands were quantified by densitometric analysis using Carestream Molecular Imaging Software (Carestream Health). The data (n=6 each group) presented reflect the density of target protein divided by the density of the β-actin endogenous control in each sample, and are expressed in arbitrary units.
Immunohistochemistry
Standard immunohistochemistry was performed on 20 μm sections at 90 days post-CCI as previously described (Piao et al., 2012). The following primary antibodies were used: rabbit anti-Iba-1 (1:1000, Wako), rat anti-CD68 (1:500; AbD serotec), mouse anti-galectin-3 (1:200, Abcam), mouse anti-gp91phox (1:200, BD), and standard immunostaining techniques were employed. Counterstaining was performed with 4′, 6-diamidino-2-phenylindole (DAPI) (1 μg/mL; Sigma). Sections to which the primary antibodies were not added served as a negative control. Fluorescence microscopy was performed using a LEICA (TCS SP5 II) confocal microscope system (Leica Microsystems, Exton, PA).
At week 5 or 9 after TBI, 50 mg/Kg BrdU (Sigma Aldrich) was injected intraperitoneally daily for 7 days (n=4 each group). For BrdU immunostaining, the 20 μm sections were pretreated with 2N HCl for 60 minutes at 37°C and neutralized in 0.1 M borate buffer, and followed by the standard immunohistochemical procedures. Rat anti-BrdU antibody (1:100, Abcam) was used as primary antibody, and co-labeled with mouse-anti NeuN antibody (1:500, Millipore), to determine newborn neurons. Three sections per brain from the same sites were used for BrdU-positive cell counting. BrdU/NeuN-double positive cells located in the granular cell layer of the DG in the hippocampus were considered as newborn neurons that have reached maturity (Komitova et al., 2002). Semi-quantification of double-labeled cells was performed by an investigator blinded to treatment group using ImageJ software, version 1.4 (NIH) as previously described (Piao et al., 2012). Briefly, each channel immunofluorescent images from independent sections were converted to 8-bit grayscale. The background was set as indicated using threshold command for each channel and double positive cells were counted using the co-localization analysis command. All images in the same series were processed using the same analysis parameters. The numbers shown in the graphs represent the average number of cells per image for each treatment.
Stereological quantification of lesion volume, surviving hippocampal neurons, and microglia phenotypes
At 90 days post-injury, mice were anesthetized and transcardially perfused with 10% buffered formalin solution (containing 4% paraformaldehyde; Fisher Scientific). Sixty and twenty μm coronal sections were cut (3 × 60 μm followed by 3 × 20 μm sections) and serially collected throughout the injured brain starting at +1.78mm from bregma and mounted onto superfrostplus slides. Every fourth 60 μm section was processed for histological or immunohistochemical analysis beginning from a random start point. Sixty-micron sections were stained with cresyl violet (FD NeuroTechnologies, Baltimore, MD), and used for analysis. Sections were analyzed using a Leica DM4000B microscope. Lesion volume was determined based on the Cavalieri method as previously described (Kabadi et al., 2012). The lesion area, including both the cavity and surrounding damaged tissue, was outlined using the Stereoinvestigator software (MBF Biosciences, Williston, VT) to obtain the final lesion volume.
Total number of surviving neurons in the CA2/3 and dentate gyrus sub-regions of the hippocampus using the optical fractionator method of unbiased stereology (Piao et al., 2012). Briefly, the optical dissector had a size of 50 μm by 50 μm in the x and y-axis with a height of 10 μm and guard zone of 4 μm from the top of the section. Dissectors were positioned every 150 μm in the x and y-axis. The sampled region for each hippocampal subfield was demarcated in the injured hemisphere and cresyl-violet neuronal cell bodies were counted. The volume of the hippocampal subfield was measured using the Cavalieri estimator method. The estimated number of surviving neurons in each field was divided by the volume of the region of interest to obtain the cellular density expressed in cells/mm3.
The number of microglia displaying ramified or activated cellular morphologies was quantified using the optical fractionator method. Microglia was immunostained with anti-Iba-1 antibody and counterstained with cresyl violet, and used for analysis. The sampled region was the ipsilateral CA2/3 and DG sub-regions of hippocampus between −1.22 mm and −2.30 mm from bregma. The optical dissector had a size of 50 μm by 50 μm in the x and y-axis with a height of 10 μm and guard zone of 4 μm from the top of the section. Dissectors were positioned every 150 μm in the x and y-axis. Microglial phenotypic classification was based on the length and thickness of the projections as previously described (Byrnes et al., 2012; Soltys et al., 2001). The volume of the region of interest was measured using a Cavalieri estimator method with a grid spacing of 100 μm. The estimated number of microglia in each phenotypic class was divided by the volume of the region of interest to obtain the cellular density expressed in cells/mm3.
Neurolucida software (MBF Biosciences) was used to trace the cell bodies and dendrites of microglia at resting or activated stage following injury as described previously (Byrnes et al., 2012). The cell body was outlined using the contour tool followed by the tracing of the individual dendrites, using the dendrite line tool.
Statistical analysis
Examiners blinded to treatment group performed lesion volume, functional data, Western blot, unbiased stereological and semi-quantification of cell counting analysis. Quantitative data are presented as mean +/− standard error of the mean. Functional data were analyzed with repeated measures one-way analysis of variance (ANOVA) and post-hoc adjustments using Tukey’s test. Remaining data were analyzed using Student’s t-test or one-way ANOVA, where appropriate. All statistical tests were performed using the GraphPad Prism Program, Version 3.02 for Mac (GraphPad Software, San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
Delayed exercise initiation improved functional recovery after traumatic brain injury
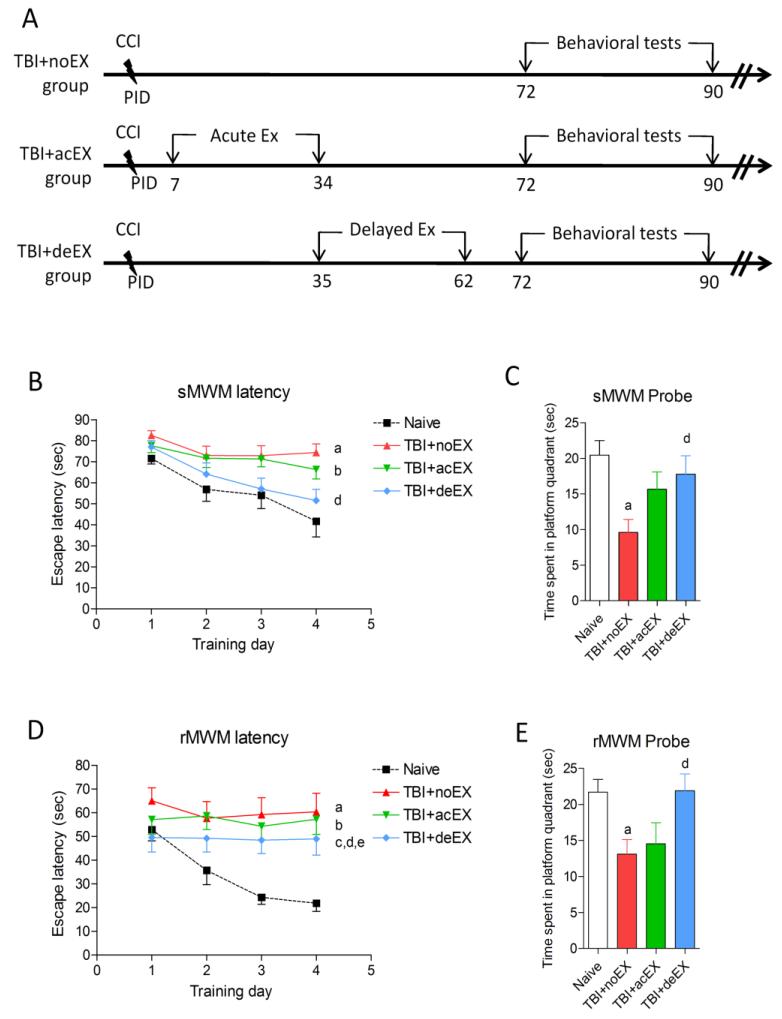
Animals were evaluated using comprehensive cognitive, motor, and depression-anxiety functional tests through three months post-injury (Fig. 1A). In pilot studies, there were no significant differences in any of the functional outcome tests between non-exercised naïve mice and exercised naïve mice that underwent the 4 week voluntary exercise protocol (data not shown). As such, non-exercised naïve mice were used as the uninjured control group. There was no significant difference in the exercise profile between the acute and delayed (340.6±28.94 and 320.7±21.49 revolution/day, respectively) exercise groups. The standard Morris water maze (sMWM) and reverse Morris water maze (rMWM) tests were employed to evaluate TBI-induced special learning and memory impairments from 72 - 81 days post-injury. The non-exercised and acute exercised TBI mice showed increased latency to locate the hidden platform during the acquisition phase when compared to uninjured naïve mice (a, p<0.01, TBI+noEX; and b, p<0.05, TBI+acEX, respectively vs. naïve). The delayed exercise group showed significantly less impairment in working memory with reduced latency to find the submerged platform when compared with non-exercised TBI mice (d, p<0.05, TBI+deEX vs. TBI+noEX), and was not significantly different from uninjured naïve mice (Fig. 1B). Delayed exercise TBI mice had significantly increased dwell time in the target quadrant when compared to non-exercised TBI mice (d, p<0.05, TBI+deEX vs. TBI+noEX), indicating reduced retention memory deficits in the probe test. Non-exercised TBI mice had significantly decreased dwell time in the target quadrant when compared to uninjured naïve mice (a, p<0.05, TBI+noEX vs. naïve) (Fig. 1C). In the rMWM test all TBI mice had significantly increased latency to find the submerged hidden platform when compared to uninjured naïve mice throughout the acquisition trials (a, b, c, p<0.05; TBI+noEX, TBI+acEX and TBI+deEX, vs. naïve, respectively), indicating that all TBI mice had reversal working memory impairments. Delayed exercise TBI mice showed significant improvements in their ability to locate the hidden platform in the rMWM test when compared to non-exercised TBI and acute exercise TBI mice (d and e, p<0.05, TBI+deEX vs. TBI+noEX and TBI+acEX, respectively) (Fig. 1D). Reference memory was assessed using a reverse probe test on the day following rMWM acquisition trials. Delayed exercise TBI mice spent more time in the target quadrant (SW) compared to non-exercised TBI mice (d, p<0.05, TBI+deEX vs. TBI+noEX). Non-exercised TBI mice spent significantly reduced time in target quadrant compared to uninjured naïve mice (a, p<0.05, TBI+noEX vs. naïve) (Fig. 1E). Swim speed did not differ across groups and all mice spent similar time to locate the flagged platform in the visual cue test (data not shown).
Figure 1.
Effect of exercise on TBI-induced neurological deficits in mice. (A) Experimental protocol. (B) TBI-induced spatial learning and working memory deficits were assessed using a standard Morris Water Maze (sMWM) test. Injured non-exercised mice (a; p<0.05 vs. naïve) and acute exercised mice (b; p<0.05 vs. naïve) showed significant impairment in working memory over time. Injured delayed exercised mice were not significantly different from naïve and showed significant improvements in working memory as compared to non-exercised animal (d; p<0.05 vs. TBI+noEX). (C) Reference memory was assessed using the sMWM probe test. There was a significant impairment in reference memory in the non-exercised injured animals (a; p<0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p<0.05 vs. TBI+noEX). (D) Reversal spatial learning was assessed using a Reverse Morris Water Maze (rMWM) test immediately after sMWM. All injured mice showed significantly increased latency to find the sub-merged hidden platform (a-TBI+noEX, b-TBI+acEX, c-TBI+deEX; p<0.05 vs. naïve). Injured delayed exercise mice showed significant improvements in reversal spatial learning as compared to non-exercised (d; p<0.05 vs. TBI+noEX) or acute exercise animals (e; p<0.05 vs. TBI+acEX). (E) Reference memory in rMWM was assessed on the day after the fourth acquisition day. There was a significant impairment in reference memory in the non-exercised injured animals (a; p<0.01 vs. naïve) and a significant improvement in reference memory in injured delayed exercised mice compared to non-exercised animals (d; p<0.05 vs. TBI+noEX). Mice spent similar time with the two identical objects during the sample phase in each group. (F) Non-spatial memory was assessed using the novel object recognition (NOR) test. Significant discrimination with preference for the novel object was observed for each group (p<0.05; novel compared to familiar object exploration). Significantly shorter exploring time was observed in injured non-exercised (a; p<0.05 versus naïve) and acute exercised mice (b; p<0.05 versus naïve). The delayed exercised injured group exploring time did not significantly differ from naïve and was significantly longer than non-exercised injured (d; p<0.05 vs. TBI+noEX) and acute exercise injured animals (e; p<0.05 vs. TBI+acEX). Data included in this figure are expressed as mean ± SEM, n = 13-15/group, statistical analysis using repeated measures one-way ANOVA (Fig.1 B and D) or one-way ANOVA (Fig.1 C, E, F) followed by post hoc adjustments using Tukey’s test.
In addition to spatial learning and memory measured by the MWM tests, hippocampal-mediated non-spatial learning and memory after TBI was assessed by a novel object recognition (NOR) test at 84 and 85 days post-injury (Fig. 1F). During the choice phase of this test uninjured naïve mice spent more time than chance (7.5 seconds) with the novel object 1 hour after the sampling phase (training) indicating intact memory. Non-exercised TBI (a, p<0.05, TBI+noEX vs. naïve) and acute exercise TBI mice (b, p<0.05, TBI+acEX vs. naïve) spent significantly shorter exploration times with the novel object during the choice phase. In contrast, the delayed exercise TBI mice had similar exploration times as uninjured naïve mice and spent a significantly longer exploring time with the novel object compare to both non-exercised TBI (d, p<0.05, TBI+deEX vs. TBI+noEX) and acute exercise TBI (e, p<0.05, TBI+deEX vs. TBI+acEX) mice.
TBI-induced locomotor activity was assessed using the open field test at 84 days post-injury. There were no significant locomotor impairments in each of the TBI groups when compared to the uninjured naïve group (data not shown). In addition, the tail suspension test was performed at 86 days post-injury to assess depressive-like behaviors in TBI mice. Immobility times were significantly increased in the non-exercised TBI group (105 ± 5.14 seconds, p<0.05, TBI+noEX vs. naïve) when compared to uninjured naïve group (78.93 ± 8.51 seconds), and acute exercise TBI group (106 ± 6.17 seconds, p<0.05, TBI+acEX vs. naïve) and delayed exercise TBI group (103.1 ± 4.85 seconds, p>0.05, TBI+deEX vs. naïve) had similar immobility times as non-exercised TBI group, indicating depressive-like behaviors in all TBI mice (data not shown).
Delayed exercise initiation reduced lesion volume after traumatic brain injury
Previously we reported that there was progressive lesion expansion between one and three months in the mouse moderate-to-severe CCI model (Byrnes et al., 2012). To evaluate whether voluntary exercise application after TBI affects progressive tissue loss, we assessed lesion volume using unbiased stereological analysis at 90 days post-injury following completion of the behavioral studies. Representative histological images from each group at three months post-injury are presented in Fig. 2A. Delayed exercise TBI mice had significantly reduced lesion volume (5.444 ± 1.0 mm3) when compared to non-exercised TBI mice (8.754 ± 0.83 mm3, d, p<0.05, TBI+deEX vs. TBI+noEX) or to acute exercise TBI mice (9.043 ± 0.86 mm3, e, p<0.05, TBI+deEX vs. TBI+acEX) (Fig. 2B).
Figure 2.
Effect of exercise on TBI-induced brain lesion at three months post-injury. (A) Brain lesion was assessed with cresyl-violet stained brain sections in TBI mice. (B) Unbiased stereological assessment showed that lesion volume was significantly reduced in the delayed exercised TBI mice compared to both non-exercised (d; p<0.05 vs. TBI+noEX) and acute exercise TBI mice (e; p<0.05 vs. TBI+acEX). Data expressed as mean ± SEM, n=7-8/group, statistical analysis using one-way ANOVA followed by post hoc adjustments using Tukey’s test).
Delayed exercise, but not early exercise initiation, reduced molecular markers of microglia-associated inflammatory response following traumatic brain injury
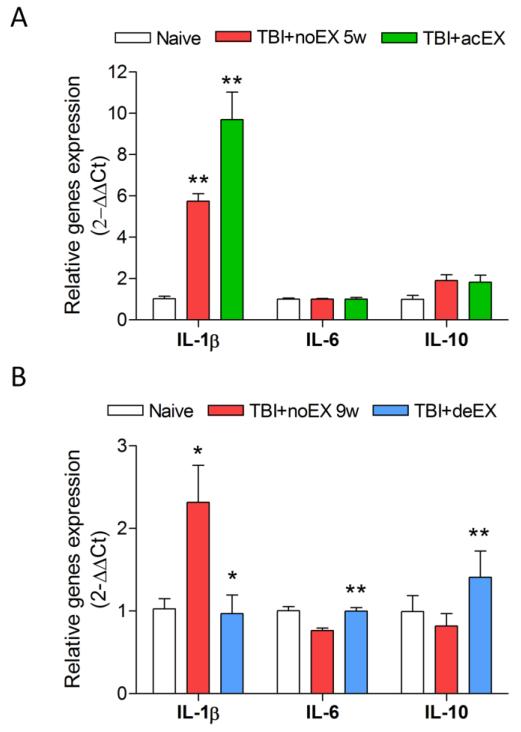
We examined the temporal profile of pro- and anti-inflammatory cytokines expression at 1 week, 5 weeks and 9 weeks after TBI (data not shown). The pro-inflammatory cytokine, IL-1β was markedly increased at 1 week (10.26±0.98), 5 weeks (5.74±0.36), and 9 weeks (2.316±0.44) after TBI compared to uninjured naïve controls. TBI increased the expression of IL-6 at 1 week (1.16±0.09) post-injury, followed by a gradual decline to control levels at 9 weeks (0.76±0.03) post-injury. TBI also caused up-regulation of the anti-inflammatory cytokine IL-10 at 1 week (4.20±0.55) post-injury, which returned to control levels at 9 weeks (0.82±0.14 versus naïve). Acute exercise initiation starting at 1 week post-injury resulted in further increase in the expression of IL-1β (p<0.01, TBI+acEX vs. TBI+noEX 5w) when compared to the non-exercised TBI group at 5 weeks post-injury (Fig. 3A), whereas there were no significant changes in IL-6 and IL-10 expression at this time point (Fig. 3A). In contrast, delayed exercise initiation starting at 5 weeks post-injury significantly reduced the expression of IL-1β compared to the non-exercised TBI group (p<0.01, TBI+deEX vs. TBI+noEX 9w) at 9 weeks post-injury (Fig. 3B). Moreover, delayed exercise resulted in a significant increase of both IL-6 (p<0.01, TBI+deEX vs. TBI+noEX 9w) and IL-10 (p<0.01, TBI+deEX vs. TBI+noEX 9w) expression at this time point (Fig. 3B).
Figure 3.
Effect of exercise on expression of IL-1β, IL-6, and IL-10 after TBI. Quantitative real-time PCR analysis was performed at 5 weeks or 9 weeks post-injury with/without exercise. (A) Acute exercise significantly up-regulated expression level of IL-1β at 5 weeks post-injury (**p<0.01, TBI+acEX vs. TBI+noEX 5w; n=4-6/group). No significant changes were observed in the expression of IL-6 and IL-10. (B) Delayed exercise significantly attenuated the TBI-induced IL-1β expression at 9 weeks post-injury. Moreover, delayed exercise resulted in a significant increase of both IL-6 and IL-10 expression (**p<0.01 TBI+deEX vs. TBI+noEX 9w; n=4-6/group). All data normalized to the expression level of the naïve group.
We also examined the long-term expression of classical markers of microglial activation: galectin-3 (MAC-2), a microglia-selective cell surface marker (Lalancette-Hebert et al., 2007); C1qB, a molecule involved in microglia/macrophage activation (Pasinetti et al., 1992); and, gp91phox and p22phox, membrane components of the NADPH oxidase enzyme (Loane et al., 2009). Brain homogenates were obtained at 3 months post-injury with/without acute or delayed exercise and were used for Western blot analysis. The non-exercised TBI group had significantly increased galectin-3 and C1qB protein levels compared to the uninjured naïve controls (a, p<0.05, TBI+noEX vs. naïve) (Fig.4A and B). In addition, the acute exercise TBI group had increased galectin-3 and C1qB protein expression (b, p<0.05, TBI+acEX vs. naïve), which was significantly higher than the levels in the non-exercised TBI group (f, p<0.05, TBI+acEX vs. TBI+noEX). Notably, the delayed exercise TBI group had reduced galectin-3 and C1qB expression; although higher than the uninjured naïve group (c, p<0.05, TBI+deEX vs. naïve), galectin-3 and C1qB expression was significantly lower in the delayed exercise TBI group than either the non-exercised TBI (d, p<0.01, TBI+deEX vs. TBI+noEX) or the acute exercise TBI groups (e, p<0.01, TBI+deEX vs. TBI+acEX) (Fig. 4A and B). Immunohistochemical analysis revealed that galectin-3 expression was up-regulated in the acute exercise TBI tissue when compared to non-exercised TBI tissue, whereas galectin-3 expression was markedly reduced in delayed exercise tissue at 3 months post-inury (Fig. 4C), and these galectin-3-positive cells were colocalized with the microglial marker Iba-1 and the activated microglia/macrophage marker CD68 (Fig. 4D).
Figure 4.
Effect of exercise on expression of microglial activation markers C1qB and Galectin-3 at three months post-injury. (A) Representative immunoblots for microglial activation markers (C1qB and galectin-3) and the loading control (β-actin). (B) Quantification of protein band intensity showed that expression of C1qB and galectin-3 was significantly increased in all TBI groups compared to naïve group (a-TBI+noEX, b-TBI+acEX, c-TBI+deEX; p<0.05 vs. naïve). The expression of C1qB and galectin-3 was significantly increased in the acute exercise injured animals compared to non-exercised TBI group (f, p<0.05 TBI+acEX vs. TBI+noEX) and attenuated by the delayed exercise compared to both non-exercise (d; p<0.05 TBI+deEX vs. TBI+noEX) and acute exercise (e, p<0.05 TBI+deEX vs. TBI+acEX) TBI groups. (C) Representative galectin-3 immunohistochemical images in the contused cortex at 3 months post-injury. (D) Galectin-3-positive cells (red) co-labeled with Iba1-positive microglia (green) and/or CD68-positive reactive microglia (magenta). Bar = 30 μm (C); 250 μm (D).
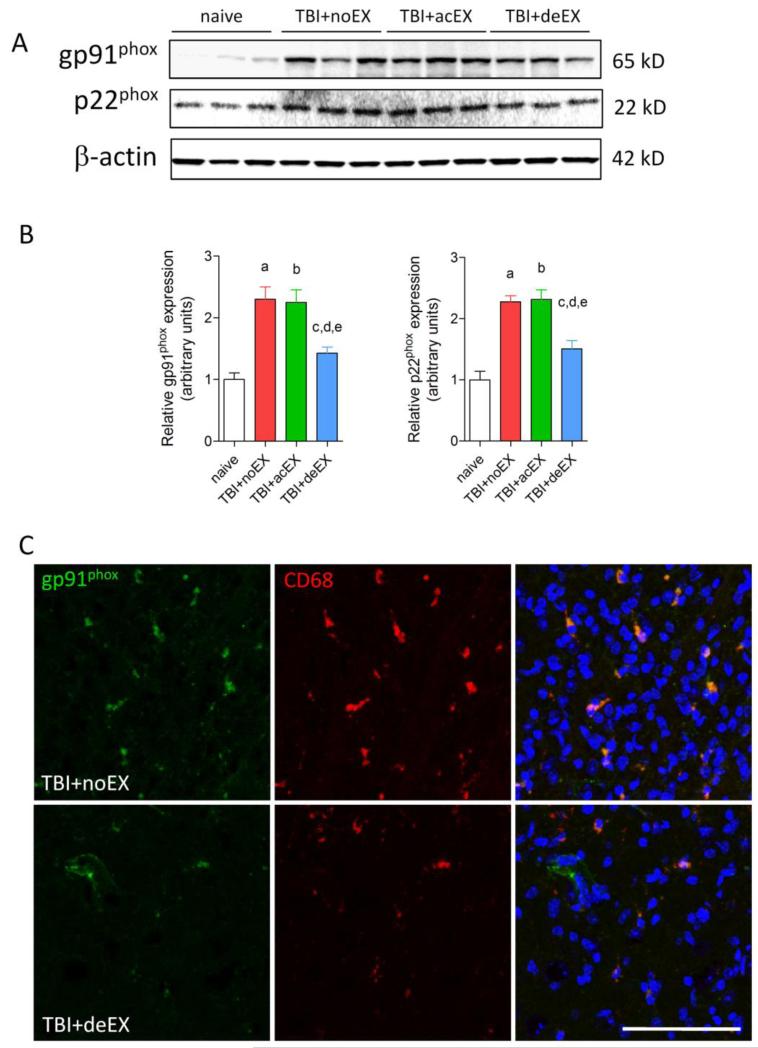
Western blot analysis showed that the protein levels for NADPH oxidase subunits gp91phox and p22phox were significantly increased in all TBI tissue with/without exercise compared to uninjured naïve control tissue (a, b, c, p<0.05, TBI+noEX, TBI+acEX and TBI+deEX, respectively vs. naïve) (Fig.5 A, B). However, delayed exercise significantly attenuated the expression of gp91phox and p22phox subunits when compared to non-exercised TBI group (d, p<0.05, TBI+deEX vs. TBI+noEX) or acute exercise TBI brain (e, p<0.05, TBI+deEX vs. TBI+acEX) (Fig. 5A, B). Immunohistochemical analysis revealed that gp91phox was highly expressed in the non-injured TBI tissue at 3 months post-injury and co-localized with the CD68 in reactive microglia (Fig. 5C). In contrast, there were reduced numbers of gp91phox+/CD68+ microglia in the delayed exercise TBI tissue at this time point.
Figure 5.
Effect of exercise on expression of NADPH oxidase at three months post-injury. (A) Representative immunoblots for the NADPH oxidase subunits gp91phox and p22phox and the loading control (β-actin). (B) Quantification of protein band intensity showed that in all injured groups expression of gp91phox and p22phox was significantly increased compared to naïve group (a-TBI+noEX, b-TBI+acEX, c-TBI+deEX; p<0.05 vs. naïve). The expression of gp91phox and p22phox was significantly attenuated by delayed exercise compared to either non-exercised TBI (d; p<0.05 TBI+deEX vs. TBI+noEX) or acute exercise TBI (e; p<0.05 TBI+deEX vs. TBI+acEX) groups. (C) Gp91phox-positive cells (green) co-localized with CD68-positive reactive microglia (red) that displayed a hypertrophic morphology in the injured cortex of non-exercised TBI sections at three months post-injury. Delayed exercise has reduced gp91phox-positive reactive microglia at this time point. Bar = 75 μm.
Delayed exercise, unlike early exercise initiation, attenuated the activated microglial phenotypes following traumatic brain injury
Stereological assessment of microglial activation phenotypes in CA2/3 and dentate gyrus sub-regions of hippocampus was performed at three months post-injury. Representative images and reconstructions (Neurolucida) of the resting (ramified, small cell body with elongated and thin projections) and activated (hypertrophic, large cell body with shorter and thicker projections) microglia were presented (Fig.6A). No significant differences were observed in the number of ramified microglia across the groups (Fig. 6B). Activated microglial phenotypes were significantly increased in the non-exercised TBI group (a, p<0.05, TBI+noEX vs. naïve) and showed a non-significant trend toward higher levels in the acute exercise TBI group (b, p<0.05, TBI+acEX vs. naïve) (Fig. 6B). In contrast, for the delayed exercise TBI group there was no significant difference in the number of activated microglia compared to controls and a significant reduction in activated microglial phenotypes compared to the acute exercise TBI group (e, p<0.05 TBI+deEX vs. TBI+acEX) (Fig. 6B).
Figure 6.
Effect of exercise on activated microglial phenotypes in the hippocampus at three months post-injury. (A) Representative Iba-1 immunohistochemical images displayed resting (ramified morphology, upper left) or activated (hypertrophic morphology, lower left) microglial phenotypes and the corresponding Neurolucida reconstructions (right). (B) Unbiased stereological quantitative assessment of microglial phenotypes in the hippocampus of each group. There was no significant difference in the number of ramified microglia in the CA2/3 and DG regions across groups. Injured non-exercised (a; p<0.05 TBI+noEX vs. naïve) or acute exercise tissue (b; p<0.05 TBI+acEX vs. naïve) showed significantly increased activated microglia in CA2/3 and DG regions compared to naïve. Delayed exercise tissue showed no significant differences in activated microglia when compared to naïve and demonstrated significantly reduced numbers of activated microglia in CA2/3 and DG compared to acute exercise (e; p<0.05 TBI+deEX vs. TBI+acEX; n=4-6/group).
Delayed exercise, but not early exercise initiation, increased plasticity-associated genes and newborn neurons in post-traumatic brain
We evaluated the effects of exercise on plasticity-associated genes including BDNF, CREB, and IGF-1. Hippocampal tissue was obtained from TBI brains with/without exercise and was used for quantitative real-time PCR analysis. Acute exercise initiation failed to increase BDNF and CREB gene expression; in contrast, delayed exercise significantly up-regulated BDNF and CREB gene expression in the hippocampus when compared to the uninjured naïve group (p<0.01, TBI+deEX vs. naïve), non-exercised TBI group (p<0.01, TBI+deEX vs. TBI+noEX 1w, 5w, 9w) or acute exercise TBI group (p<0.01, TBI+deEX vs. TBI+acEX), (Fig. 7A). No significant TBI-induced changes in the expression of BDNF and CREB were observed between non-exercised TBI (1w, 5w, and 9w) and uninjured naïve groups (Fig.7A). There was significant TBI-induced IGF-1 up-regulation at 1 week (p<0.01, TBI+noEX 1w vs. naïve) post-injury, which gradually returned to uninjured naïve levels at 9 weeks post-injury. Acute exercise initiation did not increase IGF-1 expression, whereas delayed exercise significantly increased IGF-1 (p<0.01, TBI+deEX vs. TBI+noEX 9w or naïve) expression compared to the uninjured naïve group or the non-exercised TBI groups (Fig. 7A).
Figure 7.
Effect of exercise on the expression of BDNF, CREB, IGF-1, and neurogenesis in post-traumatic brain. (A) BDNF, CREB and IGF-1 genes expression were compared across naïve, non-exercised TBI (1w, 5w, and 9w) and acute/delayed exercised TBI animals using quantitative real-time PCR. There were no differences in the expression of BDNF and CREB genes in non-exercised TBI mice compared to naïve mice. Delayed exercise significantly increased the expression of BDNF and CREB genes compared to either uninjured naïve (**p<0.01, versus naive) or injured non-exercised controls (**p<0.01, versus TBI+noEX 1w, 5w, 9w). Notably, there was significant TBI-induced IGF-1 up-regulation at 1 week (**p<0.01, TBI+noEX 1w vs. naïve) post-injury, which gradually returned to uninjured naïve levels at 9 weeks post-injury. Delayed exercise significantly increased IGF-1 (**p<0.01, TBI+deEX vs. TBI+noEX 9w or naïve) expression compared to uninjured controls or non-exercised TBI groups at 9 weeks post-injury. No significant changes in any of these genes were observed in acute exercise TBI group compared to uninjured or injured controls at 5 weeks post-injury. (B and C) Representative confocal images showed NeuN-positive (green) neurons and newly generated BrdU-positive (red) cells in ipsilateral DG with exercised or non-exercised TBI mice. Arrowheads indicated double positive NeuN+/BrdU+ cells, representing newly generated neurons that had reached maturity. (D) Semiquantitative analysis was performed for assessment of BrdU+/NeuN+- cells in the ipsilateral DG in each section at 3 months post-injury. The injured delayed exercise animals had significantly more NeuN+/BrdU+ neurons compared to non-exercised (9w) group (d; p<0.05, TBI+deEX vs. TBI+noEX (9w)). (E) Unbiased stereological assessment of surviving neurons in the DG. All injured mice showed significantly lower number of neurons compared to naïve (a-TBI+noEX, b-TBI+acEX, c-TBI+deEX; p<0.05 vs. naïve). Injured delayed exercise mice showed significantly improved DG neuronal survival after TBI compared to both injured non-exercised (d; p<0.05 TBI+deEX vs. TBI+noEX) and acute exercise (e; p<0.05 TBI+deEX vs. TBI+acEX) groups. Data represents mean ± SEM, statistical analysis using one-way ANOVA followed by post hoc adjustments using Tukey’s test, n=5-6/group. Bar = 75 μm.
To evaluate effects of exercise on neurogenesis after TBI, BrdU was administered in the 5th week post-TBI for acute exercise group (Fig. 7B) or in the 9th week post-TBI for delayed exercise group (Fig. 7C). Immunohistochemistry with co-immunolabeling of BrdU/NeuN was performed using tissue obtained from TBI mice euthanized at three months post-injury. Semi-quantitative analysis of BrdU+/NeuN+ cells in dentate gyrus of hippocampus revealed that acute exercise did not significantly increase the number of newly born neurons (BrdU+) that reached maturity (NeuN+) in this region (p>0.05 TBI+acEX vs. TBI+noEX 5w) compared to non-exercised TBI mice (Fig. 7B and D). In contrast, delayed exercise significantly increased the number of BrdU+/NeuN+ neurons in comparison to non-exercised TBI mice (p<0.05 TBI+deEX vs. TBI+noEX) (Fig. 7C and D). Finally, we performed quantitative stereological assessment of total surviving neurons in the dentate gyrus at three months post-TBI. Although there was significant neuronal loss in this region in all TBI groups compared to the uninjured naïve group (p<0.05 vs. naïve), delayed exercise significantly increased the number of surviving neurons in dentate gyrus when compared to the non-exercised TBI (p<0.05, TBI+deEX vs. TBI+noEX) or the acute exercised TBI groups (p<0.05, TBI+deEX vs. TBI+acEX) (Fig. 7E). Differences in outcome for all experiments across injured and exercise groups are summarized in table 1.
Table 1.
Summary of major findings
| TBI + noEX | TBI + acEX | TBI + deEX | |
|---|---|---|---|
| Neurological outcomes | ↓ ↓ | ↓ ↓ | ↓ |
| Lesion volume | ↑ ↑ | ↑ ↑ | ↑ |
| DG neurons | ↓ ↓ | ↓ ↓ | ↓ |
| BrdU+/NeuN+ cells | ± | ↑ | ↑ ↑ |
| Plasticity-associated genes (i.e. BDNF, CREB, IGF-1) |
± | ± | ↑ ↑ |
| Pro-inflammatory cytokines (i.e. IL-lβ) |
↑ ↑ | ↑ ↑ ↑ | ↑ |
| Anti-inflammatory cytokines (i.e. IL-6, IL- 10) |
± | ± | ↑ ↑ |
| Inflammatory markers (i.e. ClqB, Gal-3) |
↑ ↑ | ↑ ↑ ↑ | ↑ |
| Oxidative stress markers (i.e. gp91phox, p22phox) |
↑ ↑ | ↑ ↑ | ↑ |
| Activated microglial phenotype |
↑ ↑ | ↑ ↑ ↑ | ↑ |
Note: All comparisons were made to uninjured naïve controls.
Discussion
This study used mouse CCI, a well-established experimental TBI model, to demonstrate that delayed (5 week), but not early (1 week) exercise initiation reduces cognitive deficits and brain lesion volume at three months post-injury. Four weeks of delayed voluntary exercise significantly reduced the microglial-associated proinflammatory/classical inflammatory response and augmented the anti-inflammatory and/or alternative immune response in the ipsilateral hemisphere, compared to non-exercised TBI animals. Mice exposed to delayed exercise demonstrated greater increases in expression of the neurotrophic factors BDNF, CREB, and IGF-1, than early exercised animals, and showed enhanced neurogenesis and neuronal survival in the dentate gyrus. In contrast to delayed exercise initiation, early exercise initiation failed to alter cognitive outcome, lesion volume or survival of new neurons in the dentate gyrus, while increasing microglial activation and associated proinflammatory responses. These data are the first to indicate that early initiation of exercise after TBI can exacerbate the classical inflammatory response, potentially providing a less favorable microenvironment for post-traumatic neurogenesis; this effect may have contributed to the significantly reduced cognitive recovery shown for the acute exercise TBI group.
The critical importance of timing of exercise initiation after experimental TBI was previously demonstrated by Griesbach and colleagues, who showed that exercise initiation at 2 weeks induced greater up-regulation of hippocampal BDNF expression and cognitive recovery than that initiated immediately after mild fluid-percussion induced injury (FPI) in rats (Griesbach et al., 2004b). This study demonstrated that levels of CREB and synapsin I decreased when exercise was initiated immediately after injury, and that with more severe FPI, optimal exercise-induced up-regulation of plasticity-associated genes (BDNF, CREB, and synapsin I) required an ever greater delay period after trauma. Thus, the optimal timing of exercise initiation after TBI may be critically related to injury severity (Griesbach et al., 2007; Griesbach et al., 2004b). Our data are consistent with these observations and show that delayed exercise starting at 5 weeks post-injury induces greater up-regulation of BDNF, CREB, and IGF-1 expression in the ipsilateral brain, as compared to exercise initiated at 1 week in a moderately severe CCI model.
Kozlowski and colleagues, using a unilateral sensorimotor cortical injury model, were among the first to suggest that very early use-dependent physical activity can serve to increase neuronal loss and lesion volume (Kozlowski et al., 1996). Early training after permanent or transient focal cerebral ischemia has also been found to exacerbate tissue damage and results in poor sensorimotor outcome (Bland et al., 2001; Risedal et al., 1999). Similarly, Crane and colleagues reported that acute exercise following severe bilateral TBI increased deficits in a complex task and that performance did not return to the levels for non-exercised injured rats until post-TBI day 13 (Crane et al., 2012). However, such observations have not been observed uniformly in all brain injury models (Biernaskie et al., 2004; Tillerson et al., 2001; Van Keuren et al., 1998). Thus, exercise-timing effects may reflect injury mechanism as well as injury severity.
Several mechanisms may help to explain the detrimental effects of early initiation of exercise or early use-dependent exacerbation of neuronal injury. Early initiation of exercise may compromise compensatory “reactive plasticity” (Witte, 1998; Witte and Stoll, 1997). Neuronal dendritic growth was reported in contralateral motor cortex up to 18 days after unilateral cortical lesions (Jones and Schallert, 1994). Such plasticity may reflect the up-regulation of trophic factors after brain injury-including CREB (Raghavendra Rao et al., 2002), IGF-1 (Hughes et al., 1999) and/or bFGF (Rowntree and Kolb, 1997). We also found that the expression of IGF-1 gene was significantly up-regulated at 1 week post-TBI. Earlier initiation of exercise after TBI, in contrast to more delayed exercise, can limit the increased expression of such factors (Griesbach et al., 2007; Griesbach et al., 2004b).
Another potential factor that may contribute to exercise timing outcome is post-traumatic metabolic changes. Decreased glucose metabolism is found after TBI, the duration of which is related to injury severity (Ginsberg et al., 1997; Sutton et al., 1994; Yoshino et al., 1991). A decrease in free intracellular magnesium concentration also occurs after TBI and is associated with a reduction in cellular bioenergetics state that reflects injury severity (Vink et al., 1988a; Vink et al., 1988b). Given that exercise itself places an increased energetic demand, it has been suggested that injury severity may thus critically affect the consequences of physical activity timing (Griesbach et al., 2007). In contrast, others have proposed that hypermetabolism rather than hypometabolism may be a key issue. Cortical networks adjacent to a focal brain ischemia may be hyperexcitable due to an imbalance between excitatory and inhibitory synaptic function (Schiene et al., 1996). Hyperexcitability within the penumbra is first detected 1 to 3 days after focal ischemia and reaches a maximum at 28 days (Qu et al., 1998). Physical activity can further stimulate the release of glutamate and catecholamines (Vanderwolf and Cain, 1994), which in the metabolically compromised brain may be harmful. The observation that the NMDA receptor antagonist MK-801 can prevent use-dependent exaggeration of neural injury is consistent with this hypothesis (Humm et al., 1998).
Our data are also consistent with the hypothesis that differences in chronic posttraumatic neuroinflammation following acute or delayed exercise initiation may contribute to the differences in outcomes. TBI induces progressive neurodegeneration and tissue loss (Bramlett and Dietrich, 2007; Byrnes et al., 2012; Smith et al., 1997). Persistent microglial activation/inflammation and related oxidative stress appear to contribute to the development of progressive structural changes and long-term functional deficits (Byrnes et al., 2012; Gentleman et al., 2004; Ramlackhansingh et al., 2011). Our data demonstrate chronic inflammatory/immune changes in the brains of non-exercised TBI mice at 3 month post-injury, with significantly increased C1qB, galectin-3, gp91phox, and p22phox expression. Notably, early exercise amplifies posttraumatic C1qB and galectin-3 expression, as well as activated microglial phenotypes, which may contribute to progressive neurodegeneration after CNS injury (Byrnes et al., 2012; Venkatesan et al., 2010). It has been suggested that galectin-3 is a microglial specific marker (Lalancette-Hebert et al., 2007; Lalancette-Hebert et al., 2012), and extensive galectin-3 positive cells were found in the contused cortex and hippocampus; but some of these were co-labeled with CD68, which is expressed by peripheral macrophages (Galea et al., 2005) in addition to resident microglia. Therefore, we cannot rule out a systemic source of the galectin-3 positive cells. Recent studies in experimental spinal cord injury and stroke have implicated galectin-3 and C1q, respectively, in secondary injury (Pajoohesh-Ganji et al., 2012; Ten et al., 2010).
Prior reports demonstrate that exercise can modulate both classical and alternative inflammatory responses (Petersen and Pedersen, 2005; Woods, 2005). The current study shows that delayed exercise initiation after TBI not only reduced the classical inflammatory response (decreased IL-1β, C1qB, galectin-3, gp91phox, and p22phox expression), but also stimulates an alternative immune response (increased IL-6 and IL-10) that may act to limit neurotoxic effects of inflammation. In this context, it has been shown that exercise pre-conditioning inhibits pro-inflammatory (IL-1β, TNF-α) cytokine accumulation and neutrophil infiltration, and enhances anti-inflammatory (IL-10) cytokines, which may contribute to improved motor recovery after FPI (Knoblach and Faden, 1998; Mota et al., 2012; Petersen and Pedersen, 2005). In addition, voluntary exercise protects hippocampal neurons from trimethyl-tin induced injury by modulating TNFα-mediated neurotoxicity through IL-6 (Funk et al., 2011). Although persistent neuroinflammatory responses after TBI may contribute to late neurodegeneration, it has been suggested that maintaining rate-limited inflammation may be important for repair in the late phase after TBI (Ziebell and Morganti-Kossmann, 2010; Kumar and Loane, 2012). In our studies, late exercise initiation was associated with increased survival of newborn neurons in the dentate gyrus of the hippocampus, potentially reflecting a more favorable inflammatory state in those animals.
In summary, our studies compared the effects of exercise timing after brain injury using more detailed biochemical, behavioral and histological outcomes than prior studies, and with longer duration follow-up. The neuroprotective effects of exercise after TBI are critically dependent on the timing of exercise initiation, and appear to be strongly correlated to changes in chronic post-traumatic neuroinflammation. Such exercise-induced modulation of inflammation may serve to provide a more favorable microenvironment for neurogenesis and neuronal survival after TBI. The ability of markedly delayed exercise initiation to limit neurodegeneration and to enhance post-traumatic cognitive recovery challenges current concepts about the therapeutic window for neuroprotection. This is consistent with other recent work from our laboratory, demonstrating that targeted phamacological intervention to limit chronic microglial activation has at least a one-month therapeutic window (Byrnes et al., 2012).
Highlights.
Late unlike early exercise improves cognition and reduces lesion volume after TBI
Late exercise after TBI reduces chronic classical inflammatory responses
Late exercise initiation after TBI augments alternative inflammatory responses
Early exercise after TBI exacerbates chronic classical inflammatory responses
Late exercise, but not early exercise initiation enhances newborn neuron survival
Acknowledgements
We thank Titilola Akintola, Katherine Cardiff, Jeremy Bengson and Domenick Giordano for expert technical assistance. This work is supported by Maryland Exercise and Robotics Center of Excellence (MERCE) Pilot Project Award and an NIH grant, R01 (NS061839) to AIF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: The authors have no conflict of interests.
Co-authors’ contribution: CP and AIF: conceived and designed the study; CP, BAS, DJL and AIF wrote the manuscript; CP, BAS, JW, BS, ZZ and RC: executed the experiments, analyzed and interpreted data.
Reference
- Adkins DL, et al. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–82. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- Biernaskie J, et al. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–54. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, et al. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Bland DC, et al. Effectiveness of physical therapy for improving gait and balance in individuals with traumatic brain injury: A systematic review. Brain Inj. 2011;25:664–79. doi: 10.3109/02699052.2011.576306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Quantitative structural changes in white and gray matter 1 year following traumatic brain injury in rats. Acta Neuropathol. 2002;103:607–14. doi: 10.1007/s00401-001-0510-8. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–41. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, et al. Delayed mGluR5 activation limits neuroinflammation and neurodegeneration after traumatic brain injury. J Neuroinflammation. 2012;9:43. doi: 10.1186/1742-2094-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Clark RE, et al. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–60. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–71. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- Crane AT, et al. The effects of acute voluntary wheel running on recovery of function following medial frontal cortical contusions in rats. Restor Neurol Neurosci. 2012;30:325–33. doi: 10.3233/RNN-2012-120232. [DOI] [PubMed] [Google Scholar]
- Cryan JF, et al. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Faden AI. Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:345–6. doi: 10.1002/ana.22555. [DOI] [PubMed] [Google Scholar]
- Funk JA, et al. Voluntary exercise protects hippocampal neurons from trimethyltin injury: possible role of interleukin-6 to modulate tumor necrosis factor receptor-mediated neurotoxicity. Brain Behav Immun. 2011;25:1063–77. doi: 10.1016/j.bbi.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, et al. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–84. doi: 10.1002/glia.20124. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Sci Int. 2004;146:97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, et al. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 1997;272:H2859–68. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, et al. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004a;1016:154–62. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, et al. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–71. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, et al. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004b;125:129–39. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Hughes PE, et al. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog Neurobiol. 1999;57:421–50. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- Humm JL, et al. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–92. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Itoh T, et al. Exercise inhibits neuronal apoptosis and improves cerebral function following rat traumatic brain injury. J Neural Transm. 2011;118:1263–72. doi: 10.1007/s00702-011-0629-2. [DOI] [PubMed] [Google Scholar]
- Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–52. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabadi SV, et al. Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J Cereb Blood Flow Metab. 2012;32:137–49. doi: 10.1038/jcbfm.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MS, Steward O. Injury-induced physiological events that may modulate gene expression in neurons and glia. Rev Neurosci. 1997;8:147–77. doi: 10.1515/revneuro.1997.8.3-4.147. [DOI] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Exp Neurol. 1998;153:143–51. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Kobilo T, et al. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–9. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, et al. Effects of cortical ischemia and postischemic environmental enrichment on hippocampal cell genesis and differentiation in the adult rat. J Cereb Blood Flow Metab. 2002;22:852–60. doi: 10.1097/00004647-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, et al. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–86. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, et al. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. 2012;32:10383–95. doi: 10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loane DJ, Faden AI. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31:596–604. doi: 10.1016/j.tips.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, et al. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem. 2009;284:15629–39. doi: 10.1074/jbc.M806139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, et al. Randomized controlled trials in adult traumatic brain injury. Brain Inj. 2012;26:1523–48. doi: 10.3109/02699052.2012.722257. [DOI] [PubMed] [Google Scholar]
- Mota BC, et al. Exercise pre-conditioning reduces brain inflammation and protects against toxicity induced by traumatic brain injury: behavioral and neurochemical approach. Neurotox Res. 2012;21:175–84. doi: 10.1007/s12640-011-9257-8. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, et al. Characterization of inflammatory gene expression and galectin-3 function after spinal cord injury in mice. Brain Res. 2012 doi: 10.1016/j.brainres.2012.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, et al. Complement C1qB and C4 mRNAs responses to lesioning in rat brain. Exp Neurol. 1992;118:117–25. doi: 10.1016/0014-4886(92)90028-o. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Piao CS, et al. Combined inhibition of cell death induced by apoptosis inducing factor and caspases provides additive neuroprotection in experimental traumatic brain injury. Neurobiol Dis. 2012;46:745–58. doi: 10.1016/j.nbd.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, et al. Long-term changes of ionotropic glutamate and GABA receptors after unilateral permanent focal cerebral ischemia in the mouse brain. Neuroscience. 1998;85:29–43. doi: 10.1016/s0306-4522(97)00656-8. [DOI] [PubMed] [Google Scholar]
- Raghavendra Rao VL, et al. Gene expression analysis of spontaneously hypertensive rat cerebral cortex following transient focal cerebral ischemia. J Neurochem. 2002;83:1072–86. doi: 10.1046/j.1471-4159.2002.01208.x. [DOI] [PubMed] [Google Scholar]
- Ramlackhansingh AF, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–83. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- Risedal A, et al. Early training may exacerbate brain damage after focal brain ischemia in the rat. J Cereb Blood Flow Metab. 1999;19:997–1003. doi: 10.1097/00004647-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Rowntree S, Kolb B. Blockade of basic fibroblast growth factor retards recovery from motor cortex injury in rats. Eur J Neurosci. 1997;9:2432–41. doi: 10.1111/j.1460-9568.1997.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Schiene K, et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996;16:906–14. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- Smith DH, et al. Progressive atrophy and neuron death for one year following brain trauma in the rat. J Neurotrauma. 1997;14:715–27. doi: 10.1089/neu.1997.14.715. [DOI] [PubMed] [Google Scholar]
- Soltys Z, et al. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res. 2001;63:90–7. doi: 10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Faden AI. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics. 2010;7:3–12. doi: 10.1016/j.nurt.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RL, et al. Metabolic changes following cortical contusion: relationships to edema and morphological changes. Acta Neurochir Suppl (Wien) 1994;60:446–8. doi: 10.1007/978-3-7091-9334-1_122. [DOI] [PubMed] [Google Scholar]
- Ten VS, et al. Complement component c1q mediates mitochondria-driven oxidative stress in neonatal hypoxic-ischemic brain injury. J Neurosci. 2010;30:2077–87. doi: 10.1523/JNEUROSCI.5249-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, et al. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–35. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keuren KR, et al. Fixed-ratio discrimination training as replacement therapy in Parkinson’s disease: studies in a 6-hydroxydopamine-treated rat model. Brain Res. 1998;780:56–66. doi: 10.1016/s0006-8993(97)01184-0. [DOI] [PubMed] [Google Scholar]
- van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH, Cain DP. The behavioral neurobiology of learning and memory: a conceptual reorientation. Brain Res Brain Res Rev. 1994;19:264–97. doi: 10.1016/0165-0173(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Venkatesan C, et al. Chronic upregulation of activated microglia immunoreactive for galectin-3/Mac-2 and nerve growth factor following diffuse axonal injury. J Neuroinflammation. 2010;7:32. doi: 10.1186/1742-2094-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink R, et al. Changes in cellular bioenergetic state following graded traumatic brain injury in rats: determination by phosphorus 31 magnetic resonance spectroscopy. J Neurotrauma. 1988a;5:315–30. doi: 10.1089/neu.1988.5.315. [DOI] [PubMed] [Google Scholar]
- Vink R, et al. Decline in intracellular free Mg2+ is associated with irreversible tissue injury after brain trauma. J Biol Chem. 1988b;263:757–61. [PubMed] [Google Scholar]
- Witte OW. Lesion-induced plasticity as a potential mechanism for recovery and rehabilitative training. Curr Opin Neurol. 1998;11:655–62. doi: 10.1097/00019052-199812000-00008. [DOI] [PubMed] [Google Scholar]
- Witte OW, Stoll G. Delayed and remote effects of focal cortical infarctions: secondary damage and reactive plasticity. Adv Neurol. 1997;73:207–27. [PubMed] [Google Scholar]
- Woods JA. Physical activity, exercise, and immune function. Brain Behav Immun. 2005;19:369–70. doi: 10.1016/j.bbi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Yoshino A, et al. Dynamic changes in local cerebral glucose utilization following cerebral conclusion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–19. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- Zhang HT, et al. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–23. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, et al. Comparing the Predictive Value of Multiple Cognitive, Affective, and Motor Tasks after rodent TBI. J Neurotrauma. 2012 doi: 10.1089/neu.2012.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]