Abstract
Objective
To examine the long-term relationship between changes in water and beverage intake and weight change.
Subjects
Prospective cohort studies of 50 013 women aged 40-64 in the Nurses’ Health Study (NHS, 1986-2006), 52 987 women aged 27-44 in the NHS II (1991-2007), and 21 988 men aged 40-64 in the Health Professionals Follow-up Study (1986-2006) without obesity and chronic diseases at baseline.
Measures
We assessed the association of weight change within each 4-year interval with changes in beverage intakes and other lifestyle behaviors during the same period. Multivariate linear regression with robust variance and accounting for within-person repeated measures were used to evaluate the association. Results across the three cohorts were pooled by an inverse-variance-weighted meta-analysis.
Results
Participants gained an average of 1.45 kg (5th to 95th percentile, −1.87 to 5.46) within each 4-year period. After controlling for age, baseline body mass index, and changes in other lifestyle behaviors (diet, smoking habits, exercise, alcohol, sleep duration, TV watching), each 1-cup/d increment of water intake was inversely associated with weight gain within each 4-year period (−0.13 kg; 95% CI: −0.17, −0.08). The associations for other beverages were: SSBs (0.36 kg; 0.24, 0.48), fruit juice (0.22 kg; 0.15, 0.28), coffee (−0.14 kg; −0.19, −0.09), tea (−0.03 kg; −0.05, −0.01), diet beverages (−0.10 kg; −0.14, −0.06), low-fat milk (0.02 kg; −0.04, 0.09), and whole milk (0.02 kg; −0.06, 0.10). We estimated that replacement of 1 serving/d of SSBs by 1 cup/d of water was associated with 0.49 kg (95% CI: 0.32, 0.65) less weight gain over each 4-year period, and the replacement estimate of fruit juices by water was 0.35 kg (95% CI: 0.23, 0.46). Substitution of SSBs or fruit juices by other beverages (coffee, tea, diet beverages, low-fat and whole milk) were all significantly and inversely associated with weight gain.
Conclusion
Our results suggest that increasing water intake in place of SSBs or fruit juices is associated with lower long-term weight gain.
Keywords: water, beverage, body weight, prospective cohort study
INTRODUCTION
Beverages are major components of the daily diet, and may affect our health through various pathways.1 Patterns of beverage consumption have changed dramatically in the past several decades:2 sugar-sweetened beverages (SSBs) and fruit juices have increased and become major sources of fluids in the American diet, contributing to over 10% of daily calories,3 while milk consumption has decreased.2,3 There is growing evidence that intake of SSBs and fruit juices are associated with an elevated risk of obesity and weight gain.4,5
While several studies have evaluated the links between these caloric beverages and weight gain, much less is known about effects of water intake. Drinking more water has been proposed as one method to reduce weight gain.6 For example, it has been hypothesized that the volume could increase gastric distension and satiety compared to not drinking any fluids;7 or, perhaps more relevantly, that drinking of water could replace other caloric beverages and thereby reduce total calories consumed.6 Short-term feeding studies have suggested that drinking water before a meal was associated with a lower energy intake over the course of the day compared with drinking SSBs or fruit juices.6 In the setting of weight loss diets, one 12-week trial7 and a post-hoc analysis of a 12-month trial8 suggested that increasing water intake or replacing SSBs with water led to greater weight loss.
Beyond these small, short-term feeding studies and limited weight loss interventions, little is known about the long-term impact of water consumption on body weight. In particular, results from such interventions may not be generalizable to the long-term, gradual weight change seen in free-living populations. Also, little is known on the potential impact of other beverages (e.g., coffee and tea) on long-term weight gain, which generally contains fewer calories than SSBs or fruit juices and may also contain other compounds, such as caffeine, that could influence weight.9
We therefore investigated the relationship between intake of water and other beverages with long-term weight gain in three separate prospective cohort studies: the Nurses’ Health Study (NHS), NHS II, and Health Professionals Follow-up Study (HPFS), with up to 20 years of follow-up. We also estimated the association of replacing SSBs or fruit juices by water or other beverages in relation to weight change over time.
SUBJECTS AND METHODS
Study Population
The study designs of these cohorts have been described previously.10 The NHS consists of 121 700 female registered nurses from 11 US states who were enrolled in 1976. The NHS II was established in 1989 and was comprised of 116 671 younger female registered nurses from 14 states. The HPFS was initiated in 1986 with 51 529 US male health professionals from 50 states. The cohorts have been updated with biennial validated questionnaires to collect information on medical history, lifestyle and health factors. The follow-up rates of these cohorts all exceed 90% in each 2-year interval. In the present analysis, baseline was the first year when we collected detailed information on major lifestyle habits (diet, physical activity, smoking status): 1986 in the NHS and HPFS, and 1991 in the NHS II. We excluded participants with missing data on body weight, various beverages, lifestyle habits, more than nine blank responses on the baseline dietary questionnaire, and implausible energy intake (<900 or >3 500 kcal/d). Because of possible confounding by age-related and disease-related loss of muscle mass, we also excluded participants who were over 65 years of age, individuals with diabetes, cancer, cardiovascular, pulmonary, renal, or liver disease at baseline. We also excluded women who were pregnant during follow-up. These exclusions left a total of 50 013 women in the NHS, 52 987 women in the NHS II, and 21 988 men in the HPFS for this analysis. Participants who were excluded because of missing data were similar in major characteristics to those included in the analysis.10 The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health.
Assessment of water and other beverages
At baseline, food frequency questionnaires (FFQs) with over 130 items were administered to participants to collect information on their usual intake of foods and beverages over the previous year. Similar FFQs were administered every 4 years during follow-up. All FFQs asked participants how often, on average, they consumed each food using standard portion sizes (one standard serving, cup, glass, can or bottle). There were nine possible responses, ranging from “never or less than once per month” to “six or more times per day”. Questionnaire items on beverages included water (tap or bottled, including mineral or seltzer water), SSBs (“Coke, Pepsi, or other cola with sugar,” “caffeine-free Coke, Pepsi, or other cola with sugar,” “other carbonated beverages with sugar”, and “Hawaiian punch, lemonade, or other non-carbonated fruit drinks”), diet beverages (including “low-calorie cola with caffeine,” “low-calorie caffeine-free cola,” and “other low-calorie beverages”), fruit juices (“apple juice,” “orange juice,” “grapefruit juice,” and “other juice”), coffee (“decaffeinated coffee” and “coffee with caffeine”), milk (“skim or low-fat milk” and “whole milk”), and tea. The reproducibility and validity of the FFQ have been demonstrated in detail previously in the NHS11 and HPFS.12 The deattenuated correlation coefficients for various beverages between the FFQs and multiple dietary records ranged from 0.36 to 0.93 in validation studies.11,12 For example, the correlation coefficients were 0.78 for coffee, 0.84 for SSB, 0.93 for tea,11 and 0.52 for water.12
Assessment of other lifestyle factors
Other dietary factors in the analysis included fruits, vegetables, whole grain, refined grain, potatoes, potato chips, red meat, other dairy products, sweets and desserts, nuts, fried foods and trans-fat, which have been related to weight gain.10 Alcohol (beer, wine, and liquor) was also evaluated. In the biennial follow-up questionnaires, we updated information on cigarette smoking and physical activity. Sleep duration was asked in 1986, 2000 and 2002 in NHS; in 2001 in the NHS II; and in 1987 and 2000 in the HPFS. Therefore, we only used categorical variables for sleep duration in the analysis: missing data, <6, 6-7, 7-8 and >8 hours per day. The average hours per week of watching television at home was assessed in 1992 and 2004 in the NHS; in 1991, 1997, 2001 and 2005 in the NHS II; and in 1988 and every 2 years thereafter in the HPFS. Therefore, a categorical variable for television watching was used in the NHS (missing data, 0-1, 2-5, 6-20, 21-40, >40 hours per week), and absolute change (continuous variables) was used in the NHS II and HPFS.
Assessment of body weight
Height and weight were assessed in the baseline questionnaires, and weight was assessed every 2 years through questionnaires. In a validation study among 184 women from the NHS, participants were weighed 6 to 12 months after completing the mailed questionnaire. Reported weights were highly correlated with measured weights (Spearman correlation coefficient = 0.96), although they averaged 1.5 kg (3.3 lb) lower than the measured values.13
Statistical analysis
For these analyses of lifestyle changes and long-term weight gain, we utilized statistical approaches that have been described in detail previously.10 In brief, participants were followed for 20 years in the NHS (1986-2006), 16 years in the NHS II (1991-2007), and 20 years in the HPFS (1986-2006). Multivariate linear regression models (“PROC GENMOD” in SAS) were used to adjust for age, baseline BMI, and all lifestyle changes simultaneously in each 4-year period. In the models, we used the unstructured correlation matrix and accounted for within-person repeated measures. Changes in dietary factors (including beverages) were evaluated as continuous variables, and values below 0.5 percentiles and above 99.5 percentiles were censored to minimize the influence of outliers. Smoking status was treated as a categorical variable (never smoked, no change; former smoker, no change; current smoker, no change; current smoker changed to former smoker; former smokers changed to current smoker; and never smoked changed to current smoker). Changes in physical activity levels were assessed in quintiles. Given limited data on changes over time, sleep duration was assessed as a categorical variable using baseline information. Television watching was assessed as a categorical variable in the NHS because of limited repeated measures in this cohort, and as continuous variables in the NHS II and HPFS.
To minimize missing values during follow-up, we replaced missing values with carried-forward values for continuous variables, and added a missing indicator for categorical variables. Analyses among individuals without any missing values revealed similar results. The participants were censored if they reached 65 years of age, or 6 years before a diagnosis of chronic pulmonary, renal, or liver disease, or cancer (other than nonmelanoma skin cancer).10 We estimated the association of “substituting” a serving of one beverage for another by including them in the same model. The difference in their beta coefficients were used to estimate the effect size for the substitution association, and their variances and covariance matrix were used to derive the 95% confidence interval (CI).14
Stratified analyses were performed a priori by age (≤50 and >50 years), and BMI categories (<25.0, 25.0-29.9, and ≥30.0 kg/m2). The interaction was tested by including cross-product terms (e.g., changes in water intake × age group) in the models. An inverse-variance-weighted, random-effects meta-analysis was used to pool the results across cohorts. All analyses were performed using SAS software, version 9.2 (SAS Institute, North Carolina), at a two-tailed P value of 0.05.
RESULTS
The baseline characteristics and average 4-year changes in beverages and other lifestyle behaviors in the three cohorts are shown in Table 1. The mean weight change over all of the 4-year periods combined differed across the cohorts: 1.08 kg (5th to 95th percentile, −2.25 to 4.80) for women in the NHS, 2.10 kg (−1.35 to 6.75) for women in the NHS II, and 0.72 kg (−2.25 to 3.83) for men in the HPFS. The differences in weight gain across the cohorts may be due to the differences in sex and age at baseline: the mean ages were 51.8 (5th to 95th percentile, 41.0 to 63.0), 37.6 (30.0 to 44.0), and 50.6 (40.0 to 63.0), respectively.
Table 1.
Baseline characteristics and average 4-year change of beverages among 108 708 US women and men in the three prospective cohorts
| Variables | Nurses’ Health Study (n = 50 013 women) |
Nurses’ Health Study II (n = 52 987 women) |
Health Professionals Follow-up Study (n = 21 988 men) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | Changes within each 4-year period |
Baseline | Changes within each 4-year period |
Baseline | Changes within each 4-year period |
|
| Age, y | 51.8 (41.0 to 63.0) | - | 37.7 (30.0 to 44.0) | - | 50.6 (40.0 to 63.0) | - |
| Weight, kg | 67.0 (50.4 to 91.8) | 1.08 (−2.25 to 4.80) | 67.2 (49.5 to 99.0) | 2.10 (−1.35 to 6.75) | 80.4 (64.8 to 101.3) | 0.72 (−2.25 to 3.83) |
| BMI, kg/m2 | 25.1 (19.6 to 34.1) | 0.41 (−0.90 to 1.83) | 24.9 (19.1 to 36.3) | 0.78 (−0.48 to 2.50) | 25.3 (21.2 to 30.8) | 0.23 (−0.75 to 1.20) |
| Overweight, n (%) | 15531 (31.1) | 13444 (25.4) | 10111 (46.0) | |||
| Obesity, n (%) | 7981 (16.0) | 10674 (20.1) | 2021 (9.2) | |||
| Physical activity, MET-hr/wk |
14.2 (0.4 to 49.0) | 1.67 (−8.8 to 14.5) | 20.3 (0.9 to 64.9) | −0.10 (−16.7 to 15.1) | 21.7 (0.4 to 68.8) | 6.5 (−8.6 to 31.9) |
| Smoking status, n (%) | ||||||
| Never smoked | 22231 (44.5) | - | 33880 (63.9) | - | 10973 (49.9) | - |
| Past smoker | 19264 (38.5) | - | 13049 (24.6) | - | 8957 (40.7) | - |
| Current smoker | 8428 (16.9) | - | 6004 (11.3) | - | 1753 (8.0) | - |
| Missing data | 90 (0.2) | - | 54 (0.1) | - | 305 (1.4) | - |
| Alcohol intake, drinks/d | 0.51 (0 to 2.5) | −0.03 (−0.39 to 0.21) | 0.27 (0 to 1.14) | 0.04 (−0.11 to 0.27) | 0.90(0 to 3.21) | 0.00 (−0.47 to 0.43) |
| Sleep duration, hr/d | 7.0 (6.0 to 8.0) | - | 7.0 (5.0 to 9.0) | - | 7.2 (6.0 to 9.0) | - |
| Television watching, hr/wk | 4.3 (2.0-6.0) | - | 8.9 (1.0 to 30.5) | 0.36 (−3.50 to 4.75) | 10.9 (3.5 to 30.5) | −0.28 (−5.25 to 5.00) |
| Beverage intake, serving/d | ||||||
| Plain water | 2.67 (0.07 to 6.00) | 0.13 (−1.00 to 1.45) | 2.69 (0 to 6.0) | 0 (−1.09 to 0.88) | 2.54 (0.14 to 6.0) | −0.04 (−1.04 to 0.93) |
| Coffee | 2.44 (0 to 6.0) | −0.19 (−1.18 to 0.54) | 1.69 (0 to 5.0) | −0.10 (−0.84 to 0.47) | 1.95 (0 to 5.5) | −0.07 (−0.93 to 0.67) |
| SSB | 0.24 (0 to 1.07) | 0 (−0.22 to 0.25) | 0.46 (0 to 2.5) | −0.04 (−0.36 to 0.20) | 0.37(0 to 1.36) | −0.01 (−0.28 to 0.22) |
| Fruit juice | 0.83 (0 to 2.29) | 0.05 (−0.40 to 0.57) | 0.62 (0 to 2.0) | −0.04 (−0.36 to 0.25) | 0.78(0 to 2.43) | −0.01 (−0.40 to 0.45) |
| Whole-fat milk | 0.15 (0 to 1.0) | −0.03 (−0.22 to 0.01) | 0.07 (0 to 0.43) | −0.01 (−0.06 to 0.02) | 0.13 (0 to 0.79) | −0.03 (−0.19 to 0.01) |
| Low-fat milk | 0.76 (0 to 2.5) | 0.05 (−0.47 to 0.63) | 0.94 (0 to 2.5) | −0.04 (−0.52 to 0.38) | 0.73 (0 to 2.5) | −0.09 (−0.63 to 0.33) |
| Tea | 0.60 (0 to 2.5) | 0.05 (−0.49 to 0.66) | 0.74 (0 to 2.5) | 0.04 (−0.52 to 0.63) | 0.45 (0 to 2.5) | 0.01 (−0.40 to 0.47) |
| Diet beverage | 0.52 (0 to 2.5) | −0.01 (−0.41 to 0.39) | 1.06 (0 to 4.5) | −0.06 (−0.63 to 0.41) | 0.51 (0 to 2.5) | 0 (−0.36 to 0.38) |
Data were expressed as mean (5th to 95th percentile) or specified. Abbreviations: BMI, body mass index; MET: metabolic equivalent task; SSB, sugar-sweetened beverage.
At baseline, the median intake of water was 2.5 cups/d in all three cohorts. Averaged across all participants, mean water intake did not change over time, but the range of between-individual changes in water intake was large (Table 1). The mean change every 4 years was 0.13 cup/d (5th to 95th percentile, −1.00 to 1.45) in the NHS, 0 cup/d (−1.09 to 0.88) in the NHS II, and −0.04 (−1.04 to 0.93) in the HPFS. Findings for other beverages were similar, with very small average changes over time in the whole population, but large between-person differences. For example, in the NHS II, the difference in daily servings between persons in the upper and lower level of changes in beverages (95th percentile minus 5th percentile) was 1.97 for water, 1.31 for coffee, 1.15 for tea, 0.56 for SSBs, 0.61 for fruit juices, 0.90 for low-fat milk, and 1.04 for diet beverages. Notably, correlations between changes in various beverages were generally small (absolute Spearman correlation coefficients <0.10, data not shown).
Table 2 shows the relationships between changes in intake of water and beverages and weight gain in the three cohorts. The effect estimates were similar in direction and magnitude across cohorts, although small differences in certain beverages also existed. In the pooled analysis of multivariate-adjusted models, each serving per day increase of SSBs and fruit juices were significantly associated with weight gain within each 4-year period: 0.36 kg (95% CI: 0.24, 0.48) and 0.22 kg (95% CI: 0.15, 0.28), respectively. Inverse associations with weight gain were observed for water (−0.13 kg; 95% CI: −0.17,−0.08), coffee (−0.14 kg; 95% CI: −0.19, −0.09), diet beverages (−0.10 kg; 95% CI: −0.14, −0.06), and tea (−0.03 kg; 95% CI: −0.05, −0.01) for one serving per day increase within each 4-year period. Changes in low-fat or whole milk intake were not significantly related to weight change.
Table 2.
Cohort-specific and pooled results for the relationships between changes in beverage intake (1 serving/d) and absolute weight change (kg) within each 4-year period in three prospective cohortsa
| Nurses’ Health Study | Nurses’ Health Study II | Health Professionals Follow-up Study |
Pooled resultsb | P for heterogeneityc | |
|---|---|---|---|---|---|
| Plain water | |||||
| Age-adjusted changes | −0.18 (−0.19 to −0.16) | −0.21 (−0.23 to −0.19) | −0.09 (−0.11 to −0.06) | −0.16 (−0.22 to −0.09) | <0.001 |
| Multivariate-adjusted changesd | −0.15 (−0.17 to −0.14) | −0.15 (−0.17 to −0.14) | −0.07 (−0.09 to −0.05) | −0.13 (−0.17 to −0.08) | <0.001 |
| Coffee | |||||
| Age-adjusted changes | −0.13 (−0.15 to −0.11) | −0.19 (−0.22 to −0.16) | −0.09 (−0.12 to −0.07) | −0.14 (−0.19 to −0.08) | <0.001 |
| Multivariate-adjusted changesd | −0.12 (−0.14 to −0.10) | −0.20 (−0.22 to −0.17) | −0.11 (−0.14 to −0.08) | −0.14 (−0.19 to −0.09) | <0.001 |
| Sugar-sweetened beverage | |||||
| Age-adjusted changes | 0.50 (0.44 to 0.54) | 0.66 (0.61 to 0.70) | 0.38 (0.31 to 0.44) | 0.51 (0.35 to 0.67) | <0.001 |
| Multivariate-adjusted changesd | 0.36 (0.30 to 0.41) | 0.47 (0.42 to 0.52) | 0.25 (0.19 to 0.31) | 0.36 (0.24 to 0.48) | <0.001 |
| Fruit juice | |||||
| Age-adjusted changes | 0.28 (0.24 to 0.32) | 0.22 (0.19 to 0.26) | 0.12 (0.07 to 0.16) | 0.21 (0.12 to 0.30) | <0.001 |
| Multivariate-adjusted changesd | 0.24 (0.20 to 0.28) | 0.26 (0.22 to 0.30) | 0.15 (0.10 to 0.19) | 0.22 (0.15 to 0.28) | <0.001 |
| Whole-fat milk | |||||
| Age-adjusted changes | 0.14 (0.03 to 0.25) | 0.16 (−0.08 to 0.40) | 0.16 (0.01 to 0.30) | 0.15 (0.06 to 0.23) | 0.98 |
| Multivariate-adjusted changesd | 0.05 (−0.06 to 0.16) | −0.14 (−0.38 to 0.10) | 0.03 (−0.11 to 0.18) | 0.02 (−0.06 to 0.10) | 0.37 |
| Low-fat milk | |||||
| Age-adjusted changes | −0.07 (−0.10 to −0.04) | 0.01 (−0.03 to 0.05) | −0.05 (−0.09 to −0.01) | −0.04 (−0.09 to 0.01) | 0.008 |
| Multivariate-adjusted changesd | 0.02 (−0.02 to 0.05) | 0.09 (0.05 to 0.13) | −0.03 (−0.08 to 0.01) | 0.02 (−0.04 to 0.09) | <0.001 |
| Tea | |||||
| Age-adjusted changes | −0.02 (−0.05 to 0.01) | −0.01 (−0.04 to 0.02) | −0.03 (−0.07 to 0.02) | −0.02 (−0.04 to 0.01) | 0.83 |
| Multivariate-adjusted changesd | −0.04 (−0.07 to −0.01) | −0.01 (−0.04 to 0.02) | −0.04 (−0.08 to 0.00) | −0.03 (−0.05 to −0.01) | 0.34 |
| Diet beverage | |||||
| Age-adjusted changes | −0.08 (−0.11 to −0.04) | −0.03 (−0.07 to 0.00) | −0.15 (−0.19 to −0.10) | −0.08 (−0.14 to −0.02) | <0.001 |
| Multivariate-adjusted changesd | −0.11 (−0.15 to −0.08) | −0.07 (−0.10 to −0.03) | −0.14 (−0.18 to −0.09) | −0.10 (−0.14 to −0.06) | 0.03 |
Data are based on 20 years of follow-up (1986–2006) in the Nurses’ Health Study, 16 years of follow-up (1991–2007) in the Nurses’ Health Study II, and a 20 years of follow-up (1986–2006) in the Health Professionals Follow-up Study. The weight changes shown are for increased consumption; decreased consumption would be associated with the inverse of these weight changes. Increased consumption was defined as an increase in the number of servings per day for all beverages.
The results were pooled by an inverse-variance-weighted random-effects meta-analysis across the three cohorts.
P for heterogeneity was assessed by Cochrane Q statistic.
Values were adjusted for age and baseline body mass index at the beginning of each 4-year period, sleep duration, as well as for changes in physical activity, alcohol use, television watching, smoking, dietary variables (fruits, vegetables, whole grain, refined grain, potatoes, potato chips, red meat, other dairy products, sweets and desserts, nuts, fried foods and trans-fat), and all the beverage variables in the table simultaneously.
We estimated that substituting one cup/d of water for one serving/d of SSBs was associated with 0.49 kg (95% CI: 0.32, 0.65) less weight gain within each 4-year period (Figure 1), and the substitution estimate of water for fruit juices was 0.35 kg (95% CI: 0.23, 0.46). Substitution of coffee for SSBs or fruit juices was associated with similar magnitudes of less weight gain (0.50 and 0.36 kg, respectively). Replacements of tea (0.39 and 0.25 kg), diet beverages (0.47 and 0.33 kg), and low-fat milk (0.34 and 0.20 kg) for SSBs or fruit juices were all significantly and inversely related to weight gain (Figure 1).
Figure 1.
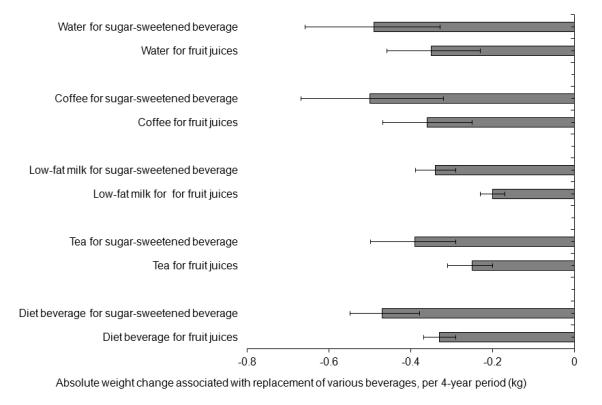
Changes in absolute body weight associated with serving-to-serving replacements between beverages per 4-year period
Data are based on 20 years of follow-up (1986–2006) in the Nurses’ Health Study, 16 years of follow-up (1991–2007) in the Nurses’ Health Study II, and a 20 years of follow-up (1986–2006) in the Health Professionals Follow-up Study. Values were adjusted for age and baseline body mass index at the beginning of each 4-year period, sleep duration, as well as for changes in physical activity, alcohol use, television watching, smoking, dietary variables (fruits, vegetables, whole grain, refined grain, potatoes, potato chips, red meat, other dairy products, sweets and desserts, nuts, fried foods and trans-fat), and all the beverage variables (water, coffee, sugar-sweetened beverages, fruit juices, whole milk, low-fat milk, tea, and diet beverages) simultaneously within each 4-year period. The results were absolute weight changes (kg) associated with serving-to-serving substitution between beverages, and were pooled by an inverse-variance-weighted random-effects meta-analysis across the three cohorts. The error bars indicate 95% confidence intervals.
In analyses stratified by age (≤50 and >50 years old; Table 3), we observed slightly larger effect estimates for water in younger participants. In participants ≤50 years of age, each one serving per day increase of water was associated with −0.17 kg (95% CI: −0.26, −0.08) weight gain, while the corresponding estimate in adults >50 years of age was −0.05 kg (95% CI: −0.14, 0.03). The interaction was significant in the NHS and NHS II, but not in the HPFS (Supplementary Table 1). Estimates for coffee, SSBs and diet beverages were also slightly stronger in younger than older participants, while estimates for other beverages were similar in magnitude between younger and older participants.
Table 3.
Pooled results for the relationships between changes in beverage intake (1 serving/d) and absolute weight change (kg) within each 4-year period: stratified by age or body mass indexa
| Stratified by age | Stratified by body mass index | ||||
|---|---|---|---|---|---|
|
|
|||||
| ≤50 years old | >50 years old | <25.0 kg/m2 | 25.0-29.9 kg/m2 | ≥30 kg/m2 | |
|
|
|||||
| Plain water | −0.17 (−0.26 to −0.08) | −0.05 (−0.14 to 0.03) | −0.07 (−0.10 to −0.05) | −0.17 (−0.26 to −0.08) | −0.23 (−0.28 to −0.19) |
| Coffee | −0.16 (−0.21 to −0.12) | −0.08 (−0.13 to −0.22) | −0.05 (−0.08 to −0.03) | −0.14 (−0.20 to −0.09) | −0.27 (−0.37 to −0.17) |
| Sugar-sweetened beverage | 0.43 (0.35 to 0.50) | 0.32 (0.21 to 0.44) | 0.14 (0.11 to 0.17) | 0.40 (0.25 to 0.54) | 0.63 (0.41 to 0.85) |
| Fruit juice | 0.23 (0.15 to 0.30) | 0.25 (0.05 to 0.46) | 0.09 (0.04 to 0.13) | 0.25 (0.16 to 0.34) | 0.57 (0.49 to 0.66) |
| Whole-fat milk | 0.06 (−0.06 to 0.19) | 0.00 (−0.19 to 0.20) | −0.07 (−0.20 to 0.05) | 0.17 (0.03 to 0.32) | 0.10 (−0.44 to 0.64) |
| Low-fat milk | 0.00 (−0.11 to 0.11) | 0.08 (−0.04 to 0.20) | 0.04 (0.00 to 0.08) | 0.00 (−0.09 to 0.09) | 0.03 (−0.09 to 0.16) |
| Tea | −0.02 (−0.04 to 0.01) | −0.12 (−0.29 to 0.05) | −0.01 (−0.03 to 0.01) | −0.01 (−0.06 to 0.05) | −0.05 (−0.11 to 0.01) |
| Diet beverage | −0.15 (−0.22 to −0.08) | 0.05 (−0.13 to 0.22) | −0.01 (−0.03 to 0.02) | −0.13 (−0.16 to −0.09) | −0.17 (−0.30 to −0.05) |
Data are based on 20 years of follow-up (1986–2006) in the Nurses’ Health Study, 16 years of follow-up (1991–2007) in the Nurses’ Health Study II, and a 20 years of follow-up (1986–2006) in the Health Professionals Follow-up Study. The weight changes shown are for increased consumption; decreased consumption would be associated with the inverse of these weight changes. Increased consumption was defined as an increase in the number of servings per day for all beverages. Values were adjusted for age and baseline body mass index at the beginning of each 4-year period, sleep duration, as well as for changes in physical activity, alcohol use, television watching, smoking, dietary variables (fruits, vegetables, whole grain, refined grain, potatoes, potato chips, red meat, other dairy products, sweets and desserts, nuts, fried foods and trans-fat), and all the beverage variables in the table simultaneously. The results were pooled by an inverse-variance-weighted random-effects meta-analysis across the three cohorts.
In analyses stratified by BMI categories (<25.0, 25.0−29.9, and ≥30.0 kg/m2; Table 3), we observed larger differences in weight gain with changes in water, coffee, SSBs, fruit juices, and diet beverages among overweight and obese participants, compared with weight gain in normal-weight persons. The pattern was similar among the three cohorts, and the P values for interaction were statistically significant in the individual cohort (Supplementary Table 2). No significant interactions were found for tea, low-fat or whole milk with body weight status.
DISCUSSION
In three separate large prospective cohorts of US women and men, we found that increasing intakes of water, coffee, and diet beverages were each inversely, and SSBs and fruit juices were positively associated with long-term weight gain. Substitution of SSBs and fruit juices by water and other beverages was significantly associated with less weight gain over time.
Very few studies have specifically evaluated the association between water consumption with body weight and obesity. To the best of our knowledge, our study is the first to report that increasing water intake per se was independently and significantly associated with less weight gain in long-term prospective cohorts. Studies have indicated that drinking water may induce thermogenesis and increase metabolic rate.15,16 thus increasing energy expenditure. However, this finding was not observed in other studies.17 It has also been hypothesized that liquid volume can increase gastric distension and satiety compared to not drinking any fluids,7 thus reducing energy intake at the subsequent meal. Several feeding studies have been conducted to contrast the effects of no water versus a water preload 30-60 minutes before or with a meal on energy intake in that meal. Results have been inconsistent with both decreased energy intake7,18,19 and neutral effects19,20,21 when water was added before or with the meal. A 12-week intervention study in 48 overweight middle-aged and elderly adults found that consuming 500 ml of water prior to each main meal when combined with a hypocaloric diet led to greater weight loss than a hypocaloric diet alone.7 Nevertheless, results from those short-term feeding studies and intervention trials were interesting, but may not be directly generalizable to the long-term effects of water on body weight.
Increasing coffee consumption was found to be inversely associated with weight gain. This is consistent with our previous analysis of 12-year weight change and coffee and caffeine intake in the NHS and HPFS.9 Caffeine is an adenosine-receptor antagonist, and studies have suggested that caffeine intake may increase basal energy expenditure and stimulate thermogenesis via the sympathetic nervous system.22,23 Most coffee was caffeinated in our cohorts, and exclusion of decaffeinated coffee from the analysis gave similar results (data not shown). Increasing tea consumption was not significantly associated with weight gain in our study. Studies have suggested that catechins in green tea may increase fat oxidation, reduce body fat and promote weight loss, if consumed in concentrated supplement form.24,25 However, consumption levels in the U.S. population are probably too low to achieve that concentration. We also observed that increasing diet beverage consumption was significantly but modestly associated with less weight gain. The results from other studies regarding noncaloric sweeteners and weight gain have been inconsistent.26,27 It is possible that there is an unmeasured confounding factor that tracks with diet beverage consumption (e.g., people who chose diet beverages instead of SSBs may be more health conscious, and implemented several other behaviors to maintain or lose weight). The association was only observed among overweight individuals in our study supported this possibility. Clearly, more research is still needed to investigate the effects of noncaloric sweeteners on body weight. Our analysis found a neutral association between milk (neither low-fat nor whole-fat) and weight gain, which is consistent with a prior analysis in the HPFS cohort.28 A recent systematic review of 19 prospective cohort studies29 and another meta-analysis of randomized clinical trials (RCTs)30 also found an inconclusive relation between dairy products and weight change.
Our findings with regard to SSBs and fruit juices are consistent with several previous observational studies.4,5,31,32 The SSB-weight relation has also been confirmed by several small-scale short-term RCTs in adults.33,34,35 Therefore, it has been proposed that SSBs or fruit juices should be replaced by other non- or low-caloric beverages such as water.6 Daniels and Popkin6 summarized data from short-term feeding trials in adults and concluded that drinking SSBs and fruit juices before a single meal was associated with 7.8% and 14.4% higher total energy intake compared to drinking water, respectively. In a secondary data analysis of the 12-month A TO Z weight loss intervention among 173 premenopausal overweight women, increasing water intake in lieu of SSBs was associated with lower energy intake,36 and significant losses of body weight and fat over time.8 A recent 6-month RCT among 318 overweight and obese adults was conducted to replace SSBs by water or diet drinks.37 Overall, the authors did not find statistically significant between-group differences in body weight, but participants in the beverage replacement groups were more likely to achieve a 5% weight loss compared to the control group.37 However, a significant reduction in energy intake in the control group resulting from health education efforts may mask the benefits of replacement of SSBs by water. Taken together, our results are consistent with the prior evidence and indicate a long-term benefit of replacing SSBs or fruit juices with water. In a recent analysis of NHS II cohort,38 we found that substituting water for SSBs or fruit juices was associated with a significantly lower risk of developing type 2 diabetes in women. Therefore, changing the beverage drinking pattern and improving the access to more water and less SSBs or fruit juices could be both effective and cost-effective, and should be prioritized in obesity prevention strategies.
Furthermore, substitutions of SSBs and fruit juices by other beverages (coffee, tea, diet beverages, and low-fat milk) were all estimated to be associated with less weight gain. However, the estimated effect sizes varied depending on the original associations between the beverages and weight gain. The major reason for the substitution benefits was the decreased total energy intake when replacing SSBs and fruit juices by other beverages. These findings indicate that a variety of choices are available to replace SSBs and fruit juices for the prevention of weight gain.
The strengths of the current study include a large sample size, prospective nature, novel analysis approach, and repeated assessments of diet and lifestyle variables. Our “change-on-change” analysis approach builds on the repeated measurements and long-term follow-up periods in the three large cohorts. This method has the feature of a quasi-experimental design, although it lacks the randomization as in a clinical trial. In the cohorts, participants chose to change their diet and lifestyles themselves without intervention from the investigators, who only observed and followed changes in body weight. Therefore, our results may be more externally generalizable to the real-world scenario as compared to a well-controlled laboratory setting. Our multivariate linear regression models simultaneously adjusted for multiple diet and lifestyle factors and also accounted for within-person correlations. In contrast to prevalent behaviors, changes in diet (including beverages) and lifestyle factors were generally not strongly correlated (|r|<0.10), suggesting that the different behaviors often change independently.10 Taken together, our results suggest that changes in beverage patterns may have moderate but significant influences on long-term weight change.
The current study has several potential limitations. First, our study populations primarily consisted of white and educated US adults, which may limit the generalizability of findings to other groups. We observed stronger effect estimates for the relations of various beverages (water, coffee, SSBs and fruit juices) with weight changes among overweight and obese individuals, thus it is possible that our results may underestimate the population impact of substitution of SSBs and fruit juices by other beverages (like water and coffee), given that the prevalence of overweight and obesity was lower in our study populations than the general US population.39 Second, because diet was assessed by FFQs, some measurement error in water and beverage assessment is inevitable, which may underestimate the true associations with weight change if the measurement error was random. As been shown in the validation study,13 self-reported body weights are generally lower than the directly-measured weights, which may underestimate the observed associations, because overweight/obese individuals or people who gained weight generally would under-report their weights. Furthermore, reverse causation is possible in that a person’s weight change could lead to changes in beverage pattern. For example, people who gained weight might reduce their SSBs intake and/or increase water intake, which would generally lead to an underestimation of the true effects. On the other hand, although we have simultaneously controlled for a series of lifestyle factors, people may attempt to lose weight by reducing SSBs intake and/or increasing water intake along with other lifestyle changes, which were not measured or inaccurately measured in our cohorts. Therefore, unmeasured or residual confounding factors are still possible to explain the associations. Finally, the observed associations could not prove causality as in any observational studies.
Conclusions
In conclusion, these data indicate that increasing plain water and coffee intake was inversely associated with weight gain, and substitution of sugar-sweetened beverages or fruit juices by non-caloric beverages, like plain water, is related to less weight gain. These findings support current recommendations to limit consumption of sugar-sweetened beverages and reduce consumption of fruit juices and increase consumption of water for the prevention of obesity.
Supplementary Material
ACKNOWLEDGEMENTS
We are indebted to the participants in the Nurses’ Health Study, Nurses’ Health Study II and Health Professionals Follow-up Study for their continuing outstanding support and colleagues working in these studies for their valuable help.
Footnotes
Funding/Support The study was supported by the National Institutes of Health grant (P01CA087969, R01CA050385, U19CA055075, R01DK058845, P30DK046200, and U54CA155626). The study sponsors were not involved in the study design and the collection, analysis, and interpretation of data, nor the writing of the article or the decision to submit it for publication. The authors were independent from the study sponsors.
Conflict of Interest The authors declare no conflict of interest.
Supplemental Information Supplementary information is available at IJO’s website.
REFERENCES
- 1.Wolf A, Bray GA, Popkin BM. A short history of beverages and how our body treats them. Obes Rev. 2008;9(2):151–164. doi: 10.1111/j.1467-789X.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27:205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988-1994 to 1999-2004. Am J Clin Nutr. 2009;89:372–381. doi: 10.3945/ajcn.2008.26883. [DOI] [PubMed] [Google Scholar]
- 4.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009;10:68–75. doi: 10.1111/j.1467-789X.2008.00523.x. [DOI] [PubMed] [Google Scholar]
- 6.Daniels MC, Popkin BM. Impact of water intake on energy intake and weight status: a systematic review. Nutr Rev. 2010;68:505–521. doi: 10.1111/j.1753-4887.2010.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis EA, Dengo AL, Comber DL, Flack KD, Savla J, Davy KP, et al. Water consumption increases weight loss during a hypocaloric diet intervention in middle-aged and older adults. Obesity. 2010;18:300–307. doi: 10.1038/oby.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stookey JD, Constant F, Popkin BM, Gardner CD. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity. 2008;16:2481–2488. doi: 10.1038/oby.2008.409. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Garcia E, van Dam RM, Rajpathak S, Willett WC, Manson JE, Hu FB. Changes in caffeine intake and long-term weight change in men and women. Am J Clin Nutr. 2006;83:674–680. doi: 10.1093/ajcn.83.3.674. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 12.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 15.Boschmann M, Steiniger J, Hille U, Tank J, Adams F, Sharma AM, et al. Water-induced thermogenesis. J Clin Endocrinol Metab. 2003;88:6015–6019. doi: 10.1210/jc.2003-030780. [DOI] [PubMed] [Google Scholar]
- 16.Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, Jordan J. Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab. 2007;92:3334–3337. doi: 10.1210/jc.2006-1438. [DOI] [PubMed] [Google Scholar]
- 17.Brown CM, Dulloo AG, Montani JP. Water-induced thermogenesis reconsidered: the effects of osmolality and water temperature on energy expenditure after drinking. J Clin Endocrinol Metab. 2006;91:3598–3602. doi: 10.1210/jc.2006-0407. [DOI] [PubMed] [Google Scholar]
- 18.Davy BM, Dennis EA, Dengo AL, Wilson KL, Davy KP. Water consumption reduces energy intake at a breakfast meal in obese older adults. J Am Diet Assoc. 2008;108:1236–1239. doi: 10.1016/j.jada.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Walleghen EL, Orr JS, Gentile CL, Davy BM. Pre-meal water consumption reduces meal energy intake in older but not younger subjects. Obesity. 2007;15:93–99. doi: 10.1038/oby.2007.506. [DOI] [PubMed] [Google Scholar]
- 20.Rolls BJ, Kim S, Fedoroff IC. Effects of drinks sweetened with sucrose or aspartame on hunger, thirst and food intake in men. Physiol Behav. 1990;48:19–26. doi: 10.1016/0031-9384(90)90254-2. [DOI] [PubMed] [Google Scholar]
- 21.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005;44:187–193. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J. Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr. 1990;51:759–767. doi: 10.1093/ajcn/51.5.759. [DOI] [PubMed] [Google Scholar]
- 23.Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, Fay LB, et al. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J Clin Nutr. 2004;79:40–46. doi: 10.1093/ajcn/79.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes. 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 25.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. Am J Clin Nutr. 2010;91:73–81. doi: 10.3945/ajcn.2009.28157. [DOI] [PubMed] [Google Scholar]
- 26.Pepino MY, Bourne C. Non-nutritive sweeteners, energy balance, and glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2011;14:391–395. doi: 10.1097/MCO.0b013e3283468e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattes RD, Shikany JM, Kaiser KA, Allison DB. Nutritively sweetened beverage consumption and body weight: a systematic review and meta-analysis of randomized experiments. Obes Rev. 2011;12:346–365. doi: 10.1111/j.1467-789X.2010.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajpathak SN, Rimm EB, Rosner B, Willett WC, Hu FB. Calcium and dairy intakes in relation to long-term weight gain in US men. Am J Clin Nutr. 2006;83:559–566. doi: 10.1093/ajcn.83.3.559. [DOI] [PubMed] [Google Scholar]
- 29.Louie JC, Flood VM, Hector DJ, Rangan AM, Gill TP. Dairy consumption and overweight and obesity: a systematic review of prospective cohort studies. Obes Rev. 2011;12:e582–592. doi: 10.1111/j.1467-789X.2011.00881.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Pan A, Malik V, Hu FB. Dairy intake and body weight: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–747. doi: 10.3945/ajcn.112.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Libuda L, Alexy U, Sichert-Hellert W, Stehle P, Karaolis-Danckert N, Buyken AE, et al. Pattern of beverage consumption and long-term association with body-weight status in German adolescents--results from the DONALD study. Br J Nutr. 2008;99:1370–1379. doi: 10.1017/S0007114507862362. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Appel LJ, Loria C, Lin PH, Champagne CM, Elmer PJ, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr. 2009;89:1299–1306. doi: 10.3945/ajcn.2008.27240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990;51:963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 34.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000;24:794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 35.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 36.Stookey JD, Constant F, Gardner CD, Popkin BM. Replacing sweetened caloric beverages with drinking water is associated with lower energy intake. Obesity. 2007;15:3013–3022. doi: 10.1038/oby.2007.359. [DOI] [PubMed] [Google Scholar]
- 37.Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, et al. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2012;95:555–563. doi: 10.3945/ajcn.111.026278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95:1454–1460. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


