Abstract
Excessive alcohol intake, a defining characteristic of an alcohol use disorder (AUD), results in neurodegeneration in the hippocampus and entorhinal cortex that has been linked to a variety of cognitive deficits. Neuroinflammation is thought to be a factor in alcohol-induced neurodegeneration, and microglia activation is a key but not sole component of an inflammatory response. These experiments investigate the effects of ethanol exposure in a well-accepted model of an AUD on both microglial activation and blood brain barrier disruption (BBB) in order to understand their relationship to classical definitions of inflammation and alcohol-induced neurodegeneration. Following a four-day binge ethanol paradigm, rat hippocampal and entorhinal cortex tissue was examined using three distinct approaches to determine microglia phenotype and BBB disruption: immunohistochemistry, autoradiography, and ELISA. After ethanol exposure, there was an increase in [3H]-PK-11195 binding and OX-42 immunoreactivity indicative of microglial activation; however, microglia were not fully activated since both OX-6 and ED-1 immunoreactive microglia were absent. This data was supported by functional evidence as there was no increase in the proinflammatory cytokines IL-6 or TNF-α but a 26% increase in the anti-inflammatory cytokine, IL-10, and a 38% increase in the growth factor, TGF-β, seven days after exposure. Furthermore, there was no evidence of a disruption of the BBB. These data suggest that the four-day binge model of an AUD, which produces neurodegeneration in corticolimbic regions, does not elicit classical neuroinflammation but instead produces partially activated microglia. Partial activation of microglia following binge ethanol exposure suggest that microglia in this model have beneficial or homeostatic roles rather than directly contributing to neurodegeneration and are a consequence of alcohol-induced-damage instead of the source of damage.
Keywords: alcoholism, alternative activation, blood brain barrier, cytokine, entorhinal cortex, ethanol, hippocampus, inflammation, microgliosis
INTRODUCTION
Whether microglial activation is the cause or consequence of neurodegeneration is hotly debated in studies of neurodegenerative disease. Although not traditionally classified as a neurodegenerative disease due to its preventable nature, alcohol use disorders (AUDs) and specifically the characteristic excessive consumption of alcohol, result in corticolimbic neurodegeneration that underlies a variety of cognitive deficits in alcoholics (Crews and Nixon, 2009; Obernier et al., 2002a; Pfefferbaum et al., 1992; Sullivan et al., 1995). As alcohol-induced neurodegeneration is thought to be a critical step in the development of an AUD (Crews, 1999; Crews and Boettiger, 2009; Koob and Le Moal, 1997), understanding how excessive alcohol consumption results in neuronal loss is crucial for the development of prevention and treatment strategies. It has been hypothesized that alcohol-induced neuroinflammation directly contributes to neurodegeneration and the development of AUDs (Crews et al., 2011). Neuroinflammation has been inferred from the upregulation of a variety of proinflammatory genes and cytokines involved in the innate immune system (Crews et al., 2006; He and Crews, 2008; Knapp and Crews, 1999; Qin et al., 2008). For example, chronic ethanol exposure induces innate immune signaling cascades through activation of the proinflammatory transcription factor nuclear factor-kappa B (NFκB; Crews et al., 2006; Crews et al., 2011; Valles et al., 2004). Others have shown that a variety of proinflammatory signals are associated with increased ethanol drinking and preference (Blednov et al., 2012) and that peripheral inflammation promotes increases in voluntary ethanol intake whereas anti-inflammatory administration reduces its consumption (Agrawal et al. 2011; Blednov et al. 2011). However, remarkably little is known about the effects of alcohol on microglia, the primary mediators of the innate immune system in the brain.
Microglial activation, the process in which microglia alter their morphology and functionally differentiate in response to changes in their environment, was traditionally described as proinflammatory and cytotoxic (Kreutzberg, 1996). In normal, non-pathologic conditions microglia are generally in a quiescent state often referred to as “resting.” Quiescent microglia, however, are not truly resting; their highly ramified morphology reflects their constant surveying of the surrounding environment (Fishman and Savitt, 1989; Nimmerjahn et al., 2005). For many neurodegenerative disorders, activated microglia are a hallmark of neuroinflammation (Banati et al., 1993; Block and Hong, 2005; Colton and Gilbert, 1987; Woodroofe et al., 1991). Howeverhowever, more recent work demonstrates that it is not just whether microglia are activated, but more importantly their phenotype during activation (Carson et al., 2007; Colton and Wilcock, 2010; Kreutzberg, 1996; Raivich et al., 1999a). Various terms have been used to describe a perceived dichotomy in microglial phenotype including M1 versus M2, classical versus alternative and classical versus partial activation. However, all classify microglia into one of two categories when it is a spectrum of phenotypes or behaviors that exist. For example, microglia phenotype varies with the type of insult, the extent of damage, and the time of recovery post injury, which makes it necessary to thoroughly examine phenotypic hallmarks within a disease before inferring their role in neuroinflammation (Harting et al., 2008; Lai and Todd, 2008; Saijo and Glass, 2012). Application of the idea of graded levels of activation allows for investigation of a potential spectrum of phenotypes. As such, Raivich defines 5 levels of microglial activation or phenotypes (Table 1): resting (stage 0), alert (stage 1), homing (stage 2), phagocytic (stage 3a) and bystander activation (stage 3b), which can be differentiated by both morphology and cytokine and/or growth factor upregulation (Raivich et al., 1999a). For example, amoeboid morphology and expression of proinflammatory factors such as TNF-α, IL-1β, prostaglandins, superoxides and nitric oxide, characterize the highest level of activation whereas microglia in lower grades of activation release neuroprotective factors such as IL-10, TGF-β, and neurotrophins and have a more ramified morphology (Block and Hong, 2005; Raivich et al., 1999b). Furthermore, although fully activated microglia are one component of classical inflammation, observation of “activated” microglia alone is not equivalent to nor very informative about the inflammation state (Graeber et al., 2011). Therefore, determining the phenotype of microglia in injury is necessary to understand their role as cytotoxic or neuroprotective and whether they are truly neuroinflammatory (Colton and Wilcock, 2010; Kreutzberg, 1996; Raivich et al., 1999a; Vilhardt, 2005).
Table 1.
Microglial activation can be differentiated based on morphology, marker expression, and cytokine secretions (derived from Raivich et al., 1999a). Immune response can occur independent of activation and may be observed in Stages 1 – 3 as evidenced by increased MHCII (OX-6).
| Ways to differentiate stages: | |||
|---|---|---|---|
| Grade | Characteristics | Morphology and Markers | Cytokines |
| Stage 0 | Normal-Ramified | Morphology: long ramified processes | |
| Stage 1 | Alert: thicker processes | Less ramified, thicker processes; ⇑OX-42 | TGF-β1 |
| Stage 2 | Homing, Proliferation | Bushy; Proliferation markers | IL-10 |
| Stage 3a | Clustered phagocytes | Amoeboid; possible ⇑MHCI, ED-1 (CD68) | IL-6, TNF-α |
| Stage 3b | Bystander activation; Lymphocyte binding | ⇑MHCI, Lower ICAM than 3a | IFN-γ |
A role for cytotoxic microglia in alcohol-induced brain damage has been suggested since the 1990s, however direct evidence of alcohol-induced full or classical microglia activation has yet to be described. The lack of classical signs of activation led some to suggest that the damage in alcoholism is “too chronic” (Streit, 1994) or too low level to affect microglia (Kalehua et al., 1992); however, there is evidence of some level of activation in both animal models and human postmortem alcoholic brain. For example, early work showed an upregulation in the microglial marker, [3H]-PK-11195, binding months after alcohol exposure in a four-day binge model of alcoholic neurodegeneration (Obernier et al., 2002b). Later, an unexpected discovery of microglial proliferation was found in this same model (Nixon et al., 2008; Obernier et al., 2002b). More recently, upregulation of various microglial markers have been described in animal models (McClain et al., 2011), and even led some to conclude that excessive alcohol exposure produces “neuroinflammation” (Qin et al., 2008; Ward et al., 2009). Importantly, although evidence of microglial activation has been observed in human alcoholic brain samples, the phenotype of these alcohol-activated microglia has yet to be described (Crews et al., 2006; Crews et al., 2011; He and Crews, 2008). Unfortunately, the pervasive theme of these and other papers is that the observation of any marker of activation is equivalent to neuroinflammation. The assessment of single markers of activation is not sufficient to characterize the activation phenotype of microglia and as discussed above, not indicative of inflammation (Colton and Wilcock, 2010).
The current experiments examine how ethanol exposure, in a well-established model of an AUD that includes significant alcohol-induced neurodegeneration, affects microglia within the context of classical definitions of inflammation. Specifically, inflammation is defined as a “multicellular process characterized by changes in the vasculature and infiltration of mobile cells.” (p. 3800; Graeber et al., 2011). This study uses an extensive assessment of immunohistochemical, morphological, and functional indices of microglial activation in order to determine their phenotype in the hippocampus and entorhinal cortex, regions consistently damaged in this binge paradigm (Collins et al., 1996; Obernier et al., 2002a). Alcohol’s effects on blood brain barrier (BBB) integrity were also examined, as macrophage and/or lymphocyte infiltration is a defining phenomenon in inflammation (Hickey, 2001).
MATERIALS AND METHODS
Alcohol Administration Model
Rats were subjected to a four-day binge model of alcohol exposure modified from Majchrowicz (1975) that was chosen for its well-documented neurodegeneration profile (Crews, 1999; Kelso et al., 2011). This model is designed to mimic the high blood alcohol levels of pattern binge drinkers (Hunt, 1993; Tomsovic, 1974). All procedures performed were in accordance with the University of Kentucky Institutional Animal Care and Use Committee and aligned with the Guidelines for the Care and Use of Laboratory Animals (NRC, 1996). A total of 214 adult male Sprague-Dawley rats (Table 2; Charles River Laboratories, Raleigh, NC) were used across all experiments. Animals were 275–300g upon arrival and single-housed in a University of Kentucky AALAC accredited vivarium with a 12h light:dark cycle and ad libitum food and water access unless otherwise noted. Rats were allowed to acclimate to the vivarium for two days and were subsequently handled for three days before the binge began.
Table 2.
| Autoradiography | |||
|---|---|---|---|
| Group | Intoxication behavior (0–5 scale) |
Dose (g/kg/day) |
BEC (mg/dl) |
| T0 (n=6) | 1.8 ± 0.3 | 9.7 ± 1.3 | 318.0 ± 14.5 |
| T2 (n=6) | 1.8 ± 0.3 | 9.8 ± 1.4 | 304.8 ± 18.4 |
| T4 (n=6) | 1.8 ± 0.3 | 9.4 ± 1.6 | 336.2 ±19.7 |
| T7 (n=6) | 1.8 ± 0.3 | 9.7 ± 1.4 | 345.1 ± 25.5 |
| Immunohistochemistry | |||
|---|---|---|---|
| Group | Intoxication behavior (0–5 scale) |
Dose (g/kg/day) |
BEC (mg/dl) |
| T0 (n=7/8) | 2.0 ± 0.3 | 9.1 ±1.2 | 361.5 ± 17.2 |
| T2 (n=6) | 1.9 ± 0.3 | 8.8 ± 1.5 | 286.7 ± 25.1 |
| T4 (n=6) | 1.7 ± 0.3 | 9.3 ± 1.5 | |
| T7 (n=7) | 1.7 ± 0.2 | 9.8 ± 1.3 | 365.8 ± 36.4 |
| T28 (n=7/8) | 2.0 ± 0.3 | 9.1 ± 1.6 | 332.9 ±26.0 |
| ELISA | |||
|---|---|---|---|
| Group | Intoxication behavior (0–5 scale) |
Dose (g/kg/day) |
BEC (mg/dl) |
| T0 (n=8) | 1.9 ± 0.3 | 9.3 ± 0.9 | 331.3 ±23.3 |
| T1 (n=8) | 2.1 ± 0.3 | 8.6 ± 1.3 | 401.5 ± 20.3 |
| T2 (n=7) | 2.1 ± 0.3 | 8.8 ± 1.3 | 411.3 ± 14.5 |
| T4 (n=7) | 2.2 ± 0.3 | 8.3 ± 1.6 | 400.5 ± 33.8 |
| T7 (n=7) | 2.3 ± 0.3 | 8.3 ± 1.7 | 365.8 ± 36.4 |
Rats were divided into two groups of comparable weights and received either ethanol (25% w/v) or control diet (isocaloric amounts of dextrose) in Vanilla Ensure Plus®. Diet was given every 8h for 4 days via intragastric gavage. Initially, each rat received a 5g/kg dose of ethanol with subsequent doses titrated based on intoxication behavior according to a 6-point scale modified from Majchrowicz (1975) but identical to previously published methods (Morris et al., 2010b; Nixon and Crews, 2004). During the four days of diet administration, chow was removed and returned 8h after the last dose. Ninety minutes after the sixth session of ethanol dosing, tail blood samples were collected, centrifuged for 5 min at 1800g, and stored at −20°C. Blood ethanol concentrations (BECs) were determined from serum using an AM1 Alcohol Analyser (Analox, London, UK) calibrated against a 300mg/dl external standard. Ten hours following the last dose of ethanol, withdrawal was observed for 30 minutes every hour for 16 intervals. Behaviors were scored based on a scale modified from Majchrowicz (Majchrowicz, 1975; Penland et al., 2001) but identical to that reported previously (Morris et al., 2010b). Because microglia respond quickly to changes in homeostasis (Davalos et al., 2005; Nimmerjahn et al., 2005) but also have the capacity for persisting memory (Bilbo and Schwarz, 2009; Bland et al., 2010; Williamson et al., 2011) this study examines microglial changes immediately following ethanol exposure through 28 days of abstinence. Therefore, rats were euthanized at various times with different methods following binge treatment: T0 (e.g. 0 days after the last dose, specifically within hours), T1, T2, T4, T7, and T28 (Table 2).
Autoradiography
Autoradiography was conducted as described in previous reports (Kelso et al., 2006; Sparks and Pauly, 1999). Briefly, rats were rapidly decapitated and extracted brains were immediately frozen in isopentane and sliced at 30µm with a cryostat. Two controls were euthanized at each time point and pooled into a single control group for comparison with ethanol treated groups (Readnower et al., 2010). Sections were mounted in a 1 in 8 series on glass slides so that every eighth section was used and stored at −80°C until processing. Slides were thawed and incubated in 50mM Tris HCl (pH=7.4) buffer with 1nM [3H]-PK-11195 (PerkinElmer, Boston, MA) for 2h followed by a series of washes in 50mM Tris HCl. [3H]-PK11195 specifically binds to the mitochondrial translocator protein 18kDa (TSPO), a protein that is highly upregulated in activated microglia (Kelso et al., 2009; Stephenson et al., 1995; Veiga et al., 2007). Similar to other studies of microglial activation after brain insult, autoradiographic localization of TSPO was used in this study because of its high sensitivity to detect activated microglia (Benavides et al., 2001; Readnower et al., 2010). After drying, the slides were exposed to BioMax film (Kodak, Rochester, NY) for 6 weeks. The film was developed with GBX developer (Kodak) and analyzed using Scion Imaging (Frederick, Maryland). Sections between approximately between Bregma −2.50mm and −4.00mm, which included both the hippocampus and entorhinal cortex, were quantified (Paxinos and Watson, 2009).
Immunohistochemistry
Rats were overdosed with anesthetic (Nembutal® 100mg/kg; i.p.) and transcardially perfused with 0.1M phosphate buffered saline (PBS, pH=7.4) followed by 4% paraformaldehyde in PBS. Brains were extracted, postfixed in paraformaldehyde for 24 hours (ED-1, OX-6, Iba-1, and IgG) or 1 hour (OX-42), and sectioned coronally at 40µm using a vibrating microtome (Leica VT1000S; Wetzlar, Germany). Sections were collected in a 1:12 series and stored in cryoprotectant at −20°C until processing so that every twelfth section was stained for all antibodies. Free floating tissue was washed in tris buffered saline (TBS, pH=7.5) and endogenous peroxidases quenched with 0.6% H2O2 in TBS. Following additional washes, sections were blocked for nonspecific binding (TBS, 0.1% triton X-100, and 3% horse or goat serum), and then incubated overnight in primary antibody at 4°C as follows: mouse anti-OX-6 (1:500, Serotec, Raleigh, NC), mouse anti-ED-1 (1:500; Serotec), rabbit anti-Iba-1 (1:1000, Wako, Richmond, VA), or mouse anti-OX-42 (1:1000; Serotec)
Primaries were chosen for their specificity for microglia (Table 1). The Iba-1 antibody recognizes a 17kDa EF hand protein that is similar in structure to other calcium binding proteins such as calmodulin (Heizmann and Hunziker, 1991; Imai et al., 1996; Ito et al., 1998). Iba-1 is used to mark all microglia, but it is upregulated during activation as it is associated with the release of cytokines, adhesion, and proliferation (Donato, 1999; Donato, 2003; Hwang et al., 2006). The OX-42 antibody is also constitutively expressed in all macrophages and recognizes complement receptor 3 (CR3 or CD11b; Robinson et al., 1986). Upregulation of this receptor is one of the first indices of activation as microglia prepare to adhere to damaged cells (Hynes, 1992; Morioka et al., 1992). Unlike Iba-1 and OX-42, ED-1 and OX-6 are not expressed in all microglia. The ED-1 antibody, also known as anti-CD68, recognizes a glycoprotein on the lysosomal membrane of macrophages and microglia that is indicative of phagocytic activity (Bauer et al., 1994; Damoiseaux et al., 1994). ED-1 is typically used to determine the presence of classically or fully activated phagocytic microglia (Graeber and Streit, 2009; O'Keefe et al., 2002; Raivich et al., 1999a). The OX-6 antibody recognizes the major histocompatibility complex class II molecules (MHC-II) associated with induction of T-helper cells (O'Keefe et al., 2002; Raivich et al., 1999a). Although OX-6 is also associated with the recruitment of phagocytes and is considered a hallmark of an immune response (Kaur and Ling, 1992; McGeer et al., 1993), recent work suggests that it may also be expressed in alternatively activated microglia (Colton and Wilcock, 2010). Microglia exhibit weak antigen presenting capabilities, but many neuroinflammatory reactions involve the upregulation of microglial MHC-II (Zhang et al., 2011)
Methods for the application of secondary antibody (biotinylated horse anti-mouse, rat adsorbed, or biotinylated goat anti-rabbit, Vector Laboratories, Burlingame, CA), avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Laboratories) and chromagen, nickel-enhanced 3,3’-diaminobenzidine tetrahydrochloride (DAB; Polysciences, Warrington, PA), were identical for all primary antibodies and followed previously published methods (McClain et al., 2011).
To determine if infiltration of macrophages and lymphocytes could occur in this model, BBB impairment was examined. Tissue was incubated in biotinylated rabbit anti-rat IgG for 2 hours followed by detection with ABC and the chromagen DAB (Rabchevsky et al., 1999). The IgG antibody is a marker of immunoglobulin G. With an intact BBB, immunoglobulins would remain in the peripheral system due to a lack of transport mechanisms (Triguero et al., 1989); thus, the presence of IgG in the brain parenchyma indicates BBB disruption. Following the final wash, all stained sections were mounted onto glass slides and dried before being coverslipped with Cytoseal® (Stephens Scientific, Wayne, NJ).
Quantification
All sections were coded to ensure the experimenter was blinded to treatment conditions during quantification. All analyses were conducted on an Olympus BX-51 microscope (Olympus, Center Valley, PA), with motorized stage (Prior, Rockland, MA), microcator and DP70 digital camera (Olympus). OX-42 immunoreactivity was analyzed using Visiomorph image analysis program (Visiomorph, Hørsholm, Denmark). Using a 10× objective lens, regions of interest were drawn around the hippocampal subregions and the entorhinal cortex approximately between Bregma −2.50mm and −4.00mm as determined by Paxinos (Paxinos and Watson, 2009), and percent area of staining was obtained. Immunoreactivity was calculated and expressed as percent control. Sections in the same stereotaxic regions were assessed qualitatively for ED-1, OX-6 and IgG using a 10× objective.
Iba-1+ cells were quantified in the entorhinal cortex by an image analysis system. Multi-panel images containing the entire entorhinal cortex were collected using Visiopharm image capturing software approximately between Bregma −2.30mm and −4.50mm (Paxinos and Watson, 2009). For each image, the number of Iba-1+ cells was determined by Image Pro Plus software. The number of cells was averaged and expressed as Iba-1+ cells/section.
Hippocampal Iba-1+ cells were estimated by unbiased stereological methods, the optical fractionator, using the newCAST Stereology System (Visiopharm, Hoersholm, Denmark) installed on a Dell Precision 380 workstation coupled to the microscope. Following parameters similar to Long and colleagues (1998), the dentate gyrus (DG), CA2/3, and CA1 regions of the dorsal hippocampus approximately between Bregma −2.30mm and −4.50mm as determined by Paxinos (Paxinos and Watson, 2009) were separately traced at 100× magnification. Section thickness was assessed at 600× using a 60× oil immersion lens and was averaged from three measurements taken at different locations within each region. The DG and CA2/3 were randomly sampled using a 70µm × 70µm counting frame with a 250µm x,y step length. The CA1 was randomly sampled using the same size counting frame and a 400µm x,y step length. After tissue processing, section thickness was approximately 24 µm, therefore, a dissector height of 20um with 2µm guard zones. Total Iba-1+ microglia in each region of interest was calculated using the following equation (West et al., 1991):
where Q is the number of cells counted, asf is the area sampling fraction (the counting frame: x,y step length ratio), tsf is the thickness sampling fraction (dissector height: section thickness ratio), and ssf is the section sampling fraction (the fraction of sections examined). For all stereological quantifications, coefficient of error ranged from 0.008 to 0.039 and averaged 0.021 ± 0.001 (Gundersen et al., 1999).
Enzyme Linked Immunosorbent Assay (ELISA)
Rats were rapidly decapitated and the brain immediately extracted. The hippocampus and entorhinal cortex were dissected on ice, snap frozen on dry ice, and stored at −80°C until assayed. Thawed tissue was manually homogenized in an ice-cold lysis buffer (1mL of buffer/50mg of tissue; pH=7.4). All reagents used in the lysis buffer were purchased from Sigma (St. Louis, MO) unless otherwise noted. It consisted of 25mM HEPES, 0.1% 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propanesulfonate, 1.3mM EDTA, 1mM EGTA, 10 µg/ml aprotinin, 10µg/ml leupeptin, 5mM MgCl2 (Fisher, Fairlawn, New Jersey), 10 µg/ml pepstatin (Fluka, Milwaukee, WI), and 1mM PMSF (Fluka; Rabuffetti et al., 2000). Homogenates were centrifuged at 20,000 × g for 15 minutes at 4°C and the supernatant stored at −80°C. Total protein content was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Cytokine protein content was determined with an ELISA kit according to the manufacturer’s instructions for rat tumor necrosis factor alpha (TNF-α; Invitrogen product #KRC3011C, Camarillo, CA), interleukin-10 (IL-10; Invitrogen product #KRC0101), interleukin-6 (IL-6; R&D Systems product #R6000B, Minneapolis, MN), or transforming growth factor beta (TGF-β; Invitrogen product #KAC1688). All samples, standards, and positive controls were run in duplicate so that all tissue for one time point fit on one plate to reduce potential variability. The cytokine protein concentration was divided by the total protein concentration obtained in the BCA assay to correct for differences in tissue volume. Protein concentration is reported as pg of cytokine/ µg of protein.
Statistical Analyses
Data were analyzed and graphed using Prism (GraphPad Software, Inc. La Jolla, Ca). All data are reported as the mean ± standard error of the mean and analyses considered significantly different if p<0.05. Behavioral scores were analyzed with a Kruskal Wallis test and BECs, autoradiography, OX-42, cytokine expression, and cell counts were analyzed by ANOVA with posthoc tests as appropriate. Each region is considered independent and therefore was analyzed separately.
RESULTS
Animal model data
Intoxication parameters across all experiments were similar as shown in Table 2. The overall mean intoxication score for all ethanol animals was 1.9 ± 0.1 on the 6-point Majchrowicz scale, which indicates that all animals were, on average, “ataxic” immediately before dosing. This level of intoxication resulted in an overall mean dose of 9.2 ± 0.3 g/kg/day of ethanol and a BEC of 354.0 ± 7.5 mg/dL for all animals used. These parameters are similar to those reported in past studies with this model (Morris et al., 2010a; Nixon and Crews, 2004) and similar to that observed in voluntary consumption (Bell et al., 2009). Neither the Kruskal – Wallis (intoxication behavior) nor one-way ANOVAs (dose, BEC) showed differences in any intoxication parameter between ethanol groups at different time points.
[3H]-PK-11195 autoradiography reveals early activation of microglia
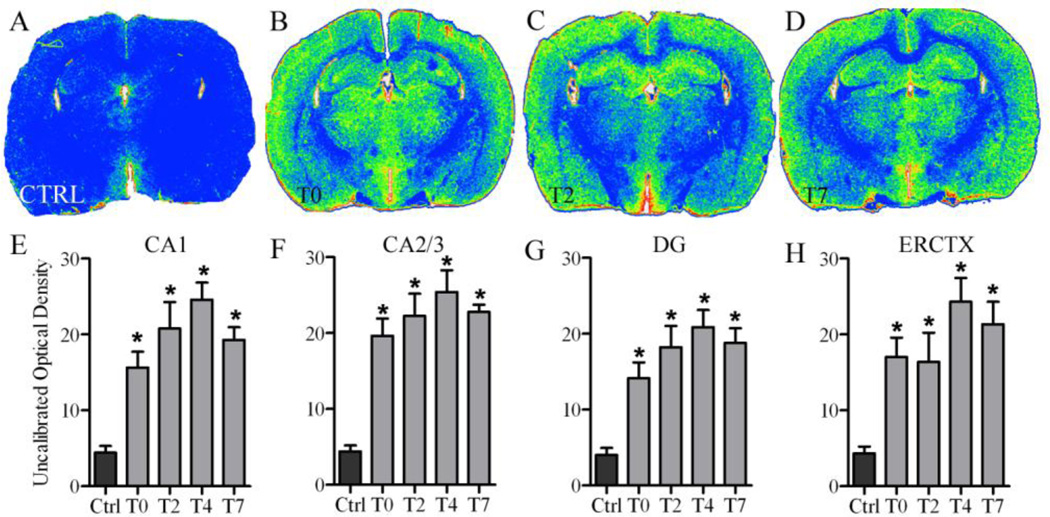
Binding of the TSPO ligand, [3H]-PK-11195, was measured by optical density at T0, T2, T4, and T7. Control levels of binding at each time point were not statistically different and therefore were pooled into a single control group (Readnower et al., 2010). As shown in representative images, ethanol treated animals have increased binding throughout the brain compared with controls (Figure 1). Specifically, one way ANOVAs showed a significant main effect of diet in each region of the hippocampus: CA1 [F(4,27) =14.93, p<0.0001], CA2/3 [F(4,27) =14.93, p<0.0001], and DG [F(4,27) =12.88, p<0.0001], as well as in entorhinal cortex [F(4,27) =9.08, p<0.0001]. Post-hoc Dunnett’s tests confirmed a significant increase (p<0.05) in the density of [3H]-PK-11195 binding in each ethanol treated time point compared to controls in all regions examined (Figure 1).
Figure 1. [3H]-PK-11195 upregulation following 4-day binge exposure.
Representative false color autoradiographs depicting [3H]-PK-11195 binding are shown for (A) controls (n=8; black bars) as well as (B) ethanol (grey bars) at T0 (n=6), (C) T2 (n=6), and (D) T7 (n=6). Quantitative analysis of the extent of binding are graphed for the (E) CA1, (F) CA2/3, (G) DG, and (H) entorhinal cortex. *p<0.05
Immunohistochemical markers of microglia indicate partial activation phenotype
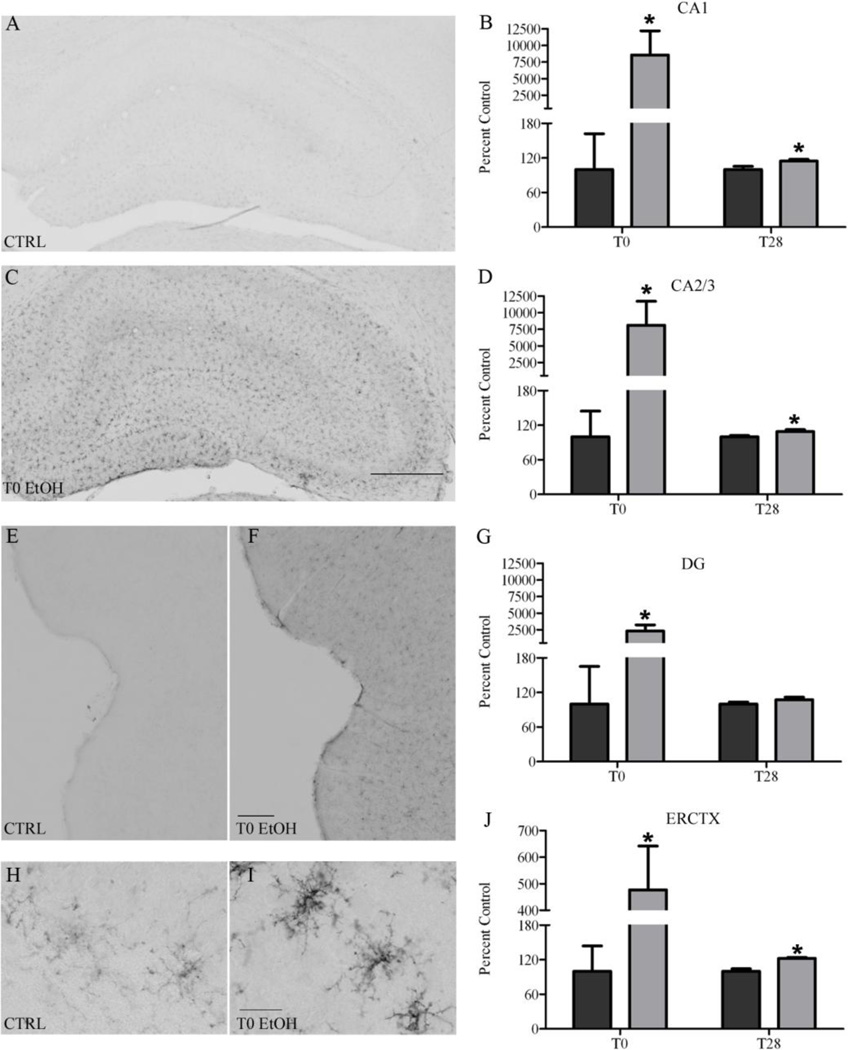
In order to see the earliest signs of activation, we examined OX-42 expression immediately after the last dose of alcohol (T0; rats are still intoxicated) and in a separate group after four weeks of abstinence (T28). OX-42 positive cells were apparent in both ethanol and control tissue which is consistent with its constitutive expression (Akiyama and McGeer, 1990). However, there was a visibly distinct increase in immunoreactivity at T0, reflecting a reduction in the ramification but a thickening of the processes in the ethanol animals compared with the controls (Figure 2). Two-way ANOVAs indicated a significant interaction between treatment and time point in the CA1 [F(1,25) =5.81, p=0.0236], CA2/3 [F(1,26) =5.71, p=0.0244] DG [F(1,25) =5.90, p=0.0227] fields, as well as in entorhinal cortex [F(1,25) =4.65, p=0.0409]. Planned posthoc t-tests indicated a significant increase after ethanol exposure in all regions at T0: CA1 [t(12) =2.39, p=0.0345], CA2/3 [t(12) =2.23, p=0.0453], DG [t(12) =2.35, p=0.0367] and entorhinal cortex [t(12) =2.21, p=0.0472]. Although the contrast between ethanol and controls was not as distinct at T28, ethanol animals maintained a significant increase compared with controls in all regions except the DG: CA1 [t(13) =2.45, p=0.0288], CA2/3 [t(13) =2.25, p=0.0427], and entorhinal cortex [t(13) =4.80, p<0.0003].
Figure 2. CD11b (OX-42) upregulation following 4-day binge exposure.
CD11b is upregulated in both the hippocampus and entorhinal cortex at T0 as shown in representative photomicrographs for (A, E) controls (T0: n=7; T28: n=7; black bars) and rats exposed to binge (C, F) ethanol (T0: n=8; T28: n=8; grey bars). Higher magnification of microglia seen in the hippocampus are shown for both (H) control and (I) ethanol. Quantifications of OX-42 immunoreactivity for the subregions of the hippocampus were significantly different and are shown: (B) CA1, (D), CA2/3, and (G) DG as well as the (J) entorhinal cortex. Scale bar in B=500µm; H=300µm; J=10µm. *p<0.05
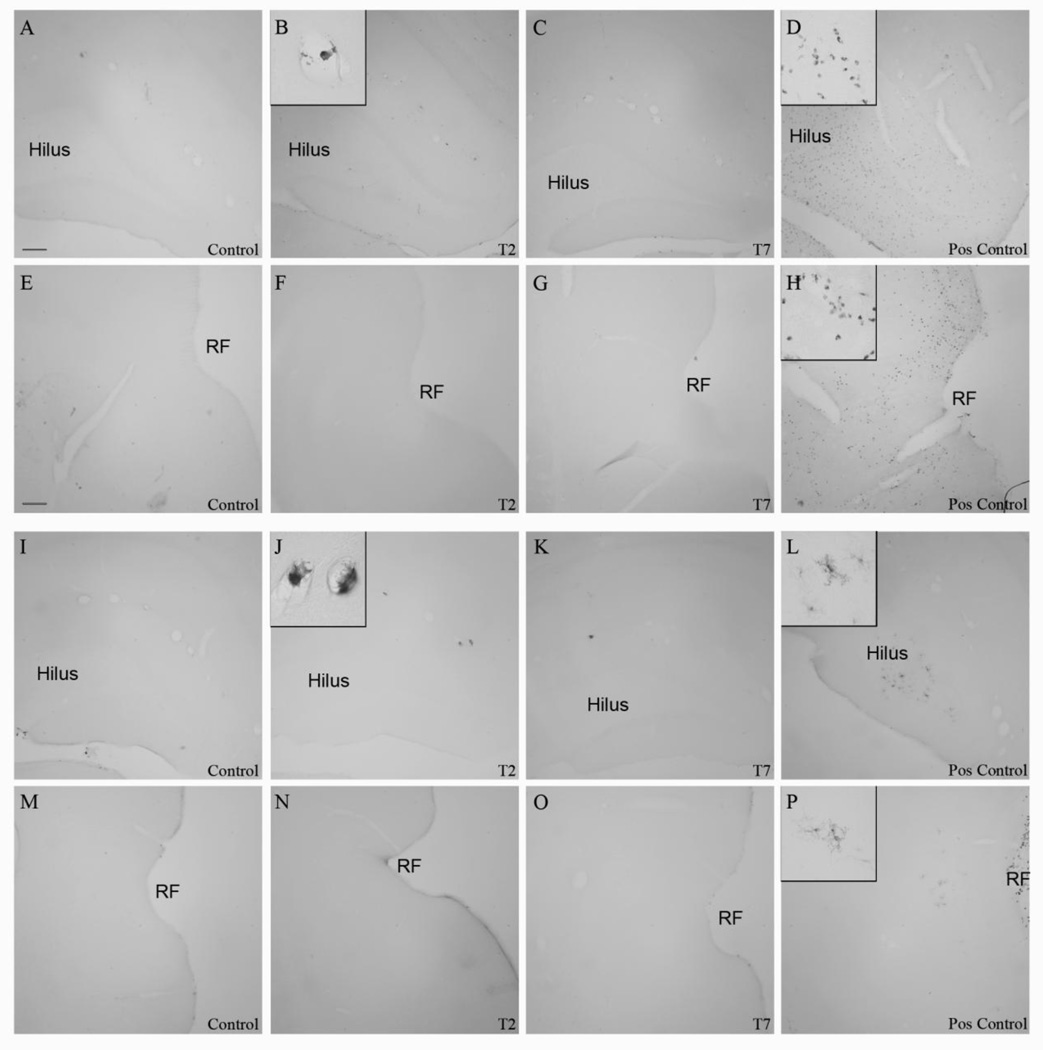
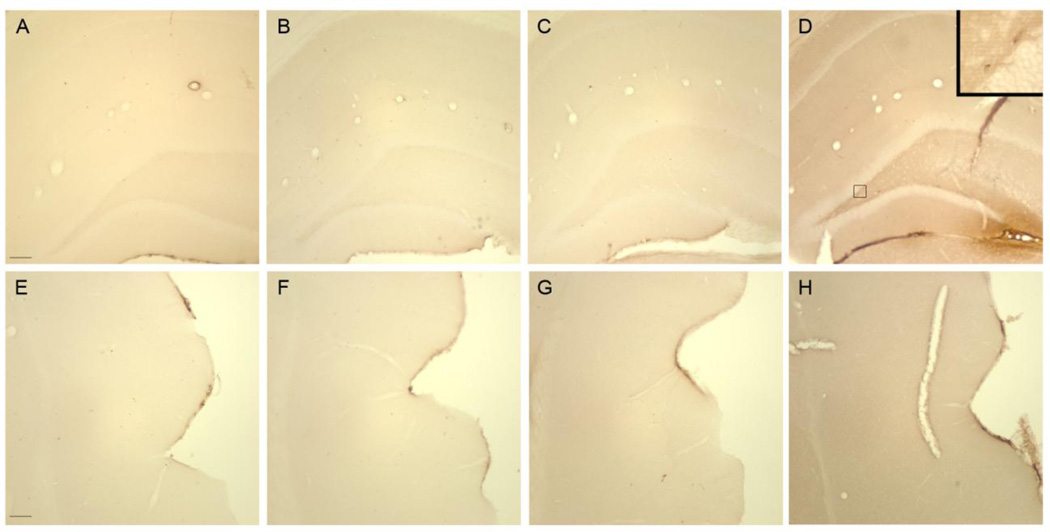
The ED-1 antibody was used to recognize phagocytic microglia (Graeber and Streit, 2009), whereas the OX-6 antibody was used to visualize the upregulation of MHC II. Neither ethanol nor control animals had ED-1 nor OX-6 positive cells within the parenchyma of the hippocampus or entorhinal cortex at T0, T2, T4, T7, or T28 (Figure 3). However, ED-1 and OX-6 positive cells were visible in blood vessels and along the meninges in both control and ethanol treated animals (Figure 3), similar to that previously reported in this model (McClain et al., 2011; Nixon et al., 2008). Thus, four-day ethanol treatment failed to induce phagocytic-stage microglia or increased MHC II in the brain parenchyma at any time point.
Figure 3. Lack of fully activated microglia following 4-day binge exposure.
ED-1 is not visible in the (A–D) hippocampus or (E–H) entorhinal cortex as seen in representative photomicrographs for (A, E) controls (T2: n=7; T7: n=8) or (B, F) ethanol (T2: n=6 ;T7: n=7) rats at (B, F) T2 or (C, G) T7. No OX-6 positive cells were visualized in the (I–L) hippocampus or (M–P) entorhinal cortex as seen in representative photomicrographs for (I, M) controls or ethanol rats at (J, N) T2 or (K, O) T7. Phagocytic and immune responsive macrophages were visible in the blood vessels as seen in insets (C) of ED-1 and (J) OX-6, respectively. ED-1 and immunopositive cells were visible in the (D, H, L, P) positive control tissue from a rat treated with kainic acid. RF=rhinal fissure. Scale bar= 150µm
Microglia proliferation increases the number of microglia
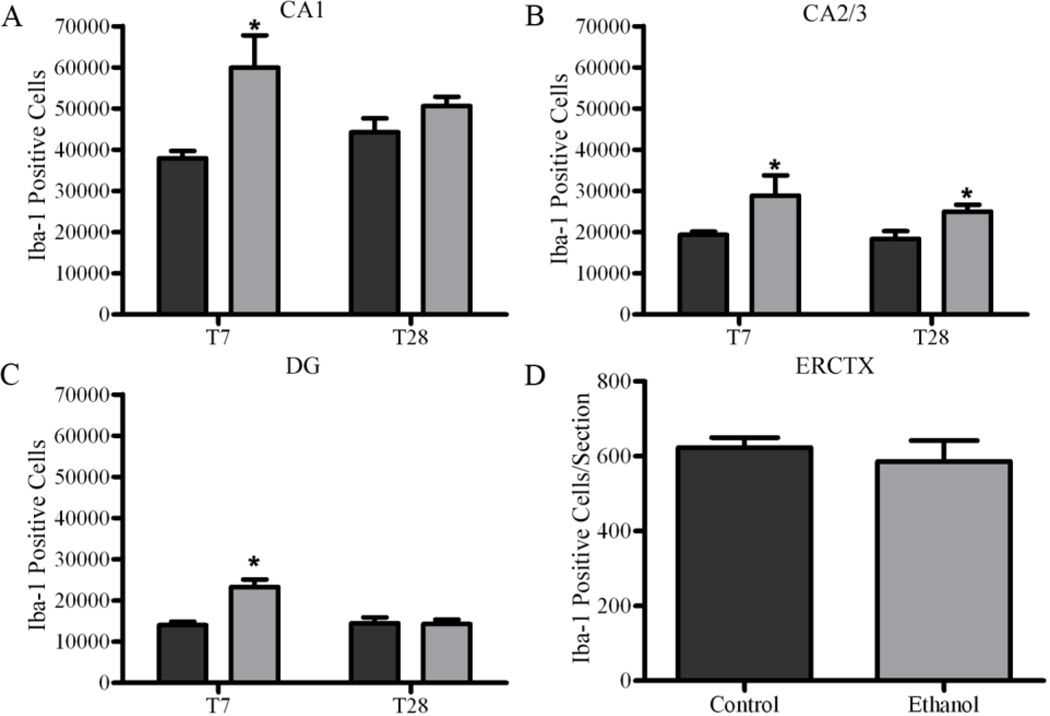
We have previously shown that microglia proliferate two days after a four-day alcohol binge (McClain et al., 2011; Nixon et al., 2008); therefore stereological estimates of Iba-1-positive microglia were conducted at seven (T7) and twenty eight (T28) days following the last ethanol dose in the hippocampus. The total number of microglia was increased in the hippocampus of ethanol treated animals compared with controls seven days after ethanol exposure (T7; Figure 4). Two-way ANOVAs indicated a significant main effect of diet {CA1 [F(1,23) =14.39 p=0.0009], CA2/3 [F(1,23) =12.14 p=0.0020], DG [F(1,23) =12.16 p=0.0020]}, time {DG [F(1,23) =10.88 p=0.0031]}, and a significant interaction between diet and time in the CA1 [F(1,23) =4.37 p=0.0477], and DG [F(1,23) =13.32 p=0.0013]. Planned post-hoc t-tests indicated a significant increase after ethanol exposure in all regions of the hippocampus at T7: CA1 [t(10) =3.22, p=0.0092], CA2/3 [t(10) =2.28, p=0.0457], and DG [t(10) =5.038, p=0.0005] However, by T28, the number of hippocampal microglia returns to control levels in all regions except the CA2/3 [t(13) =2.66, p=0.0195]. In the entorhinal cortex, microglial cell number was estimated by an automated cell count, where no change was seen in the number of microglia between ethanol (586.5±55.4 microglia/section, n=7) and control animals (623.3±26.7 microglia/section, n=7) at T7, therefore no further time point was examined.
Figure 4. Increase in microglia number following 4-day binge exposure.
Stereological estimates indicate an increase in the number of microglia in ethanol treated animals (n=7; grey bars) compared with control (n=8; black bars) at T7 in the (A) CA1 (B) CA2/CA3, and (C) DG. This increase persists twenty-eight days later in the (B) CA2/3 in ethanols (n=7) compared with controls (n=7). There was no difference in cell counts determined by an image analysis program between ethanol and control at T7 in the (D) entorhinal cortex. *p<0.05
Cytokine expression also suggests low grade activation phenotype
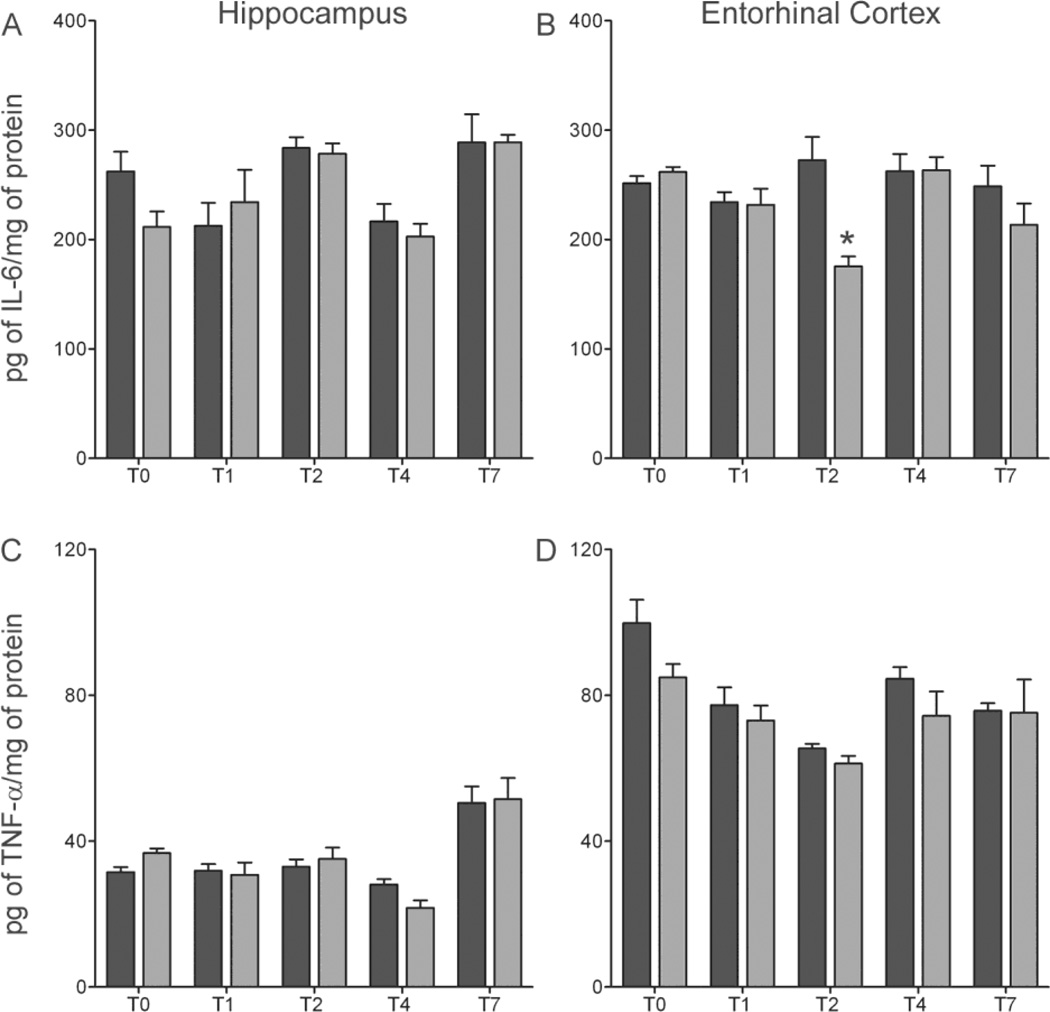
In order to assess the functional state of microglia, cytokine levels were assayed via ELISA. Increases in the proinflammatory cytokines IL-6 and TNF-α, are associated with classically activated microglia, but not partially activated microglia, and can be used to differentiate the two phenotypes of microglia (Table 1). IL-6 is a proinflammatory cytokine secreted by activated microglia in response to brain injury but can also act in an autocrine function to stimulate surrounding microglia into a phagocytic state (Chiang et al., 1994; Woodroofe et al., 1991). Two-way ANOVA’s showed a main effect of time in the hippocampus [F(4,59) =8.18, p<0.0001], but Bonferroni corrected post-hoc t-tests showed no statistical difference between ethanol and control animals in the region. However in the entorhinal cortex, two-way ANOVA indicated a significant main effect of diet [F(1,54) =7.13 p=0.01], time [F(4,54) =2.88 p=0.03], and a significant interaction between diet and time point [F(4,54) =4.72 p=.002] (Figure 5). Bonferroni corrected post-hoc t-tests show a significant 36% decrease [t(11) =3.97, p=0.011] in IL-6 in ethanol animals compared to controls in the entorhinal cortex at T2. Taken together, these results indicate that inhibition of basal IL-6 expression occurs after ethanol withdrawal in a temporally and regionally specific manner. In addition to IL-6, TNF-α is a proinflammatory cytokine expressed by fully activated microglia and increased after many forms of injury (Vitarbo et al., 2004). Two-way ANOVA analysis of the hippocampus showed a main effect of time [F(4,63) =20.77, p<0.0001], but there was no statistical differences between ethanol and control animals after Bonferroni corrected post-hoc t-tests. Despite significant main effects of both diet [F(1,54) =4.77 p=0.03], time [F(4,59) =8.86 p<.0001] in the entorhinal cortex, Bonferroni corrected post-hoc t-tests indicated no difference between ethanol and control animal at any time point. This lack of TNF-α upregulation in brain is consistent with previous reports in rats (Ehrlich et al., 2012; McClain et al., 2011; Zahr et al., 2010), but not mice (Qin et al., 2008).
Figure 5. No pro-inflammatory cytokine expression in the 4-day binge.
Concentrations of (A, B) IL-6, (C, D) TNF-α were determined by ELISA in both the hippocampus (A, C)) and entorhinal cortex (B, D). A 36% decrease of IL-6 was measured in the (B) entorhinal cortex at T2 in ethanol animals (n=7) [175pg/mg ± 8.9] compared to controls (n=7) [272pg/mg ± 21.2]; however, no change in TNF-α was seen in either the (E) hippocampus or the (F) entorhinal cortex. *p<0.05
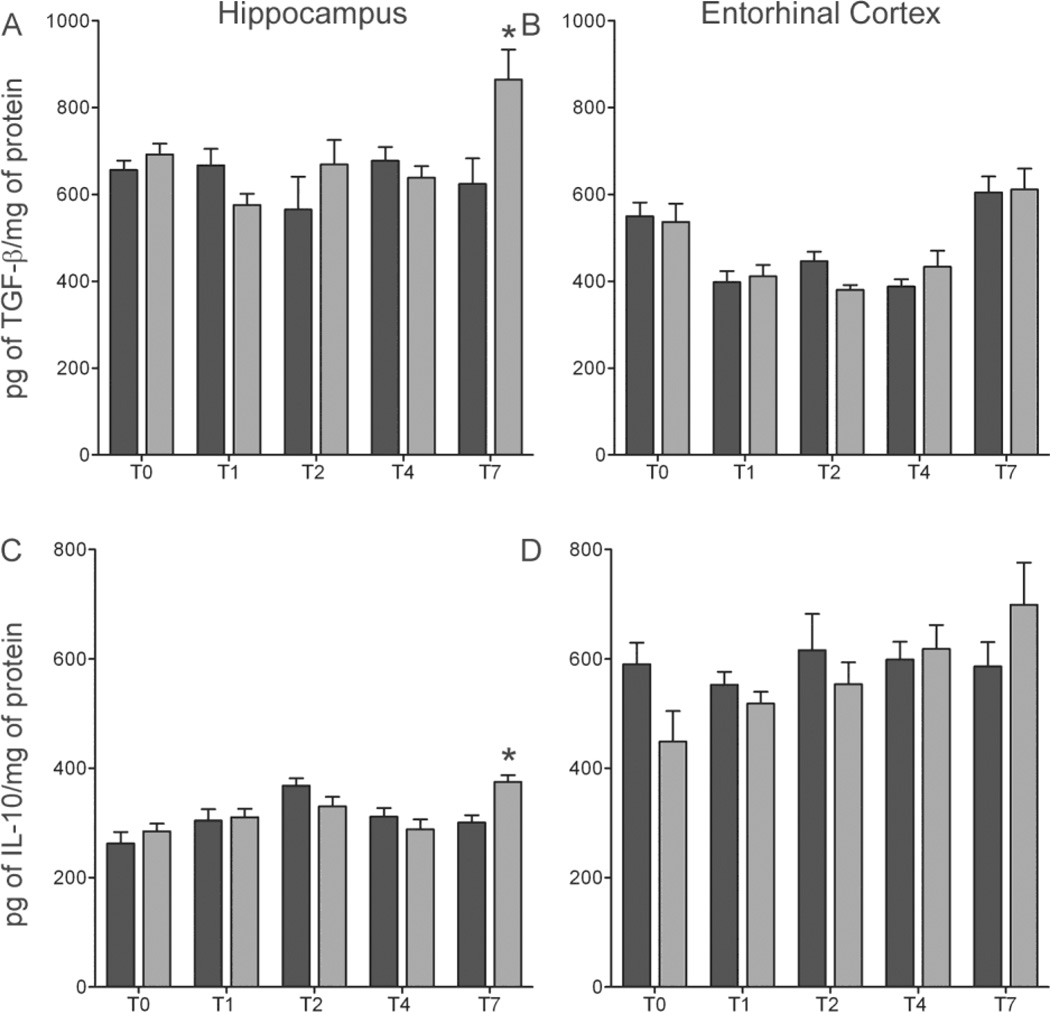
Basal expression of TNF-α and IL-6 were not increased following four-day ethanol exposure, suggesting the lack of a proinflammatory response. Therefore, we examined the effects of ethanol on TGF-β, a growth factor, and IL-10, an anti-inflammatory cytokine (Fiorentino et al., 1991; Polazzi et al., 2009). A significant interaction between diet and time point was shown in the hippocampus using a two-way ANOVA of TGF-β [F(4,53) =4.20 p=0.005]. Bonferroni corrected post-hoc t-tests revealed a significant 26% increase [t(11) =2.673, p=0.0434] in TGF-β in ethanol animals compared to controls at T7 (Figure 6). Despite a significant main effect of time point in the entorhinal cortex [F(4,47) =18.65 p<0.0001], no difference in TGF-β was observed between ethanol and control treated animals. In the hippocampus, a two-way ANOVA of IL-10 concentrations indicated a main effect of time point [F(4,59) =6.71 p=0.0002], plus a significant interaction between treatment and time point [F(4,64) =3.24, p=0.01]. Bonferroni corrected post-hoc t-tests revealed a significant 26% increase [t(11) =3.97, p=0.011] in IL-10 in ethanol animals compared to controls in the hippocampus at T7 (Figure 6). A two-way ANOVA showed no statistically significant main effects or interaction between diet and time point in the entorhinal cortex indicating no significant difference in the mean protein concentration between ethanol treated animals and controls (Figure 6).
Figure 6. Increase in anti—inflammatory cytokine expression after 7 days of abstinence.
Concentrations of (A, B) TGF-β (C, D) IL-10 were determined by ELISA in both the hippocampus (A, C)) and entorhinal cortex (B, D). An increase in both (A) TGF-β (38%) (C) IL-10 (26%) was seen in ethanol animals (n=6,7) (grey bars) compared with controls (n=7;black bars) in the hippocampus at T7. *p<0.05
Blood brain barrier remains intact following four-day binge ethanol exposure
In order to assess, whether the BBB is possibly breached by four-day binge ethanol exposure, we examined the penetration of IgG molecules during intoxication and at T2. Penetration of IgG into the parenchyma was observed in the ventral hypothalamus around the 3rd ventricle, a region known to lack an intact BBB under physiological conditions (Schmidt and Grady, 1993). However, qualitative analysis of IgG immunoreactivity between Bregma −2.30mm and −4.50mm (Paxinos and Watson, 2009) showed that both ethanol and control animals had few, if any IgG positive cells or diffusion in the parenchyma of either the hippocampus or entorhinal cortex at T0 or T2 (Figure 7). Therefore, the BBB does not appear to be breached in this model.
Figure 7. No disruption in the blood brain barrier (BBB).
There is no disruption in the BBB following ethanol as there is little to no IgG staining in either the (B, C, F, G) ethanol (T0: n=8; T2: n=6) or (A,E) control (T0 n=6; T2 n=7) compared with a (D,H) kainate positive control. Scale bar = 400 µm.
DISCUSSION
Microglia take on a variety of phenotypes, which can be used to predict the cell’s role in brain insult or neurodegenerative disease. The major finding of this work is that both morphological and functional evidence from these experiments support the conclusion that binge ethanol exposure does not classically activate microglia and is consistent with definitions of partial activation. The lack of classically activated microglia therefore does not meet the criteria for classical definitions of inflammation. Of Raivich’s five levels of microglial activation (Raivich et al., 1999a), these data support that four-day binge ethanol exposure only appears to activate cells up to stage 2. A step-wise progression is noted beginning while the animals are intoxicated (T0) where stage 1 (Table 1) or low level “alert” activation begins to occur and persists for at least twenty eight days according to [3H]-PK-11195 autoradiography for the TSPO receptor and OX-42 (CR3) immunoreactivity. Both markers are upregulated during and after four-day binge alcohol exposure. In addition, the morphology of OX-42 positive cells in ethanol-exposed brains supports that microglia are “alert” and “homing” as they appear less ramified with thicker, bushier processes (Figure 2). A stage 2 level of activation, or “proliferation and homing,” was suggested previously with the observation of proliferating microglia (Nixon et al., 2008). That microglia proliferate and home to sites of damage is further supported by the increased numbers of Iba-1+ microglia observed at T7 in all regions of the hippocampus, which persists in the CA2/3 at T28 (Figure 4). Importantly, the highest indices of activation, proliferation and increased number, are observed well after the peak of alcohol-induced cell death (T0; Crews et al., 2000; Kelso et al., 2011), which suggests that alcohol-induced microglial activation is a consequence of alcohol-induced cell death.
However, neither TSPO nor CR3 upregulation indicates the level of activation. Therefore, in order to determine microglia phenotype, more classical markers of full activation were evaluated. Neither OX-6 nor ED-1 were detected in the brain parenchyma, which indicates that few, if any, microglia have been activated to either a phagocytic or bystander activation state (Kato et al., 1995). Indeed, with the addition of these data, ED-1 has been exhaustively examined following four-day binge ethanol exposure, the most acutely damaging model of an AUD, and at no time point examined have ED-1-positive cells ever been found inside the brain parenchyma (McClain et al., 2011; Nixon et al., 2008). Therefore, morphology, number and marker data converge to support that microglia are only partially activated to a stage 2, proliferation and homing state.
Activated microglia not only change morphologically but also functionally as they secrete cytokines and growth factors that may impact the surrounding environment. Similarly, these cytokines can have either damaging or protective/reparative effects depending on the phenotype or level of microglial activation (Raivich et al., 1999a; Suzumura et al., 2006). Therefore, we examined key cytokines at critical time points of previously reported cellular events following four-day binge exposure. Cytokine expression following binge ethanol exposure also indicated that microglia are only partially activated. Proinflammatory TNF-α was not changed at any time point, IL-6 was selectively decreased at T2 in entorhinal cortex, the time of microglial proliferation, whereas anti-inflammatory cytokines, IL-10 and TGF-β, which can be secreted by alert/homing microglia, were selectively increased at T7 in the hippocampus. Alternatively activated microglia secrete both TGF-β and IL-10, and are known to suppress microglia activation and subsequent neuronal damage (Ledeboer et al., 2000; Sharma et al., 2011; Spittau et al., 2012). The increase IL-10 and TGF-β seven days after ethanol exposure (T7) in the hippocampus comes after significant neuronal damage in this region and, intriguingly, coincides with reactive neurogenesis (Kelso et al., 2011 Nixon and Crews, 2004; Obernier et al., 2002a). However, TNF-α and IL-6, released in the highest levels of activation, were not increased at any time point in either the hippocampus or entorhinal cortex (Bethea et al., 1999; Stoll et al., 2000). The lack of effect on TNF-α is consistent with recent reports from multiple laboratories that TNF-α is not increased in rats following excessive alcohol exposure (Ehrlich et al., 2012; McClain et al., 2011; Zahr et al., 2010), though conflicts with reports in mice (Alfonso-Loeches et al., 2010; Qin and Crews, 2012; Qin et al., 2008). It is important to note that the source of these cytokines was not determined in the present study or the cited reports as reactive astrocytes also secrete many of the same cytokines (Lau and Yu, 2001). Astrocytes are activated in the four-day binge model used and other alcohol models, though in a more delayed time course than that observed for microglia (Kelso et al., 2011). Because of the overlap in microglia and astroglia activation at T7 in this model, it is impossible to definitively link microglia activation with the secretion of particular cytokines. An important future discovery will be to show the cellular source of these cytokines in vivo. In summary, cytokine expression patterns following four-day binge alcohol exposure are consistent with that observed in immunohistochemical and morphological analyses – microglia phenotype is not one of classical activation, but merely partialactivation.
The activation state of microglia is critical to understanding their role in alcoholic neuropathology. Microglia progress stepwise through these various phenotypes, each of which is predictive of the cell’s role in homeostasis/neuroprotection versus neurodegeneration (Raivich et al., 1999a; Schwartz et al., 2006; Vilhardt, 2005). Although the concept of a graded state of activation (phenotype) has resolved the debate as to whether microglia are “good” or “bad,” each insult still results in a distinct response (Harting et al., 2008; Lai and Todd, 2008; Saijo and Glass, 2012). Even various patterns of alcohol intake produce a distinct response. As shown here, four-day binge ethanol exposure, which is an acutely damaging event compared to more chronic models, only produces partially activated microglia. Partially activated or low level phenotypes are more closely associated with roles in homeostasis and neuroprotection and therefore alcohol-activated microglia may be playing a role in neuroprotection, repair, or in the hippocampal DG, regeneration (Battista et al., 2006; Engelsberg et al., 2004). Although it may seem surprising that a brain insult as severe as high blood alcohol concentrations and alcohol-induced neurodegeneration, does not result in an overt, phagocytic level of reactive microgliosis, not all types of brain injury result in a full phagocytic, i.e. classical, microglial response (Graeber et al., 1988). Indeed, a recent report details phagocytosis independent of fully activated microglia (Sierra et al., 2010) and multiple reports show that partially activated microglia are necessary in neuroprotection and axonal regeneration (Shokouhi et al., 2010; Wainwright et al., 2009).
Intriguingly, intermittent exposure to ethanol results in evidence of more classically activated microglia such as TLR4 upregulation (Alfonso-Loeches et al., 2010; Fernandez-Lizarbe et al., 2009). Greater levels of activation with intermittent exposure models leads us to speculate that the initial exposure may serve as a priming stimulus to microglia such that subsequent exposures result in over-response as seen in other neurodegenerative disease models (Bilbo and Schwarz, 2009; Perry et al., 2003). The concept of microglia priming would explain why more classic-like activation is observed with multiple exposures or multiple intoxication/withdrawal cycles as that used by Qin (Qin et al., 2008), as opposed to our single cycle of prolonged intoxication then withdrawal and why the pattern of drinking is more associated with gliosis than the level of consumption (Riikonen et al., 2002). Unfortunately, these and other data support that microglia remain “primed” or partially activated for long periods of time after exposure. For example, [3H]-PK-11195 remains upregulated months after alcohol exposure (Obernier et al., 2002b; Syapin and Alkana, 1988) and the number of microglia remains increased at least a month after the binge in some regions (Figure 4). The long-term persistence of some level of activation supports the theory that cells could be “primed” by the initial damaging binge exposure. Furthermore, repeated cycling could also change the microglia response to secondary neuroimmodulators such as systemic inflammation (Qin et al., 2008; Zahr et al., 2010) which could be crucial when considering the large number alcoholics have systemic inflammation associated with liver disease (Polednak, 2012; Seth et al., 2011; Wang et al., 2012). This observation is important clinically as human binges tend to occur in an episodic nature and binge-pattern drinkers have a greater likelihood of neurodegeneration (Hunt, 1993). Thus, our data is consistent with the idea that an initial “hit” of binge-induced damage appears to partially activate microglia as a consequence of damage, but if this partial activation primes microglia, secondary “hits” or binge exposure could “polarize” or result in a more classical activation phenotype and/or inflammation. Although this study did not address polarization of microglia, nor the specific definitions associated with alternative or M2 activation, this could be a logical next step of the current work. A defining hallmark of classical inflammation is a compromised blood brain barrier, which, based on an examination of IgG expression, is not evident in the four-day binge model, the most severe of AUD models. Indeed, these data agree with evidence from less acutely damaging but longer term, chronic models of exposure such as 12 month 20% ethanol in the drinking water (Ehrlich et al., 2012). Other alcohol models, that have enhanced proinflammatory cytokine expression, do show BBB disruption, further supporting the theory that BBB disruption is necessary for a true neuroinflammatory event (Abdul Muneer et al., 2012). Importantly, the lack of evidence for a BBB compromise in this model strongly supports that classical inflammation does not occur with four-day binge exposure. Although this is only one model of an AUD, the well-defined cell death and degeneration profile coupled with data reported here does not indicate that classical inflammation drives alcohol-induced brain damage or that inflammation, according to classical definitions, occurs at all in this model.
The timecourse of expression of these various microglial markers and cytokine effects coupled with published timecourses of alcohol-induced cell death in this model (Crews et al., 2000; Kelso et al., 2011) support that alcohol-induced microglia activation is a consequence, not a cause of alcohol neurotoxicity. Alcohol-induced partial activation suggests a beneficial role of microglia in this model of an AUD, especially as no reports to date have observed fully activated, phagocytic microglia in brains from alcoholics. Indeed, if you remove microglia in many forms of neurodegeneration, worsened outcomes occur (Wainwright et al., 2009). Microglia have diverse roles in homeostasis, including newly defined roles in synaptic plasticity and neurotransmission (Tremblay and Majewska, 2011) and it is not known, nor revealed by these data, how partial activation might affect their homeostatic actions in synaptic plasticity. Intriguingly, the lack of phagocytic microglia could have implications for synaptic pruning and remodeling, especially in ongoing neurogenesis in the DG (Tremblay and Majewska, 2011). Thus, the inflammation hypothesis of AUD and targeting microglia in the treatment of AUDs must be considered with caution. Neuroinflammatory responses alone do not lead to AUDs and many of the reported microglial activation markers are expressed in the beneficial alternatively activated or acquired deactivated microglia that help to resolve and repair damage (Colton and Wilcock, 2010). Thus, it is not just that these microglia are activated by excessive alcohol exposure; the critical information is their phenotype. Therefore, these data do not rule out a role for microglia in AUDs, but do not support a direct relationship between alcohol, microglial activation and inflammation driven neurotoxicity. Careful consideration of these various current and previous studies, however, suggest that this partial activation phenotype could be consistent with a “primed” state such that repeated bouts of damaging, excessive alcohol intake, which is consistent with binge-benders in AUDs, may eventually result in highly or classically activated microglia and a proinflammatory state. The immediacy of microglial activation during alcohol intoxication, which was observed here, suggests that controlling the activation state of microglia during ethanol exposure may be a potential therapeutic target for AUDs. If microglia can be limited to only partial activation, perhaps they may be beneficial to endogenous repair systems after alcohol-induced neurodegeneration.
Highlights.
Alcohol activates microglia to a low-grade, anti-inflammatory phenotype.
Increase in number of microglia, OX-42, [3H]PK-11195, TGF-β, and IL-10 suggest stage 1 and 2.
Lack of ED-1, OX-6, TNF-α, and IL-6 upregulation indicate that microglia are not fully activated.
Absence of fully activated microglia and BBB disruption suggest neuroinflammation does not occur.
Microglia events are a consequence and not a cause of alcohol-induced neurodegeneration.
ACKNOWLEDGEMENTS
The authors thank M. Ayumi Deeny and David Eaves for their technical assistance. This research was supported by NIAAA R01AA016959 and NIDA T32DA016176.
Abbreviations
- ABC
Avidin-Biotin Complex
- AUD
Alcohol Use Disorder
- BBB
Blood Brain Barrier
- BEC
Blood Ethanol Concentration
- CA
Cornu Ammonis
- DAB
3, 3’-diaminobenzidine tetrahydrochloride
- DG
Dentate Gyrus
- ELISA
Enzyme-Linked Immuno-Sorbent Assay
- MHC-II
Major HistoCompatibility Complex II
- TLR
Toll-like Receptor
- TSPO
Translocator Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. The mechanisms of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol. 2012;32:1167–1177. doi: 10.1161/ATVBAHA.112.247668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Behav Immun. 2011;25(Suppl 1):S165–S169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Gehrmann J, Schubert P, Kreutzberg GW. Cytotoxicity of microglia. Glia. 1993;7:111–118. doi: 10.1002/glia.440070117. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38:365–375. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, McClintick JN, Strother WN, Carr LG, Liang T, Rodd ZA, Mayfield RD, Edenberg HJ, McBride WJ. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–147. doi: 10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides J, Dubois A, Scatton B. Peripheral type benzodiazepine binding sites as a tool for the detection and quantification of CNS injury. Curr Protoc Neurosci. 2001;Chapter 7(Unit7):16. doi: 10.1002/0471142301.ns0716s09. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, Bilbo SD. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;24:329–338. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25:S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM. A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics. 2007;4:571–579. doi: 10.1016/j.nurt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CS, Stalder A, Samimi A, Campbell IL. Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev Neurosci. 1994;16:212–221. doi: 10.1159/000112109. [DOI] [PubMed] [Google Scholar]
- Collins MA, Corse TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic "binge" intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS & Neurological Disorders – Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Reviews. 1999;5:379–394. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;25:S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Pirchl M, Humpel C. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsberg K, Ehinger B, Wasselius J, Johansson K. Apoptotic cell death and microglial cell responses in cultured rat retina. Graefes Arch Clin Exp Ophthalmol. 2004;242:229–239. doi: 10.1007/s00417-003-0780-z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Fishman PS, Savitt JM. Selective localization by neuroglia of immunoglobulin G in normal mice. J Neuropathol Exp Neurol. 1989;48:212–220. doi: 10.1097/00005072-198903000-00008. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. FEBS Lett. 2011;585:3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2009;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harting MT, Jimenez F, Adams SD, Mercer DW, Cox CS., Jr Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW, Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Basic principles of immunological surveillance of the normal central nervous system. Glia. 2001;36:118–124. doi: 10.1002/glia.1101. [DOI] [PubMed] [Google Scholar]
- Hunt WA. Are binge drinkers more at risk of developing brain damage? Alcohol. 1993;10:559–561. doi: 10.1016/0741-8329(93)90083-z. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Kim DW, Choi SY, Kang TC, Kim YS, Won MH. Ionized calcium-binding adapter molecule 1 immunoreactive cells change in the gerbil hippocampal CA1 region after ischemia/reperfusion. Neurochem Res. 2006;31:957–965. doi: 10.1007/s11064-006-9101-3. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Kalehua AN, Streit WJ, Walker DW, Hunter BE. Chronic ethanol treatment promotes aberrant microglial morphology in area CA1 of the rat hippocampus. Alcohol Clin Exp Res. 1992;16:401. [Google Scholar]
- Kato H, Kogure K, Araki T, Itoyama Y. Graded expression of immunomolecules on activated microglia in the hippocampus following ischemia in a rat model of ischemic tolerance. Brain Res. 1995;694:85–93. doi: 10.1016/0006-8993(95)00769-m. [DOI] [PubMed] [Google Scholar]
- Kaur C, Ling EA. Activation and re-expression of surface antigen in microglia following an epidural application of kainic acid in the rat brain. J Anat. 1992;180:333–342. [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Liput DJ, Eaves DW, Nixon K. Upregulated vimentin suggests new areas of neurodegeneration in a model of an alcohol use disorder. Neuroscience. 2011;197:381–393. doi: 10.1016/j.neuroscience.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Scheff SW, Pauly JR, Loftin CD. Effects of genetic deficiency of cyclooxygenase-1 or cyclooxygenase-2 on functional and histological outcomes following traumatic brain injury in mice. BMC Neurosci. 2009;10:108. doi: 10.1186/1471-2202-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso ML, Wehner JM, Collins AC, Scheff SW, Pauly JR. The pathophysiology of traumatic brain injury in alpha7 nicotinic cholinergic receptor knockout mice. Brain Res. 2006;1083:204–210. doi: 10.1016/j.brainres.2006.01.127. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–643. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia. 2008;56:259–270. doi: 10.1002/glia.20610. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Breve JJ, Poole S, Tilders FJ, Van Dam AM. Interleukin-10, interleukin-4, and transforming growth factor-beta differentially regulate lipopolysaccharide-induced production of pro-inflammatory cytokines and nitric oxide in co-cultures of rat astroglial and microglial cells. Glia. 2000;30:134–142. doi: 10.1002/(sici)1098-1136(200004)30:2<134::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Long JM, Kalehua AN, Muth NJ, Hengemihle JM, Jucker M, Calhoun ME, Ingram DK, Mouton PR. Stereological estimation of total microglia number in mouse hippocampus. J Neurosci Methods. 1998;84:101–108. doi: 10.1016/s0165-0270(98)00100-9. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25:S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Kawamata T, Walker DG, Akiyama H, Tooyama I, McGeer EG. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- Morioka T, Kalehua AN, Streit WJ. Progressive expression of immunomolecules on microglial cells in rat dorsal hippocampus following transient forebrain ischemia. Acta Neuropathol. 1992;83:149–157. doi: 10.1007/BF00308474. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010a;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010b;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: The National Academies Press; 1996. [PubMed] [Google Scholar]
- O'Keefe GM, Nguyen VT, Benveniste EN. Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: Implications in neurological diseases. J Neurovirol. 2002;8:496–512. doi: 10.1080/13550280290100941. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002a;26:547–557. [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002b;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinate. New York: Elsevier Academic; 2009. [Google Scholar]
- Penland S, Hoplight B, Obernier J, Crews FT. Effects of nicotine on ethanol dependence and brain damage. Alcohol. 2001;24:45–54. doi: 10.1016/s0741-8329(01)00142-2. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Altamira LE, Eleuteri S, Barbaro R, Casadio C, Contestabile A, Monti B. Neuroprotection of microglial condition medium on 6-hydroxydopamine-induced neuronal death: role of transforming growth factor beta-2. J Neurochem. 2009;2:545–556. doi: 10.1111/j.1471-4159.2009.06117.x. [DOI] [PubMed] [Google Scholar]
- Polednak AP. U.S. mortality from liver cirrhosis and alcoholic liver disease in 1999–2004: regional and state variation in relation to per capita alcohol consumption. Subst Use Misuse. 2012;47:202–213. doi: 10.3109/10826084.2011.635462. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 2012;9:130. doi: 10.1186/1742-2094-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky AG, Degos JD, Dreyfus PA. Peripheral injections of Freund's adjuvant in mice provoke leakage of serum proteins through the blood-brain barrier without inducing reactive gliosis. Brain Res. 1999;832:84–96. doi: 10.1016/s0006-8993(99)01479-1. [DOI] [PubMed] [Google Scholar]
- Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone induces long-lasting neuroprotection in cerebral ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J Neurosci. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999a;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Werner A, Bluthmann H, Doetschmann T, Kreutzberg GW. Molecular signals for glial activation: pro- and anti-inflammatory cytokines in the injured brain. Acta Neurochir. 1999b:21–30. doi: 10.1007/978-3-7091-6391-7_4. [DOI] [PubMed] [Google Scholar]
- Readnower RD, Chavko M, Adeeb S, Conroy MD, Pauly JR, McCarron RM, Sullivan PG. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J Neurosci Res. 2010;88:3530–3539. doi: 10.1002/jnr.22510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A. Intermittent ethanol exposure increases the number of cerebellar microglia. Alcohol. 2002;37:421–426. doi: 10.1093/alcalc/37.5.421. [DOI] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2012;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma. 1993;10:415–430. doi: 10.1089/neu.1993.10.415. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29:68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089–1105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI. IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res. 2011;1373:189–194. doi: 10.1016/j.brainres.2010.11.096. [DOI] [PubMed] [Google Scholar]
- Shokouhi BN, Wong BZ, Siddiqui S, Lieberman AR, Campbell G, Tohyama K, Anderson PN. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci. 2010;11:13. doi: 10.1186/1471-2202-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology (Berl) 1999;141:145–153. doi: 10.1007/s002130050818. [DOI] [PubMed] [Google Scholar]
- Spittau B, Wullkopf L, Zhou X, Rilka J, Pfeifer D, Krieglstein K. Endogenous transforming growth factor-beta promotes quiescence of primary microgli in vitro. Glia. 2012;12 doi: 10.1002/glia.22435. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–5274. doi: 10.1523/JNEUROSCI.15-07-05263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm. 2000:81–89. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia in the Pathological Brain. National Institute Alcohol Abuse and Alcoholism. 1994:55–68. 1994 Report nr 94–3742. [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia-derived cytokines on neuronal degeneration and regeneration. Ann N Y Acad Sci. 2006;1088:219–229. doi: 10.1196/annals.1366.012. [DOI] [PubMed] [Google Scholar]
- Syapin PJ, Alkana RL. Chronic ethanol exposure increases peripheral-type benzodiazepine receptors in brain. Eur J of Pharmacol. 1988;147:101–109. doi: 10.1016/0014-2999(88)90638-3. [DOI] [PubMed] [Google Scholar]
- Tomsovic M. "Binge" and continuous drinkers. Characteristics and treatment follow-up. Q J Stud Alcohol. 1974;35:558–564. [PubMed] [Google Scholar]
- Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4:220–222. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triguero D, Buciak JB, Yang J, Pardridge WM. Blood-brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989;86:4761–4765. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga S, Carrero P, Pernia O, Azcoitia I, Garcia-Segura LM. Translocator protein 18 kDa is involved in the regulation of reactive gliosis. Glia. 2007;55:1426–1436. doi: 10.1002/glia.20558. [DOI] [PubMed] [Google Scholar]
- Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Vitarbo EA, Chatzipanteli K, Kinoshita K, Truettner JS, Alonso OF, Dietrich WD. Tumor necrosis factor alpha expression and protein levels after fluid percussion injury in rats: the effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Mesnard NA, Beahrs TR, Politis CM, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve axotomy in CCR3-deficient mice. ASN Neuro. 2009;1:e00024. doi: 10.1042/AN20090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in Alcoholic Liver Disease. Annu Rev Nutr. 2012;21:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of 'binge drinking' rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Sholar PW, Mistry RS, Smith SH, Bilbo SD. Microglia and memory: modulation by early-life infection. J Neurosci. 2011;31:15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, Cuzner ML. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991;33:227–236. doi: 10.1016/0165-5728(91)90110-s. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine induction, thiamine levels, and hepatopathology in rats exposed to a 4-Day alcohol binge protocol. Alcohol Clin Exp Res. 2010;34:1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]