Abstract
Most patients with hematologic malignancies have received extensive chemotherapy before hematopoietic cell transplantation (HCT), resulting in neutropenia, lymphocytopenia, and use of antibiotics. Accordingly, patients have a wide range of neutrophil counts, lymphocyte counts, and prior antibiotic use. The minimal toxicity of the current conditioning regimen allowed asking whether peritransplant neutrophil or lymphocyte levels influenced the risks of acute graft-versus-host disease (GVHD) or relapse. We analyzed outcomes in 459 patients aged 7 to 75 (median 57) years who were conditioned with fludarabine and low-dose total body irradiation for HLA-matched HCT. We made two key findings. First, low neutrophil nadirs within the first 3 weeks after HCT had significant associations with increased risks of acute GVHD and 5-year non-relapse mortality (NRM), but showed no associations with the risk of relapse. Second, high lymphocyte counts immediately before transplantation had significant associations with reduced risks of relapse and overall mortality but showed no associations with the risks of GVHD or NRM. The findings suggested that the immunological mechanisms involved in acute GVHD might differ from those initiating graft-versus-tumor effects.
INTRODUCTION
Graft-vs.-host reactions occur after allogeneic hematopoietic cell transplantation (HCT) despite advances both in human leukocyte antigen (HLA) matching of the donor and the recipient and in immunosuppressive agents. Graft-vs.-host reactions can affect the recipient hematopoietic system, which may translate into graft-vs.-tumor (GVT) effects among patients with hematologic malignancies. These reactions can also affect the skin, gut and liver and the resulting graft-vs.-host disease (GVHD) can increase the risk of mortality. Among patients treated with conventional HCT, acute GVHD is initiated by the effects of high-intensity conditioning regimens, which cause tissue damage and subsequent production of inflammatory cytokines, translocation of bacterial products into the circulation, and heightened donor T-cell recognition of host antigens (reviewed in [1]). In a second phase donor T-cells are stimulated by minor histocompatibility antigens presented directly by host antigen-presenting cells [2,3] and indirectly by donor cells. In the third step, activated effector T-cells cause tissue injury that is recognized clinically as GVHD.
We developed a mild HCT-conditioning regimen that minimized tissue damage and could be tolerated by patients who were older or had serious comorbidities [4–7]. This regimen made it feasible to assess GVHD and GVT effects apart from the effects of the conditioning. In these patients, GVHD involvement of the liver has become very infrequent, especially after the introduction of prophylactic ursodiol [8,9]. Consequently, acute GVHD is now largely confined to skin and gut, both of which interface with the environment and possess characteristic microbiomes consisting of symbiotic, commensal bacteria [10,11]. The likely disturbance of microbiomes by neutropenia and antibiotics in patients undergoing chemotherapy before HCT, and the suspected roles of altered microbiomes in the development of autoimmune diseases (reviewed in [10,11]) raise the possibility that changes in commensal flora may modulate GVHD. In fact, a study in mice showed that pre-transplant treatment with the antibiotic ampicillin resulted in a loss of lactobacilli and aggravation of GVHD, whereas re-introduction of lactobacilli protected against GVHD [12]. Simultaneous studies in human patients identified increased microbial disturbances early after allogeneic HCT as a potential risk factor for GVHD [12].
Most patients with hematological malignancies have received extensive chemotherapy resulting in pancytopenia and use of broad-spectrum antibiotics. Accordingly, at arrival at our transplant center, patients have a wide range of neutrophil counts, lymphocyte counts, and prior antibiotic use. The widely ranging blood counts and the minimal toxicity of the conditioning regimen, allowed us to ask whether peri-transplant neutrophil or lymphocyte levels influenced the risks of acute GVHD or relapse (GVT effect) after HLA-matched, related and unrelated HCT.
METHODS
Patients
Between September 1, 2002 and December 31, 2010, 459 consecutive patients with hematologic malignancies were entered on prospective, minimal-intensity conditioning trials at the Fred Hutchinson Cancer Research Center (FHCRC) that were registered with ClinicalTrials.gov. All patients signed consent forms that were approved by the institutional review board of the FHCRC. Median follow-up of patients was 5 (range 1.5 to 9.9) years.
Table 1 shows patient and disease characteristics. The median patient age was 57 (range 7–75) years. Unmodified grafts consisted of granulocyte colony stimulating factor (G-CSF)-mobilized blood mononuclear cells (G-PBMC) and contained medians of 8.6 (range 0.2–29.9) × 106 CD34+ cells/kg and 3.0 (range 0.1–10.8) × 108 CD3+ cells/kg. Patients had received a median of 3 (range 0–23) chemotherapy regimens before HCT. HCT Comorbidity Index (HCT-CI) scores were assigned as described [13]. Fifty-one percent of patients had serious comorbidities with scores of 3 and higher. The designation of low, standard or high risk of relapse or progression for each disease and disease stage followed a previously described algorithm [14].
Table 1.
Patient and disease characteristics (n=459)*
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 183 (40) |
| Male | 276 (60) |
| Patient age, years | |
| 0–19 | 7 (2) |
| 20–29 | 17 (4) |
| 30–39 | 28 (6) |
| 40–49 | 68 (15) |
| 50–59 | 152 (33) |
| 60–69 | 169 (37) |
| 70–79 | 18 (4) |
| Median (range) | 57 (7–75) |
| Diagnosis | |
| ALL | 27 (6) |
| AML | 111 (24) |
| CLL | 50 (11) |
| CML | 2 (<1) |
| HL | 29 (6) |
| MDS | 53 (12) |
| MPD | 5 (1) |
| MM | 86 (19) |
| NHL | 91 (20) |
| Waldenstrom’s | 5 (1) |
| Relapse risk group [14] | |
| Low | 111 (24) |
| Standard | 220 (48) |
| High | 128 (28) |
| Conditioning regimen | |
| 2Gy TBI | 87 (19) |
| 2Gy TBI + Flu | 343 (75) |
| 3Gy TBI + Flu | 24 (5) |
| 4Gy TBI + Flu | 5 (1) |
| Prior high-intensity transplant | |
| No | 243 (53) |
| Yes† | 216 (47) |
| Donor | |
| HLA-id sibling | 217 (47) |
| Other HLA-matched related | 4 (1) |
| HLA-matched unrelated | 216 (47) |
| HLA-mismatched unrelated‡ | 22 (5) |
| HCT-CI | |
| 0 | 83 (18) |
| 1 | 45 (10) |
| 2 | 96 (21) |
| 3 | 113 (25) |
| 4 | 45 (10) |
| 5 | 38 (8) |
| 6 | 25 (5) |
| 7+ | 12 (3) |
| CMV seropositive | |
| Patient | 250 (54) |
| Donor | 184 (40) |
All patients reported here were entered on prospective clinical trials that were registered with ClinicalTrials.gov. Data on other patients, for example those whose insurance denied participation in clinical trials and who were therefore placed on treatment plans, are not included in this analysis.
Autologous HCT as part of tandem autologous/allogeneic HCT: n=114; failed autologous HCT: n=87; preceding autologous HCT for other disease: n=9; failed allogeneic HCT: n=15.
One HLA-allele mismatched.
Abbreviations, e.g., ALL=acute lymphocytic leukemia, AML=acute myeloid leukemia, CLL=chronic lymphocytic leukemia, CML=chronic myeloid leukemia, HL=Hodgkin lymphoma, MDS=myelodysplastic syndrome, MPD=myeloproliferative disorder, MM=multiple myeloma, NHL=non-Hodgkin lymphoma, TBI=total body irradiation, Flu=fludarabine, HLA=human leukocyte antigen, HCT-CI=Hematopoietic Cell Transplantation-Comorbidity Index, CMV=cytomegalovirus.
Conditioning and postgrafting immunosuppression
Four hundred thirty patients received 2 Gy TBI with (n=343) or without (n=87) fludarabine, 30 mg/m2/day, on days 4, 3, and 2 before HCT (Table 1). Twenty-four patients received 3 Gy and five patients received 4 Gy TBI. Immunosuppression after HCT included MMF (28 days for related [4] and at least 96 days for unrelated [5,6] recipients) and a calcineurin inhibitor (up to 180 days; either cyclosporine [CSP; n=270] or tacrolimus [TAC; n=189]). Thirty-seven unrelated recipients also received sirolimus as part of a randomized trial [15]. All patients received prophylactic ursodiol from approximately 14 days before transplantation until at least day 180 after transplantation. Infection prophylaxis and treatment followed FHCRC standard practice guidelines including acyclovir for herpes simplex and varicella zoster virus prophylaxis, fluconazole for yeast prophylaxis, trimethoprim-sulfamethoxazole for Pneumocystis jirovecii prophylaxis, and monitoring for cytomegalovirus reactivation. Antibacterial prophylaxis with levofloxacin was started when neutrophil counts declined below 500 cells/μL and was continued until neutrophil counts recovered to above 500 cells/μL for 2 consecutive days in the absence of infection. Transplantations were planned as outpatient procedures.
Monitoring after transplantation
As a rule, patients had marrow aspirations to assess disease status on days 28, 84, 180, 365, and then as indicated. Donor chimerism was evaluated on days 28, 84, and 365 after HCT. Acute and chronic GVHD were graded as described [16–20]. The diagnoses of relapse, defined as recurrence of malignancy, or of progression, were based on previously published criteria [14].
Causes of death
Relapse mortality included deaths after relapse or progression of pre-transplant disease, regardless of other events. Non-relapse mortality (NRM) included all deaths without relapse or progression. GVHD mortality included all deaths in patients with a history of GVHD who died during immunosuppressive therapy. Infection was listed as a cause of death in patients without relapse, progression, or a history of GVHD.
Statistical analysis
Overall survival was estimated by the Kaplan-Meier method [21]. Cumulative incidence rates of acute and chronic GVHD, NRM, and relapse mortality were estimated by methods previously described [22]. Death was a competing risk for all other endpoints. Relapse was a competing risk for NRM, and NRM was a competing risk for relapse and relapse-related mortality. Since some time-to-event endpoints also occurred during this time period, progressive neutrophil nadir was treated as a time-dependent covariate when analyzed as a risk factor. Cox regression was used for risk factor analysis for all time-to-event endpoints. Logistic regression was used to analyze risk factors for neutropenia nadir <500 cells/μL. For this analysis pre-transplant neutropenia was defined as the lowest value from day −15 through day −1 before starting the conditioning regimen. For purposes of display and characterization, neutrophil nadirs during days 1 through 20 after transplant were divided in four ranges.
All statistical analyses were adjusted to account for possible uneven distribution of the following variables: related/unrelated donor; HLA allele-level mismatch; patient age in years (<50; ≥ 50); donor/patient gender (F→M, other); fludarabine use (no, yes); calcineurin inhibitor (CSP, TAC); diagnosis (multiple myeloma, lymphoma, other); preceding high-intensity transplant (yes, no). Statistical analyses involving neutrophil nadir as a risk factor for acute and chronic GVHD, relapse, NRM, and overall mortality were also adjusted for pre-transplant lymphocyte counts. In separate analyses pre-transplant lymphocyte counts were analyzed as a risk factor for the same outcomes with adjustments both for post-transplant neutrophil counts and the other variables. Pre-transplant lymphocyte levels were determined by the minimum value between day −15 to day −1 before start of the conditioning regimen.
RESULTS
Post-transplantation neutrophil nadir
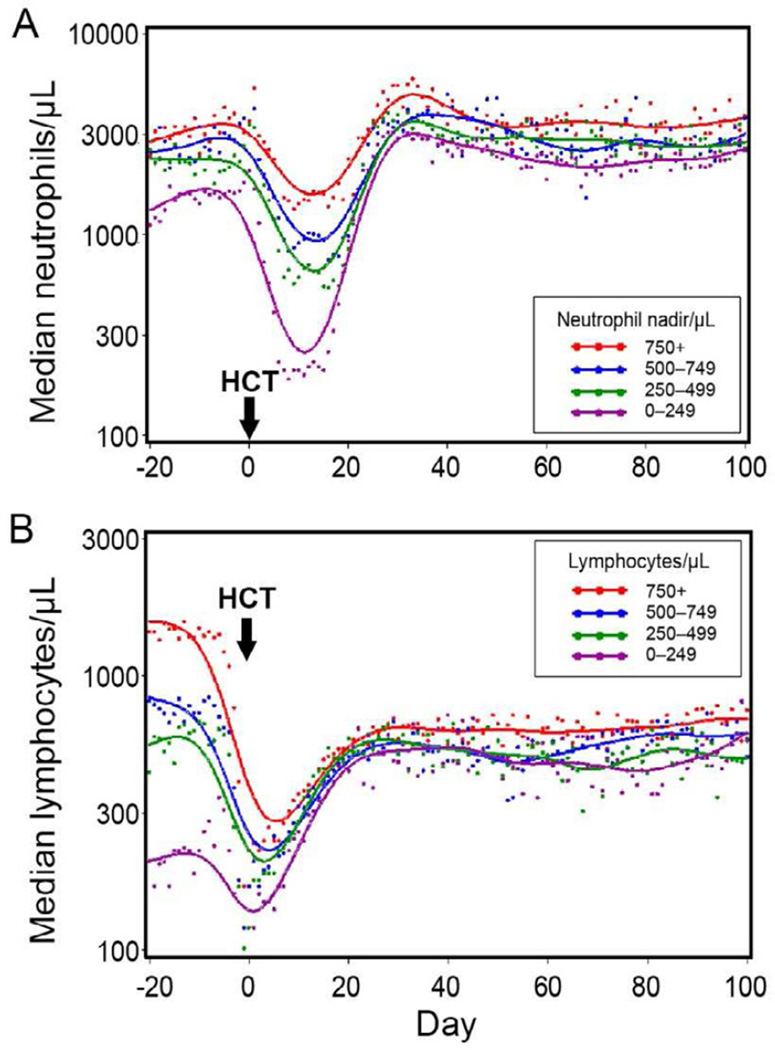
Neutrophil counts declined exponentially after HCT reaching nadirs between days 10–15, followed by exponential recoveries to the normal range by day 30. This is shown in Figure 1A, where the neutrophil nadirs were divided in four ranges for purposes of illustration.
Figure 1.
Pre- and post-transplant neutrophil changes among patients in four post-transplant neutrophil nadir ranges (A). Pre- and post-transplant lymphocyte changes among patients in four pre-transplant lymphocyte count ranges (B).
The strongest predictors for a neutrophil nadir of <500/μL after HCT were low neutrophil counts before HCT (Table 2). Compared to patients with pre-transplant neutrophil counts >2000/μL, those with counts between 500 and 1999/μL had an overall risk (O.R.) of 3.55 (95% C.I. 2.0–6.2; p<0.0001) and those with counts <500/μL an O.R. of 5.94 (95% C.I. 2.1–17; p=0.0009). Another risk factor for low neutrophil nadirs after HCT was having transplantation from an unrelated donor (O.R. 3.62; 95% C.I. 1.9–6.8; p<0.0001). Patients with MM (O.R. 0.24; 95% C.I. 0.1–0.6; p=0.002) and those with prior high-intensity transplantation (O.R. 0.46; 95% C.I. 0.2–0.9; p=0.03) were significantly less likely to have a nadir of <500 neutrophils/μL after HCT. No significant associations with neutrophil nadir were seen with donor age, patient age, CD34 cell dose, number of preceding chemotherapy regimens and HCT-CI scores. In order to estimate the effect size associated with these risk factors, the post-transplantation neutrophil nadir was analyzed as a continuous outcome on a log scale and the results were expressed as nadir multipliers (Table 2). For example, a pre-transplant neutrophil count <500/μL reduced the post-transplantation neutrophil nadir by 82%, and a diagnosis of multiple myeloma increased the nadir by 54%.
Table 2.
Multivariate analysis of risk factors for neutrophil nadir < 500/µL
| OR (95% Ci) | P | Nadir multiplier† | P | |
|---|---|---|---|---|
| Pre-transplant neutrophil level (µL) | ||||
| >2000 | 1.0 | |||
| 500–1999 | 3.55 (2.0–6.2) | <0.0001 | 0.56 (0.42–0.75) | <0.0001 |
| <500 | 5.94 (2.1–17) | 0.0009 | 0.18 (0.12–0.27) | <0.0001 |
| Disease group | ||||
| ALL,AML,MDS,CML | 1.0 | |||
| HL,NHL,CLL | 0.57 (0.3–1.1) | 0.11 | 0.91 (0.66–1.27) | 0.58 |
| MM | 0.24 (0.1–0.6) | 0.002 | 1.54 (0.96–2.47) | 0.08 |
| Donor | ||||
| Related | 1.0 | |||
| Unrelated | 3.62 (1.9–6.8) | <0.0001 | 0.56 (0.39–0.80) | 0.001 |
| Patient age, years | ||||
| <50 | 1.0 | |||
| 50–64 | 1.51 (0.8–2.8) | 0.18 | 0.74 (0.53–1.05) | 0.09 |
| 65+ | 2.40 (0.8–7.2) | 0.12 | 0.72 (0.45–1.16) | 0.18 |
| Donor age, years | ||||
| <50 | 1.0 | |||
| 50+ | 0.87 (0.5–1.7) | 0.68 | 0.87 (0.59–1.27) | 0.47 |
| CD34 cell dose × 106/kg | ||||
| 5.9+ | 1.0 | |||
| <5.9 (lowest quartile) | 1.29 (0.7–2.4) | 0.41 | 0.91 (0.67–1.23) | 0.55 |
| Prior high-intensity transplant | ||||
| No | 1.0 | |||
| Yes* | 0.46 (0.2–0.9) | 0.03 | 1.68 (1.18–2.39) | 0.004 |
| No. of prior chemotherapy regimens | ||||
| 0–3 | 1.0 | |||
| 4+ | 0.95 (0.5–1.7) | 0.87 | 0.92 (0.69–1.23) | 0.59 |
| HCT-CI score | ||||
| 0–2 | 1.0 | |||
| 3+ | 1.12 (0.7–1.9) | 0.66 | 0.89 (0.68–1.15) | 0.37 |
Autologous HCT as part of tandem autologous/allogeneic HCT: n=114; failed autologous HCT: n=87; preceding autologous HCT for other disease: n=9; failed allogeneic HCT: n=15.
The nadir is a continuous outcome on a log scale – the effects of risk factors are expressed as multipliers. For example, a pre-transplant count <500 reduces the nadir by 82%; a diagnosis of MM increases the nadir by 54%.
GVHD
Two hundred sixty-four patients developed grade II-IV acute GVHD, of whom 101 had isolated gut GVHD, 60 had isolated skin GVHD, 102 had both skin and gut GVHD, and one had isolated, stage 1 liver GVHD. Eight patients had liver GVHD in addition to gut involvement. Among the 203 patients with gut GVHD, 159 (78%) had upper gut involvement. The distributions of organ involvement in patients were comparable over the range of neutrophil nadirs. The overall cumulative incidence rates of grades II–IV and III-IV acute GVHD were 56% and 8%, respectively.
Table 3A shows post-transplantation neutrophil nadirs, per log decrease in neutrophil counts, as risk factors for GVHD. Significantly increased hazard ratios were seen for the association of neutrophil nadirs with grades II-IV and III-IV acute GVHD and de novo chronic GVHD.
Table 3.
Post-transplantation neutrophil nadir (per log decrease in absolute neutrophil count as continuous variable) and pre-transplantation absolute lymphocyte counts (per log decrease in absolute lymphocyte levels as continuous variable) as risk factors for various outcomes*
| Outcome | No. Events | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| A. Post-transplant neutrophil nadirs | |||||
| GVHD | |||||
| Acute† | Grade | II–IV | 263 | 1.45 (1.2–1.7) | <0.0001 |
| III–IV | 39 | 1.63 (1.1–2.4) | 0.02 | ||
| Chronic | Any | 270 | 1.08 (0.9–1.3) | 0.42 | |
| De Novo | 103 | 1.81 (1.2–2.8) | 0.007 | ||
| Relapse or progression | 207 | 1.11 (0.9–1.4) | 0.39 | ||
| NRM | 87 | 1.54 (1.2–2.0) | 0.001 | ||
| Overall mortality | 241 | 1.46 (1.21–1.8) | <0.0001 | ||
| B. Pre-transplant absolute lymphocyte levels | |||||
| GVHD | |||||
| Acute† | Grade | II–IV | 263 | 1.17 (0.9–1.5) | 0.23 |
| III–IV | 39 | 0.94 (0.5–1.7) | 0.84 | ||
| Chronic | Any | 270 | 0.94 (0.7–1.2) | 0.62 | |
| De Novo | 103 | 0.82 (0.5–1.3) | 0.40 | ||
| Relapse or progression | 207 | 1.48 (1.1–1.9) | 0.003 | ||
| NRM | 87 | 1.00 (0.6–1.6) | 0.99 | ||
| Overall mortality | 241 | 1.41 (1.1–1.8) | 0.007 | ||
Adjusted for: donor (related, unrelated); HLA allele-level mismatch (no, yes); patient age in years (<50, ≥ 50); donor/patient gender (F→M, other); HCT-CI (0–2, ≥ 3); fludarabine use (no, yes); GVHD prophylaxis (CSP, TAC), diagnosis (multiple myeloma, lymphoma, other); post-transplant neutrophil nadir (continuous); prior high-intensity transplant (no, yes).
The analysis of post-transplantation neutrophil nadir was also adjusted for pre-transplantation lymphocyte counts (continuous).
The analysis of pre-transplantation lymphocyte levels was also adjusted for post-transplantation neutrophil nadir (continuous).
In one patient, GVHD grade was not available. This case was excluded from multivariate analysis.
In Table 4, cumulative incidence estimates of grades II-IV and III-IV acute GVHD were analyzed within four subgroups of patients according to their neutrophil nadir. The results suggested that the neutrophil nadir represented a continuous predictor of the risk for acute GVHD. For example, the overall risk of grade II-IV acute GVHD rose from 36% in the highest nadir group to 69% in the lowest nadir group, and the risk of grade III-IV acute GVHD tripled from 3.5% to 11%.
Table 4.
Cumulative incidences of acute GVHD, relapse mortality, and NRM, and Kaplan-Meier estimates of overall survival in relation to four ranges of post-transplantation neutrophil nadir
| % Acute GVHD, grades |
% 5-year |
|||||
|---|---|---|---|---|---|---|
| Post-transplant neutrophil nadir, cells/µL |
No. of patients | II–IV | III–IV | Relapse mortality |
NRM | Overall survival |
| >750 | 58 | 36 | 3.5 | 39 | 3 | 58 |
| 500–749 | 56 | 46 | 5.4 | 33 | 9 | 58 |
| 250–499 | 118 | 54 | 9.3 | 32 | 15 | 53 |
| 0–249 | 227 | 69 | 11.0 | 35 | 25 | 40 |
Relapse or progression, NRM and overall survival at 5 years
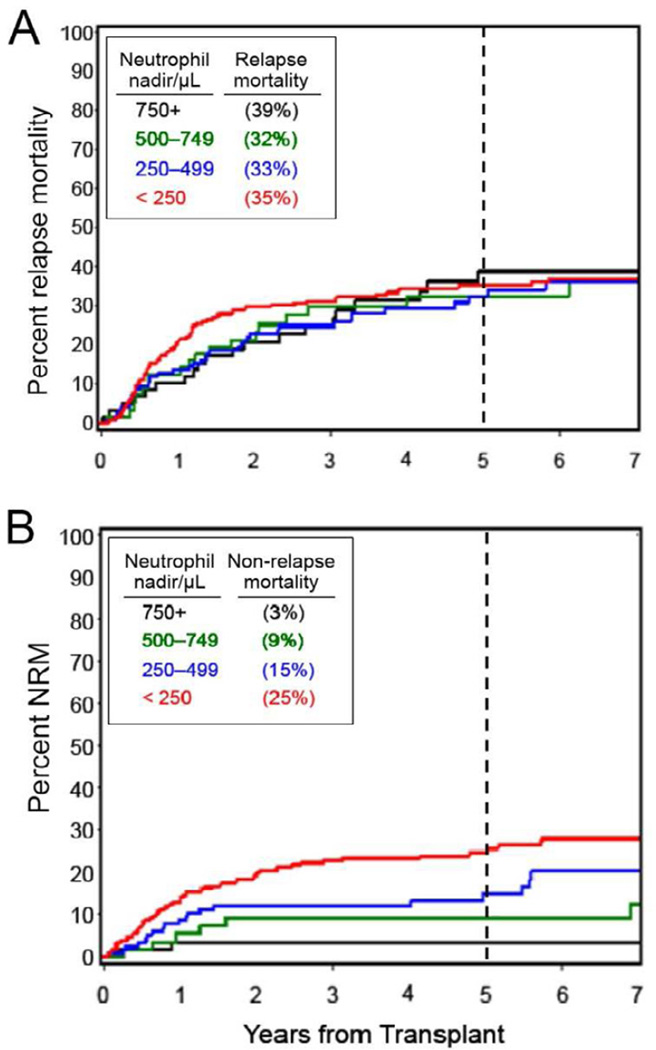
Table 3A also examined the neutrophil nadir as a time-dependent risk factor for relapse or progression, NRM and overall mortality. No statistically significant association between neutrophil nadir and relapse or progression was seen (HR 1.11; p=0.39) while associations with increased NRM and overall mortality were statistically significant (HRs 1.54 and 1.46, respectively; p=0.001 and <0.0001, respectively). Table 4 shows comparable 5-year relapse mortality in the four nadir groups ranging from 32–39% (see also Figure 2A). In contrast, 5-year NRM steadily increased from 3% in the highest nadir range to 25% in the lowest nadir range (see also Figure 2B). Five-year overall survival, affected both by relapse mortality and NRM, ranged from 58% in the highest nadir range to 40% in the lowest nadir range.
Figure 2.
Cumulative incidences of relapse mortality (A) and non-relapse mortality (B) in relation to four neutrophil nadir ranges. The percentages shown within the boxes are 5-year data.
Causes of NRM
Eighty-seven patients died of nonrelapse causes. Of these, 78 (90%) died from complications related to GVHD and its treatment. Among the 78, 59 died from bacterial, fungal, or viral infections and 19 died from organ failure including bronchiolitis obliterans organizing pneumona. Among the nine patients without GVHD, two experienced late graft failure and died of infections after receiving pentostatin and donor lymphocyte infusions (days 158 and 344, respectively). Four patients died of solid tumors (in two cases recurrent) 2.9, 5.0, 5.1, and 5.5 years after HCT, and two died in car accidents 1.3 and 2.5 years after HCT. One patient with a history of CNS radiation, intrathecal chemotherapy, and two preceding high-intensity conditioning transplantations died on day 97 from leukoencephalopathy.
Pre-transplantation lymphocyte counts
In the preceding multivariate analyses, we used lymphocyte counts immediately before transplantation as a continuous adjustment factor. Here, we analyzed pre-transplant lymphocyte counts as continuous variable and transplantation outcomes (Table 3B) with the post-transplantation neutrophil nadir used as an additional adjustment factor. While no statistically significant associations were seen with acute GVHD, chronic GVHD or NRM, low lymphocyte counts predicted significantly higher relapse risk (HR 1.48; p=0.003) and increased overall mortality (HR 1.41; p=0.007).
DISCUSSION
The study yielded two major, new results. First, the depth of the neutrophil nadirs within the first 3 weeks after transplantation was highly associated with the risks of acute GVHD and associated NRM but not with GVT effects. Second, peripheral blood lymphocyte counts immediately before transplantation were associated with GVT effects but not with acute GVHD or NRM. These findings suggested that the immunological mechanisms involved in acute GVHD differed from those initiating GVT effects.
The first result showed that patients with the lowest neutrophil nadirs had significantly increased risks of both acute GVHD and 5-year NRM compared to patients with higher nadirs. For example, the risk of grades III-IV acute GVHD rose steadily from 3.5% in the highest neutrophil nadir range to 11% in the lowest nadir range, and the risk of 5-year NRM rose from 3% to 25%. The data suggested that the neutrophil nadir might serve as a continuous predictor of risks for GVHD and NRM. While the risks of acute GVHD and NRM increased significantly with decreasing nadir, both the cumulative incidence rates and the hazard ratios of relapse were unchanged across the range of neutrophil nadirs. It could be argued that some diseases, which are especially susceptible to GVT effects such as B-cell malignancies, were unevenly distributed among the nadir ranges, but the multivariate analyses were adjusted for the type of malignancy.
Unsurprisingly, the most significant factor predicting low post-transplantation neutrophil nadirs was pre-transplant neutropenia. Other factors included underlying malignancies, having received a prior transplant with high-intensity conditioning, and unrelated grafts, but these were included as adjustments in all analyses. Low pre-transplant neutrophil counts likely resulted from extended chemotherapy and impaired marrow function before referral to HCT. Typically, neutropenic patients are treated with prophylactic, broad-spectrum antibiotics. Antibiotics can impart unintended collateral damage to symbiotic microorganisms that live in the human gastrointestinal tract [23]. Specifically, repeated exposure to broad-spectrum antibiotics can dramatically alter the composition of human gut microbial communities, and these changes can persist for long periods of time [24,25]. It is possible that both the neutropenia and the altered microbiota emerging after antibiotic treatments can lead to a pro-inflammatory state which induces production of cytokines, such as interferon-γ, TNF- α, IL-1, and IL-6, and increased expression of HLA-DR and costimulatory molecules, not only on antigen-presenting cells, but also on skin and gut epithelia. In this hypothetical sequence of events, skin and gut epithelia might conceivably present minor histocompatibility antigens to donor T-cells, thereby amplifying GVHD manifestations specifically in these two organs at the exclusion of GVT effects or immune reactions against other organs, such as those typically involved in autoimmune diseases. Alternatively, indirect antigen-presentation from these cells might be enhanced by low-grade inflammation associated with neutropenia and altered microbial flora.
Results from previous studies support the hypothesis that gut and skin microbiomes can influence the development of GVHD. “Germ-free” murine recipients of H-2 incompatible HCT had less clinically detectable GVHD and survived significantly better than conventionally raised mice, and their histologic GVHD lesions were less pronounced [26–28]. Human aplastic anemia patients given marrow grafts from HLA-identical siblings experienced both significantly less acute GVHD and better survival when the transplantation was carried out in a protective, “bacteria-free” environment compared to a conventional environment [29]. Similarly, a pediatric study showed improved prevention of GVHD with suppression of microbial flora [30]. In another study, human HCT recipients randomly assigned to antibiotic prophylaxis with ciprofloxacin and metronidazole had less acute GVHD than those given ciprofloxacin alone [31]. The authors suggested that anaerobic flora, favored by ciprofloxacin monotherapy, played a role in the pathogenesis of acute GVHD.
In mice, outgrowth of Escherichia (E.) coli bacteria was observed during gut GVHD and GVHD was ameliorated when E. coli growth was curtailed by oral polymyxin B, consistent with the hypothesis that microorganisms could modulate GVHD [32]. Another murine study confirmed outgrowth of E. coli during GVHD, a finding that was interpreted to represent a shift toward pro-inflammatory bacterial species [33]. Probiotics appeared to reduce GVHD in experimental models [34], as did oral administration of lactobacillus rhamnosus, while administration of ciprofloxacin led to worse survival, possibly as a result of a disturbed microbiome [35]. These results were consistent with the adverse effect on GVHD caused by pretreatment of mice with ampicillin and the reversal of this effect by introduction of lactobacilli [12]. Findings in human allogeneic HCT recipients did not identify special bacterial populations as potential risk factors for subsequent GVHD but found greater microbial disturbance early after transplantation in patients who developed GVHD [12]. Sequential high-resolution 16s rRNA studies of skin and gut microbiomes, especially among patients with high vs. low post-transplantation neutrophil nadirs, might provide insight into whether microbiome changes could be implicated in the causal pathway leading to development of acute GVHD.
The second major finding of the current study was that lymphocyte counts immediately before transplantation had significant associations with the risk of relapse and, consequently, with overall mortality but showed no associations with the risks of acute GVHD, chronic GVHD or NRM. Specifically, patients with higher lymphocyte counts had significantly less risk of relapse or progression and better overall survival than those with lower lymphocyte counts. Because pretransplant flow cytometry examinations of the peripheral blood were focused on detecting malignant cells, we have no detailed information on lymphocyte or mononuclear subsets in these patients. In the absence of that knowledge, we would hypothesize that lymphocyte levels might serve as a proxy for the numbers of host antigen-presenting cells with the result that larger numbers of these cells are associated with stronger GVT effects. Even though our findings are currently unexplained, they are consistent with the possibility that GVT effects can be separated from acute GVHD, and that they are initiated by different mechanisms. Alternatively, it could be argued that pre-transplant lymphocyte counts were a reflection of the intensity of the preceding chemotherapy used to reduce the tumor burden. Thus, low lymphocyte counts might be a surrogate marker for “resilience” of the malignancy to respond to therapy. In order to offset this confounder, the analysis was adjusted for underlying diagnoses.
Four studies in patients treated with either myeloablative or reduced-intensity conditioning regimens showed that the risk of relapse was not reduced by acute GVHD [7,36–38]. In contrast, a recent large study in patients with leukemias and myelodysplastic syndromes treated with high-intensity regimens did find significant incremental effects of acute GVHD on risk of relapse, although this effect was observed only beyond the first 18 months [39]. Others showed GVT effects associated with acute GVHD in patients with ALL and more advanced AML but not AML in first remission [16,40]. Ishiyama also reported GVT effects associated with acute GVHD [41]. A large, retrospective CIMBTR study [42] analyzed results in patients given allogeneic HCT for AML and MDS. Acute GVHD after myeloablative conditioning did not affect relapse, but both chronic GVHD alone and acute plus chronic GVHD were associated with reduced relapse risks. In contrast, all three manifestations of GVHD were associated with highly significant reductions in risk of relapse in patients given a variety of reduced-intensity conditioning regimens. The discordance between current results after minimal-intensity conditioning and some of the previous results after high-intensity conditioning might, in part, reflect differences in the diseases treated, which might have different susceptibilities to GVT effects. Previous studies included mainly patients with acute leukemias and chronic myeloid leukemia, while more than half of the current patients had B-cell malignancies, multiple myeloma, or Hodgkin lymphoma, and only two patients had CML.
In conclusion, low posttransplant neutrophil counts predicted increased risks of acute GVHD, de novo chronic GHVD and NRM, while high pretransplant lymphocyte counts were associated with a lower risk of relapse and improved survival. These results suggested that different mechanisms were involved in the two types of localized, immunologic graft-vs.-host reactions.
ACKNOWLEDGEMENTS
The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD, grants P01CA078902, P01HL036444, P01CA018029, P30CA015704 and HL108307. Further support came from grants from the Leukemia/Lymphoma Society (7008-08), and the Laura Landro Salomon Endowment Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
We are grateful to all research nurses and data coordinators for implementation of protocols. We also thank our administrative staff for their assistance with manuscript preparation. We are grateful to the many physicians, nurses, physician assistants, nurse practitioners, pharmacists, and support staff who cared for our patients, and to the patients who allowed us to care for them and who participated in our ongoing clinical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE STATEMENT
The authors have no primary financial relationships with any company that has a direct financial interest in the subject matter or products discussed in the submitted manuscript, or with a company that produces a competing product.
AUTHORS’ CONTRIBUTIONS
R.S.: Designed the research and wrote the paper.
B.G.: Assisted with data analysis and was principal investigator in one of the clinical trials.
B.E.S.: Carried out all statistical analyses.
D.G.M.: Principal investigator on clinical trials and contributed in writing the manuscript.
M.L.S.: Principal investigator on clinical trials and contributed in writing the manuscript.
M.M.: Principal investigator on clinical trials and contributed in writing the manuscript.
P.J.M.: Supervised long-term follow-up of patients and contributed in writing the manuscript.
B.M.S.: Principal investigator on clinical trials and contributed in writing the manuscript.
REFERENCES
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease (Review) Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 3.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 4.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 5.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 6.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Gyurkocza B, Storer BE, et al. Graft-vs.-host disease and graft-vs.-tumor effects after hematopoietic cell transplantation. J Clin Oncol. (in press) [Google Scholar]
- 8.Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–1983. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 9.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grice EA, Segre JA. The skin microbiome [Erratum appears in Nat Rev Microbiol. 2011 Aug;9(8)626] Nature Reviews Microbiology. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosiewicz M, Zirnheld AL, Alard P. Gut microbiota, immunity, and disease: a complex relationship. Frontiers in Microbiology. 2011;2:180. doi: 10.3389/fmicb.2011.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;doi doi: 10.1084/jem.20112408. [Epub ahead of print]- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandmaier BM, Maris M, Storer B, et al. A randomized 3-arm phase II study to determine the most promising postgrafting immunosuppression for prevention of acute graft-versus-host disease (GVHD) after unrelated donor hematopoietic cell transplantation (HCT) using nonmyeloablative conditioning for patients with hematologic malignancies: a multi-center trial. Blood. 2009;114:147. #348[abstr.] [Google Scholar]
- 16.Sullivan KM, Weiden PL, Storb R, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 17.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 19.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Flowers MED, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Blaser M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature. 2011;476:393–394. doi: 10.1038/476393a. [DOI] [PubMed] [Google Scholar]
- 24.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota (Review) Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 25.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45:577–588. [PubMed] [Google Scholar]
- 27.Heit H, Heit W, Kohne E, Fliedner TM, Hughes P. Allogeneic bone marrow transplantation in conventional mice: I. Effect of antibiotic therapy on long term survival of allogeneic chimeras. Blut. 1977;35:143–153. doi: 10.1007/BF00996294. [DOI] [PubMed] [Google Scholar]
- 28.Heidt PJ, Vossen JM. Experimental and clinical gnotobiotics: influence of the microflora on graft-versus-host disease after allogeneic bone marrow transplantation (Review) J Med. 1992;23:161–173. [PubMed] [Google Scholar]
- 29.Storb R, Prentice RL, Buckner CD, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308:302–307. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 30.Vossen JM, van den Berg H, Gerritsen EJ, Hermans J, Dooren LJ. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis. 1990;9:14–23. doi: 10.1007/BF01969527. [DOI] [PubMed] [Google Scholar]
- 31.Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 32.Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120:223–231. doi: 10.1182/blood-2011-12-401166. [DOI] [PubMed] [Google Scholar]
- 33.Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 34.Gerbitz A, Schultz M, Wilke A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103:4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 35.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor-alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 37.Thepot S, Zhou J, Perrot A, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia. 2010;24:1852–1858. doi: 10.1038/leu.2010.187. [DOI] [PubMed] [Google Scholar]
- 38.Cho BS, Lee SE, Song HH, et al. Graft-versus-tumor effect according to type of graft-versus-host disease defined by National Institutes of Health consensus criteria and associated outcomes. Biol Blood Marrow Transplant. 2012;18:1136–1143. doi: 10.1016/j.bbmt.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Inamoto Y, Flowers MED, Lee SJ, et al. Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood. 2011;118:456–463. doi: 10.1182/blood-2011-01-330217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 41.Ishiyama K, Takami A, Shiobara S, Koizumi S, Nakao S Kanazawa University Hospital Haematopoietic Stem Cell Transplantation group. Graft-versus-leukemia effect of allogeneic stem cell transplantation; a Japanese single center study. Haematologica. 2004;89:887–889. [PubMed] [Google Scholar]
- 42.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-Host Disease Induced Graft-versus-Leukemia Effect: Greater Impact on Relapse and Disease-Free Survival after Reduced Intensity Conditioning. Biol Blood Marrow Transplant. 2012;18:1727–1733. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]