Abstract
Aphids commonly harbor bacterial facultative symbionts that have a variety of effects upon their aphid hosts, including defense against hymenopteran parasitoids and fungal pathogens. The soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), is infected with the symbiont Arsenophonus sp., which has an unknown role in its aphid host. Our research goals were to document the infection frequency and diversity of the symbiont in field-collected soybean aphids, and to determine whether Arsenophonus is defending soybean aphid against natural enemies. We performed diagnostic PCR and sequenced four Arsenophonus genes in soybean aphids from their native and introduced range to estimate infection frequency and genetic diversity, and found that Arsenophonus infection is highly prevalent and genetically uniform. To evaluate the defensive role of Arsenophonus, we cured two aphid genotypes of their natural Arsenophonus infection through ampicillin microinjection, resulting in infected and uninfected isolines within the same genetic background. These isolines were subjected to parasitoid assays using a recently introduced biological control agent, Binodoxys communis [Braconidae], a naturally recruited parasitoid, Aphelinus certus [Aphelinidae], and a commercially available biological control agent, Aphidius colemani [Braconidae]. We also assayed the effect of the common aphid fungal pathogen, Pandora neoaphidis (Remaudiere & Hennebert) Humber (Entomophthorales: Entomophthoraceae), on the same aphid isolines. We did not find differences in successful parasitism for any of the parasitoid species, nor did we find differences in P. neoaphidis infection between our treatments. Our conclusion is that Arsenophonus does not defend its soybean aphid host against these major parasitoid and fungal natural enemies.
Introduction
Maternally inherited bacterial endosymbionts are common in arthropods [1]–[5]. Many insects are infected with obligate nutritional endosymbionts that are required for survival, e.g. Buchnera aphidicola in aphids [1], [2], [6]. In contrast, facultative endosymbionts are not strictly required for insect survival, but can provide a selective advantage in certain ecological contexts [7]. For example, facultative endosymbionts have been shown to provide their hosts with heat shock resistance [8], modify host color [9], and potentially facilitate host plant colonization [10]. A subset of these facultative endosymbionts can also defend their insect hosts against natural enemies such as parasitoids, entomopathogenic fungi, viruses, and nematodes [11]–[14].
Bacterial symbionts in the genus Arsenophonus are estimated to infect approximately 5% of arthropods [4], [15]. In the parasitoid wasp Nasonia vitripennis, Arsenophonus nasoniae acts as a male killing reproductive parasite [16]–[19]. Other strains are thought to be obligate symbionts of triatomine bugs, hippoboscid and streblid flies, and lice [20]–[22], and yet others are plant pathogens [23]–[25]. Arsenophonus is also found in multiple whitefly, psyllid, and aphid species [26]–[30], but its function among these hosts remains uncharacterized. However, there have been suggestions that Arsenophonus may play a defensive role. In a geographic survey of the lerp psyllid, Glycaspis brimblecombei, Hansen et al. (2007) found a positive correlation between parasitism and the frequency of Arsenophonus infection, potentially indicating that Arsenophonus provides the psyllid with a selective advantage in populations under heavy parasitism pressure [31].
If Arsenophonus provides defense against natural enemies, then it could be an important consideration in biological control programs against Arsenophonus-bearing pests. For example, a defensive symbiont that is present at low prevalence within a population could become common under selective pressure provided by a newly released classical biological control agent, thus undercutting the efficacy of the agent [32], [33]. Alternatively, laboratory populations, which experience vastly different selective environments and frequent population bottlenecks [34], might be expected to have a different frequency of symbiont infection than field populations. In such a case, conclusions about natural enemy efficacy drawn from laboratory studies may have little bearing on natural enemy performance in the field.
Multiple important pest species are infected with Arsenophonus, including the lerp psyllid, the cotton aphid, Aphis gossypii, the sweet potato whitefly, Bemisia tabaci, and the soybean aphid, Aphis glycines [26], [31], [35], [36]. Soybean aphid is a serious invasive pest of soybeans in North Central United States, causing extensive yield loss and requiring intensive pesticide applications to a crop that required little pesticide input prior to the introduction of the soybean aphid [37]. Early parasitism surveys in North America found that soybean aphids were infrequently parasitized [38]–[40], leading to ongoing biological control investigations that incorporate augmentation of ambient fungal pathogens and introduction of parasitoids from the aphid's native range [41]–[43]. The function and prevalence of Arsenophonus in field populations of soybean aphid has the potential to affect these pest management tactics.
The goals of this study were 1) to document the frequency and diversity of Arsenophonus infection in field-collected soybean aphids from the aphids' native and introduced range and 2) to investigate whether Arsenophonus protects soybean aphid against parasitoid wasps or entomopathogenic fungi by assessing natural enemy efficacy against infected versus experimentally cured aphid isolines. For the former goal, we performed Arsenophonus diagnostic PCR on six native and seven introduced populations of soybean aphid, followed by multi-locus strain typing (MLST) of Arsenophonus using 3 bacterial genes [30], [44]. For the latter goal, we assayed three species of parasitoid wasp and one species of fungal pathogen. The first parasitoid species assayed was Binodoxys communis, which currently is the only exotic parasitoid to have been intentionally released in the United States to control the soybean aphid as part of a classical biological control program [42]. The second wasp, Aphelinus certus, has been identified from parasitized North American soybean aphids, although estimates of parasitism rates are still forthcoming. This parasitoid is native to China, was potentially co-introduced with soybean aphid, and is of interest as a biological control agent [45]. The third wasp, Aphidius colemani, is a commercially-available generalist parasitoid of aphids that is known to be susceptible to a defensive symbiont in pea aphid [46]. The aphid fungal pathogen, Pandora neoaphidis, is also known to be susceptible to defensive symbionts in pea aphid, and is being investigated for augmentative biological control of the soybean aphid [12], [47], [48].
Results
Geographic survey
When the prevalence of Arsenophonus in native and introduced populations of the soybean aphid was surveyed, we found that the symbiont was very common in all examined populations (Table 1). In the introduced North American range, a mean (±S.E.) of 98±1% of aphids were infected, which was slightly, but significantly, higher than the 85±6% infection found in the native Asian range (Wald = 2.128, df = 11, P = 0.0334).
Table 1. Soybean aphid, Aphis glycines, collection locations, year collected, collector, and Arsenophonus prevalence.
| Locality | Year | Collector | Arsenophonus positive/Aphids screened |
| Native | |||
| Hebei Province, China | 2008 | Wu Kongming | 8/8 |
| Shangdong Province, China | 2008 | Wu Kongming | 9/10 |
| Guangxi Province, China | 2008 | Wu Kongming | 10/10 |
| Hangzou District, China | 2008 | Wu Kongming | 7/10 |
| Yangling District, China | 2008 | Wu Kongming | 9/10 |
| Harbin Province, China | 2008 | Wu Kongming | 5/8 |
| Introduced | |||
| Whitley Co., Indiana, USA | 2008 | Marc Rhainds | 23/25 |
| Tippecanoe Co., Indiana, USA | 2008 | Marc Rhainds | 10/10 |
| Wabash Co., Indiana, USA | 2008 | Marc Rhainds | 5/5 |
| Huntington Co., Indiana, USA | 2008 | Marc Rhainds | 5/5 |
| Olmsted Co., Minnesota, USA | 2008 | Fritz Breitenbach | 5/5 |
| Waseca Co., Minnesota, USA | 2008 | George Heimpel | 5/5 |
| Fayette Co., Kentucky, USA | 2011 | Jason Wulff | 27/28 |
Arsenophonus MLST
Arsenophonus fbaA, ftsK, yaeT genes were sequenced from one aphid from each of our surveyed populations [30], [44]. We did not detect any genetic variation among sequences from the native and introduced populations, giving no evidence for multiple strains of Arsenophonus within soybean aphid.
Parasitism assays
The influence of Arsenophonus on soybean aphid susceptibility to parasitism was assessed using three different parasitoids. Parasitism by the introduced biological control agent B. communis did not differ significantly between Arsenophonus-infected and experimentally cured aphids of a Kentucky (KY) origin isoline within either a cage assay (t = 0.88, df = 18, P = 0.39), or an observation assay (t = 0.22, df = 22, P = 0.83; Figure 1A). Parasitism of a Minnesota (MN) origin isoline of aphids was substantially lower than the KY isoline, but again did not differ between Arsenophonus-infected and experimentally cured aphids in either the cage assay (t = 0.86, df = 22, P = 0.40), or the observation assay (t = 0.12, df = 22, P = 0.90).
Figure 1. Mean (±SE) proportion of soybean aphids parasitized by Binodoxys communis (A), Aphelinus certus (B), and Aphidius colemani (C).
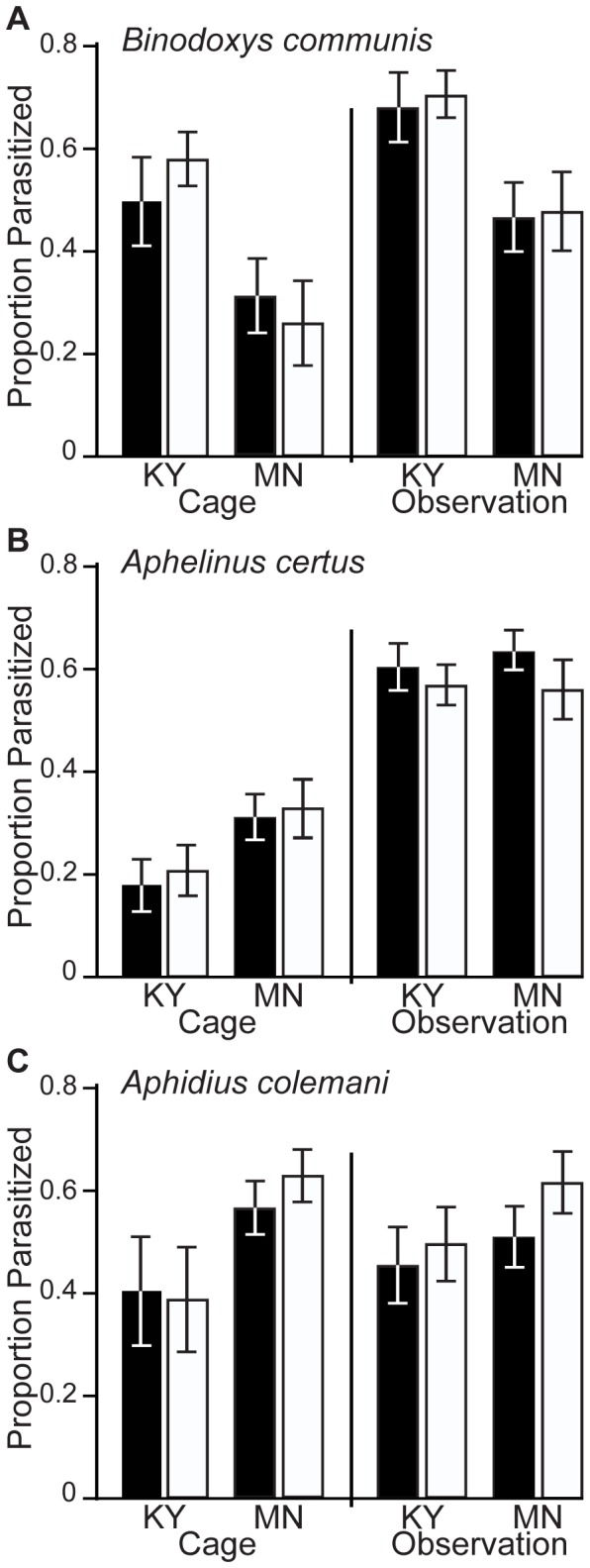
Black bars represent naturally Arsenophonus-infected soybean aphids and white bars represent experimentally cured isolines with the same genetic background. Two isoline pairs (KY and MN) were each evaluated in two experiments (cage and observation assays) for each parasitoid species. No significant differences were detected in any assay.
There were no differences in A. certus parasitism of the KY isoline in the cage assay (t = 0.38, df = 22, P = 0.71) or the observation assay (t = 0.52, df = 20, P = 0.61), nor of the MN isoline in the cage assay (t = 0.02, df = 19, P = 0.98) or the observation assay (t = 0.99, df = 18, P = 0.33; Figure 1B). A. certus had the greatest disparity in performance between the two assays, with very low rates of parasitism for cage assays compared to the observation assays.
There were also no differences in proportion parasitism by A. colemani between infected and experimentally cured soybean aphid for either isoline or parasitism assay (KY cage assay: t = 0.33, df = 20, P = 0.75; KY observation assay: t = 0.29, df = 24, P = 0.77; MN cage assay: t = 0.97, df = 20, P = 0.34; MN observation assay: t = 1.87, df = 18, P = 0.07; Figure 1C).
Fungal assays
In a challenge using the entomopathogenic fungus P. neoaphidis, observed proportions of infection were highly variable, ranging from 0 to 0.76 per replicate. Mean (± SE) proportion P. neoaphidis infection in the Arsenophonus infected and uninfected aphids in the KY isoline were 0.15±0.05 and 0.12±0.06 respectively, and arcsine squareroot transformed values did not differ significantly from one another (t = 0.58, df = 18, P = 0.57). Likewise, Arsenophonus infected and uninfected aphids in the MN isoline had 0.22±0.07 and 0.13±0.05 proportion infected, and again did not differ significantly from one another (t = 1.46, df = 18, P = 0.16).
Discussion
Our primary goal was to assess whether Arsenophonus defends soybean aphid against natural enemies. Using three parasitoid wasp species, we found no evidence that Arsenophonus provides this defense in either of two genotypes of soybean aphid. All three species of parasitoids were able to successfully attack soybean aphid, and there were no significant differences in successful parasitism of Arsenophonus-infected versus cured aphids in either cage or observation assays. Likewise, we found no difference in aphid mortality from the fungus P. neoaphidis based on Arsenophonus infection.
Our aggregated results indicate that Arsenophonus is likely not a defensive symbiont in soybean aphid, but some caveats should be considered. First, we used only two genotypes of aphids, which were infected with the same strain type of Arsenophonus, based on identical Arsenophonus ribosomal and MLST sequences. It is possible that other Arsenophonus strains may provide protection to other genotypes of soybean aphid host. For example, different strains of the bacterial endosymbiont Hamiltonella defensa provide differential protection against parasitism to pea aphid based on the presence or absence and type of APSE phage [49]. Additionally, a strain of Regiella insecticola was recently shown to protect its aphid host against parasitism, a trait not previously associated with the symbiont [46], indicating that bacterial strains can vary in their defensive properties. However, in soybean aphid our broad MLST survey of Arsenophonus did not identify any additional bacterial strains in either the native or introduced range, indicating that hypothetical alternate strain types are rare, if they exist at all. Furthermore, soybean aphid is a recent introduction to North America, and is notably lacking in genetic diversity [50]; consequently, it seems unlikely that additional sampling of aphid/symbiont genotypes in the invaded range would yield different results.
We limited our parasitism assays to wasp species relevant to the North American introduced range of soybean aphid. B. communis and A. certus are both of interest for biological control and represent two different families of parasitoids (Braconidae and Aphelinidae, respectively), the latter being a more generalized parasitoid species [45], [51]. However, there is growing evidence that defensive symbiont-mediated selection can favor parasitoid genotypes that are insensitive to the symbiont [52]. The high prevalence of Arsenophonus infection in the field makes it likely that field-collected parasitoids of soybean aphid have encountered and potentially adapted to the symbiont. A. colemani, the third wasp we assayed, was commercially cultured on other aphid species and presumably naïve to soybean aphid, yet it was also unaffected by Arsenophonus.
Although our results indicate that Arsenophonus does not defend its host against these natural enemies, it does have a very high infection rate in both the introduced and native populations. Several possible explanations could underlie this widespread infection. First, Arsenophonus could manipulate host reproduction. Reproductive manipulation is a common means by which endosymbionts promote their own infection, and has recently been documented in the sexual generation of pea aphid by the endosymbiont Spiroplasma [53], [54]. Second, Arsenophonus could be providing other context-specific benefits to soybean aphid, e.g. heat tolerance, defense against other pathogens [8], [14], or general fecundity or longevity effects [55]. Third, Arsenophonus may be transmitted horizontally, either directly between aphids or indirectly through the plant [56], [57]. Finally, high fidelity vertical transmission, coupled with a very low metabolic cost to the host, could permit Arsenophonus to persist in a population without any benefit to the host [58]. However, other endosymbionts that had been considered previously to be neutral passengers were subsequently found to be extremely beneficial to their hosts under certain circumstances [13], [59]. Given the very high prevalence of Arsenophonus in soybean aphid, it is therefore reasonable to presume that Arsenophonus, too, provides soybean aphid with a context-specific benefit that remains to be elucidated.
Materials and Methods
Geographic survey
To evaluate the prevalence of Arsenophonus, soybean aphids were collected from the Asian native range and North American invasive range. Collections were made either at university agricultural stations or on private lands with landowner permission (Table 1). For each population, 30 adult aphids were collected from plants at least 1 meter apart to minimize sampling of siblings, and immediately placed in 95% ethanol. Five aphids were selected at random from each introduced range population and ten aphids were selected from each native range population for molecular analysis. We extracted DNA by homogenizing individual aphids in 100 µl of 10% w/v Chelex (Sigma-Aldrich, St Louis, MO, USA) in PCR-grade purified water. We added 6 µl of proteinase K to each sample, vortexed, incubated overnight at 56°C, and then incubated samples at 96°C for ten minutes. We screened for the presence of Arsenophonus using a diagnostic PCR protocol modified from Thao and Baumann [26], which uses Arsenophonus specific primers to amplify the intervening region between 16S and 23S rDNA: Ars23S-1 (5′-CGTTTGATG ATTCATAGTCAAA-3′) and Ars23S-2 (5′-GGTCCTCCAGTTAGTGTTACCCAAC -3′). Reactions totaled 10 µl, containing: 2.0 µl of DNA template, 1.0 µl of 25 mM MgCl2, 1.0 µl of 10 mM dNTP mixture, 1.0 µl of Invitrogen 10× buffer (MgCl2 free), 0.8 µl of 5.0 pmole µl−1 of each primer, 0.1 µl of 5 U/ µl Invitrogen Taq polymerase, and ddH2O to 10 µl. PCR conditions were: initial denature at 95°C for 5 min; followed by 30 cycles of (95°C, 30 s; 55°C, 30 s; 72°C, 45 s); and final elongation at 70°C for 10 min. All PCRs included negative and positive controls. Product from multiple samples was sequenced to confirm Arsenophonus. All sequences were identical and the shared sequence was submitted to Genbank (Accession number KC019882). As a further control of extraction quality, we ran samples with the primers CAIF (5′-GCCTGATGCAGCCATGCCGCGTGTATG-3′) and CAIR (5′-GTCATCCCCACCTTCC-3′) with the same PCR conditions as previously listed. These primers were developed by Dale et al. [60] to target Arsenophonus 16S sequence in the hippoboscid fly, Pseudolynchia canariensis. However, they reliably detected 16S sequence from the obligate symbiont Buchnera aphidicola in soybean aphid, as confirmed by sequencing results (Accession number KC019881). Because this obligate symbiont should be present in all extractions, any samples that failed to amplify B. aphidicola were considered to be of poor quality and discarded. To compare Arsenophonus infection prevalence between the native and introduced ranges, we used logistic regression (Arc v. 1.06). To avoid overrepresentation of heavily sampled geographic regions, aphids collected from within the same county were considered to come from a single population, and pooled prior to statistical analysis.
MLST
We investigated potential genetic diversity in Arsenophonus using an MLST approach. We randomly selected a single extraction from each native and introduced population (Table 1), as well as from our two experimental colonies (KY and MN). We amplified DNA from each sample with the following primer sets: fbaAf (5′-GCYGCYAAAGTTCRTTCCC-3′) and fbaAr2 (5′-GGCAAATTAAATTTCTGCGCAACG-3′), ftsKf (5′-GTTGTYATGGTYGATGAATTTGC-3′) and ftsKr (5′-GCTCTTCATCACYTCAWAACC-3′), yaeTf (5′-GCATACGGTTCAGACGGGTTTG-3′) and yaeTr (5′-GCCGAAACGCCTTCAGA AAAG-3′).The PCR reaction recipe followed the protocol above and PCR conditions were: initial denature at 93°C for 3 min; 30 cycles of (93°C, 30 s; 52°C, 30 s; 72°C, 1 min); and final elongation at 72°C for 5 min [30], [44]. Because sequences generated from each population were identical for each of the genes, fbaA, ftsK, and yaeT, a single sequence per gene was submitted to Genbank (KC701199, KC701198, KC701197).
Arsenophonus curing and colony maintenance
We used two soybean aphid clones for experimental manipulations. These clones were collected independently of the geographic survey specimens. One aphid clone, "KY", was initially collected in Fayette County, KY in 2009.The second clone, "MN", was originally collected in Ramsey County, MN and was maintained in culture at the University of Minnesota prior to transfer to Kentucky in 2010 (USDA Permit # P526P-10-00818). In addition to Arsenophonus, each aphid clone was screened diagnostically for other known bacterial symbionts of aphids [29], and examined for total bacterial diversity using denaturing gradient gel electrophoresis (DGGE) of bacterial 16S sequences [61]. The only bacterial endosymbionts detected were Arsenophonus and Buchnera (J. Wulff, unpublished data).
We cured these aphid clones of Arsenophonus infection using antibiotic microinjection, following a protocol modified from Oliver et al. [11]. Individual aphids from each clone were immobilized on a screen-covered pipette tip attached to vacuum, under a stereo microscope. Antibiotic was fed into a borosilicate microinjection needle attached to a syringe via tubing. Fourth-instar aphids were injected with 1.0 mg ml−1 ampicillin solution [62]. Arsenophonus is susceptible to ampicillin, but the aphid's primary symbiont, Buchnera aphidicola, is not [63]. After the initial injection, aphids were individually placed on excised soybean leaves maintained on 1% w/v agar, monitored for survivors, and a subset of offspring were checked for Arsenophonus via diagnostic PCR. This procedure was repeated for two subsequent generations using offspring of survivors from the previous bout of injections. Cured and infected isoline colonies were kept at 25± 1°C and 16L:8D on Asgrow® G4303 variety commercial soybeans in 10 cm pots. Plants were individually caged in 3.78 liter plastic jars that had panels of mesh to allow ventilation while preventing aphid escape. Aphids were transferred to new plants as needed, approximately twice per month, to avoid overcrowding and prevent alate production. All aphid isolines were maintained in this manner for at least 3 months prior to experiments. Five individuals from each soybean aphid isoline were screened with diagnostic PCR at least every 2 months to assure that the isoline retained the expected infection status. The cured aphid isolines never tested positive for Arsenophonus.
Parasitism assays
We evaluated the influence of Arsenophonus in soybean aphid on parasitism success by three parasitoid wasp species. The classical biological control agent Binodoxys communis was initially collected in August 2002 near Harbin, in the Chinese province of Heilongjaing, and was maintained in quarantine in St. Paul, Minnesota prior to initiation of our colony in Kentucky (USDA-APHIS permit P526P-10-01532) [64]. Aphelinus certus was collected locally in Lexington, KY in August 2010 from parasitized soybean aphids. Aphidius colemani is a commercially available biological control agent of aphids (APHIPAR, Koppert Biological Systems, The Netherlands). Each species of parasitoid was maintained in culture with Arsenophonus-cured soybean aphids and soybean plants at 25 ±1°C and 16L:8D in the previously described culture jars with supplemental honey and water for at least two generations prior to use in parasitism assays.
Cage parasitism assays
We conducted cage parasitism assays using methodology adapted from Oliver et al. [11]. For each Arsenophonus infected/cured isoline pair, we assayed parasitism success by each of the three parasitoid species in separate experiments (6 assays total). For each assay, 12 vegetative stage 2 (V2) soybean plants were infested with Arsenophonus-infected aphids and 12 V2 soybean plants were infested with Arsenophonus-cured aphids. We transferred a leaf with >100 juvenile aphids to each experimental plant. Experimental plants were covered with cup cages, constructed from 947 ml translucent plastic containers, organza screening material, and weather stripping to provide a tight seal between cage and pot. After allowing 24 h for aphid establishment, we culled the aphids to either 30 aphids (A. certus assays), or 50 aphids (B. communis and A. colemani assays). B. communis and A. certus assays were conducted primarily with 2nd and 3rd instar aphids, whereas A. colemani assays were conducted primarily with 3rd and 4th instar aphids [64], [65]. A single mated female wasp was introduced to each cup cage and removed after 24 h. If the wasp was dead or missing after this interval, the replicate was discarded. After 10 d, parasitized aphids (mummies) were counted, and proportion parasitism was calculated by dividing the number of mummies observed by the initial aphid number for that replicate. For each assay, the effect of aphid infection status on proportion parasitism was assessed using a t-test (IBM SPSS v20). Proportion data required an arcsine square-root transformation to satisfy the assumptions of the model.
Observation assays
Six observation assays were conducted in parallel to the cage assays, using the same three parasitoid species and two aphid genotypes. For each experiment, soybean leaves infested with either Arsenophonus-infected or cured aphids of all instars were embedded, adaxial side, in 1% agar in 100×15 mm petri dishes. Five to ten wasps of the same species were aspirated onto the embedded leaf. Wasps were allowed to settle and then culled to four actively parasitizing wasps. Wasps were observed continuously under a dissecting microscope. When oviposition was observed, each stung aphid was moved to a 35 mm leaf disk embedded in 1% agar, until a total of 10–15 aphids were parasitized, constituting a replicate. This procedure was repeated with fresh wasps until 10 replicates were generated per treatment per assay.
We regularly removed aphid progeny from leaf disks to avoid confusing progeny with the original stung aphids. Wasp mummies typically formed within 5–7 days, regardless of the parasitoid species. On day 10, we calculated proportion parasitism by dividing the number of mummies by the number of aphids that had survived until just prior to mummy formation. Aphids that died prior to day 5 were excluded from the data. Proportions were arcsine square-root transformed and analyzed using a t-test for each assay.
Fungal assays
To assess the effect of Arsenophonus infection status on soybean aphid susceptibility to the entomophthoralean fungus P. neoaphidis, we conducted bioassays of Arsenophonus-infected versus cured aphids using the same two aphid genotypes as the parasitism assays. For each replicate, we transferred 25, 3rd–4th instar alatoid nymphs to a 100×15 mm, sterile, polystyrene petri dish containing moistened filter paper and an excised soybean leaflet (variety S19R5; NK, Golden Valley, MN). The petiole of each leaflet was placed in moist florist foam to prevent leaflet desiccation. To measure aphid exposure to fungal conidia, a glass cover slip was attached to each leaflet to allow for enumeration of conidia after aphid exposure to cultures.
We initiated a total of 20 replicates for each aphid isoline pair, 10 each from the infected and cured isolines. We used actively sporulating P. neoaphidis cultures to inoculate aphids. Subcultures used in the assays had been established 30–40 days prior to use and were only used after sporulation became evident (i.e., when conidia became visible on culture lids). All fungal cultures originated from the same P. neoaphidis isolate, which had been initially isolated from an infected, field-collected pea aphid (Acyrthosiphon pisum). The field collected isolate was used to infect soybean aphids in the laboratory, after which, the fungus was recovered from a single infected soybean aphid. The resulting isolate was periodically passed through and recovered from single soybean aphid individuals prior to use in the assays. Such periodic infection and recovery was necessary to maintain culture pathogenicity. Cultures used to infect soybean aphids in these assays originated from a single culture recovered from an infected soybean aphid immediately prior to assay initiation. The P. neoaphidis isolate has been deposited in the USDA, Agricultural Research Service's Collection of Entomopathogenic Fungal Cultures (ARSEF 11663).
Fungal cultures were inverted over each soybean aphid replicate. After 2 h, the fungal cultures and coverslip were removed from each replicate, and the dishes were sealed with parafilm to maintain the humidity required for fungal disease initiation. Each cover slip was stained with aceto-orcein stain, and examined at 200× magnification. Spores had been deposited on all, indicating that all replicates were exposed to fungal conidia. We then counted spores in 10 randomly chosen fields of view per replicate, and calculated mean spore number per field as an estimate of fungal exposure.
We examined the aphids once per day over the next 5 days. Dead or apparently infected aphids were removed from the experimental dish and transferred to a 50 mm tissue culture dish containing 1% water agar to induce sporulation. If sporulation occurred, the aphid was considered to be infected. We confirmed fungal species identity for two aphids exhibiting successful sporulation on each of the 5 days that assays were monitored. Conidia were stained with aceto-orcein stain and species identity was confirmed via spore morphology at 200× magnification [66].
We calculated the proportion of aphids infected per replicate, and used Pearson's correlation coefficient to determine whether this value was significantly associated with fungal exposure per replicate. We observed substantial variation in both variables, but they were not strongly correlated (R = 0.067, P = 0.72), so we proceeded to compare fungal infection between treatments without including fungal exposure as a covariate. We arcsine square-root transformed the proportion of aphids infected by P. neoaphidis, and performed t-tests (IBM SPSS v20) to determine whether this proportion differed as a function of Arsenophonus presence/absence in either aphid isoline.
Acknowledgments
We thank J. McCord for laboratory assistance, J. Obrycki and R. Perry for comments on earlier drafts of this manuscript, and R. Bessin for providing soybean seed. This is publication 13-08-029 of the Kentucky Agricultural Experiment Station. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Funding Statement
This research was supported in part by a grant from the Kentucky Science and Engineering Foundation as per Grant Agreement #KSEF-148-502-09-248 with the Kentucky Science and Technology Corporation, USDA-AFRI grant # 2011-67014-3015, and the University of Kentucky. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.hner P (1965) Endosymbiosis of animals with plant microorganisms. Interscience, New York.
- 2. glas AE (1998) Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera . Ann Rev of Entom 43: 17–37. [DOI] [PubMed] [Google Scholar]
- 3. dstrom JP, Russell JA, White JP, Moran NA (2001) Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol Ecol 10: 217–228. [DOI] [PubMed] [Google Scholar]
- 4. Duron O, Bouchon D, Boutin S, Bellamy L, Zhou LQ, et al. (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia? - a statistical analysis of current data. FEMS Microbiol Lett 281: 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, et al. (2002) Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia . Nat Genet 32: 402–407. [DOI] [PubMed] [Google Scholar]
- 7. Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55: 247–266. [DOI] [PubMed] [Google Scholar]
- 8. Russell JA, Moran NA (2006) Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc Biol Sci 273: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, et al. (2010) Symbiotic bacterium modifies aphid body color. Science 330: 1102–1104. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari J, Scarborough CL, Godfray HCJ (2007) Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 153: 323–329. [DOI] [PubMed] [Google Scholar]
- 11. Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci U S A 100: 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scarborough CL, Ferrari J, Godfray HCJ (2005) Aphid protected from pathogen by endosymbiont. Science 310: 1781–1781. [DOI] [PubMed] [Google Scholar]
- 13. Hedges LM, Brownlie JC, O'Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322: 702–702. [DOI] [PubMed] [Google Scholar]
- 14. Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329: 212–215. [DOI] [PubMed] [Google Scholar]
- 15. Novakova E, Hypsa V, Moran NA (2009) Arsenophonus, an emerging clade of intracellular symbionts with a broad host distribution. BMC Microbiol 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huger AM, Skinner SW, Werren JH (1985) Bacterial infections associated with the son-killer trait in the parasitoid wasp, Nasonia ( = Mormoniella) vitripennis (Hymenoptera, Pteromalidae). J Invertebr Pathol 46: 272–280. [DOI] [PubMed] [Google Scholar]
- 17. Werren JH, Skinner SW, Huger AM (1986) Male-killing bacteria in a parasitic wasp. Science 231: 990–992. [DOI] [PubMed] [Google Scholar]
- 18. Gherna RL, Werren JH, Weisburg W, Cote R, Woese CR, et al. (1991) Arsenophonus nasoniae gen-nov, sp-nov, the causative agent of the son-killer trait in the parasitic wasp Nasonia vitripennis. . Int J Syst Bacteriol 41: 563–565. [Google Scholar]
- 19. Duron O, Wilkes TE, Hurst GD (2010) Interspecific transmission of a male-killing bacterium on an ecological timescale. Ecol Lett 13: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 20. Hypsa V (1993) Endocytobionts of Triatoma infestan- distribution and transmission. J Invertebr Pathol 61: 32–38. [Google Scholar]
- 21. Trowbridge RE, Dittmar K, Whiting MF (2006) Identification and phylogenetic analysis of Arsenophonus- and Photorhabdus-type bacteria from adult Hippoboscidae and Streblidae (Hippoboscoidea). J Invertebr Pathol 91: 64–68. [DOI] [PubMed] [Google Scholar]
- 22. Perotti MA, Allen JM, Reed DL, Braig HR (2007) Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J 21: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 23. Zreik L, Bove JM, Garnier M (1998) Phylogenetic characterization of the bacterium-like organism associated with marginal chlorosis of strawberry and proposition of a Candidatus taxon for the organism, 'Candidatus Phlomobacter fragariae' . Int J Syst Bacteriol 48: 257–261. [DOI] [PubMed] [Google Scholar]
- 24. Bressan A, Semetey O, Arneodo J, Lherminier J, Boudon-Padieau E (2009) Vector transmission of a plant-pathogenic bacterium in the Arsenophonus clade sharing ecological traits with facultative insect endosymbionts. Phytopathology 99: 1289–1296. [DOI] [PubMed] [Google Scholar]
- 25. Bressan A, Terlizzi F, Credi R (2012) Independent origins of vectored plant pathogenic bacteria from arthropod-associated Arsenophonus endosymbionts. Microb Ecol 63: 628–638. [DOI] [PubMed] [Google Scholar]
- 26. Thao MLL, Baumann P (2004) Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr Microbiol 48: 140–144. [DOI] [PubMed] [Google Scholar]
- 27. Subandiyah S, Nikoh N, Tsuyumu S, Somowiyarjo S, Fukatsu T (2000) Complex endosymbiotic microbiota of the citrus psyllid Diaphorina citri (Homoptera: Psylloidea). Zoolog Sci 17: 983–989. [Google Scholar]
- 28. Thao ML, Clark MA, Baumann L, Brennan EB, Moran NA, et al. (2000) Secondary endosymbionts of psyllids have been acquired multiple times. Curr Microbiol 41: 300–304. [DOI] [PubMed] [Google Scholar]
- 29. Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA (2003) Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12: 1061–1075. [DOI] [PubMed] [Google Scholar]
- 30. Jousselin E, Coeur d'acier A, Vanlerberghe-Masutti F, Duron O (2013) Evolution and diversity of Arsenophonus endosymbionts in aphids. Mol Ecol 22: 260–270. [DOI] [PubMed] [Google Scholar]
- 31. Hansen AK, Jeong G, Paine TD, Stouthamer R (2007) Frequency of secondary symbiont infection in an invasive psyllid relates to parasitism pressure on a geographic scale in California. Appl Environ Microbiol 73: 7531–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clay K, Holah J, Rudgers JA (2005) Herbivores cause a rapid increase in hereditary symbiosis and alter plant community composition. Proc Natl Acad Sci U S A 102: 12465–12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oliver KM, Campos J, Moran NA, Hunter MS (2008) Population dynamics of defensive symbionts in aphids. Proc Biol Sci 275: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heimpel GE, Lundgren JG (2000) Sex ratios of commercially reared biological control agents. Biol. Control 19: 77–93. [Google Scholar]
- 35. Carletto J, Gueguen G, Fleury F, Vanlerberghe-Masutti F (2008) Screening the bacterial endosymbiotic community of sap-feeding insects by terminal-restriction fragment length polymorphism analysis. Entomol Exp Appl 129: 228–234. [Google Scholar]
- 36. Wille BD, Hartman GL (2009) Two species of symbiotic bacteria present in the soybean aphid (Hemiptera: Aphididae). Environ Entomol 38: 110–115. [DOI] [PubMed] [Google Scholar]
- 37. Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of soybean aphid in North America. Annu Rev Entomol 56: 375–399. [DOI] [PubMed] [Google Scholar]
- 38. Costamagna AC, Landis DA, Brewer MJ (2008) The role of natural enemy guilds in Aphis glycines suppression. Biol Control 45: 368–379. [Google Scholar]
- 39. Kaiser ME, Noma T, Brewer MJ, Pike KS, Vockeroth JR, et al. (2007) Hymenopteran parasitoids and dipteran predators found using soybean aphid after its midwestern United States invasion. Ann Entomol Soc Am 100: 196–205. [Google Scholar]
- 40. Noma T, Brewer MJ (2008) Seasonal abundance of resident parasitoids and predatory flies and corresponding soybean aphid densities, with comments on classical biological control of soybean aphid in the Midwest. J Econ Entomol 101: 278–287. [DOI] [PubMed] [Google Scholar]
- 41. Heimpel GE, Ragsdale DW, Venette R, Hopper KR, O'Neil RJ, et al. (2004) Prospects for importation biological control of the soybean aphid: Anticipating potential costs and benefits. Ann Entomol Soc Am 97: 249–258. [Google Scholar]
- 42. Wyckhuys KAG, Koch RL, Kula RR, Heimpel GE (2009) Potential exposure of a classical biological control agent of the soybean aphid, Aphis glycines, on non-target aphids in North America. Biol Invasions 11: 857–871. [Google Scholar]
- 43. Nielsen C, Hajek AE (2005) Control of invasive soybean aphid, Aphis glycines (Hemiptera: Aphididae), populations by existing natural enemies in New York State, with emphasis on entomopathogenic fungi. Environ Entomol 34: 1036–1047. [Google Scholar]
- 44.Wilkes T, Duron O, Darby AC, Hypsa V, Novakova E, Hurst GDD (2011) The genus Arsenophonus. In: Bourtzis K, Zchori-Fein E, editors. Manipulative Tenants. Boca Raton, Florida: CRC Press.225–244.
- 45. Heimpel GE, Frelich LE, Landis DA, Hopper KR, Hoelmer KA, et al. (2010) European buckthorn and Asian soybean aphid as components of an extensive invasional meltdown in North America. Biol Invasions 12: 2913–2931. [Google Scholar]
- 46. Vorburger C, Gehrer L, Rodriguez PA (2009) A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol Lett 6: 109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lukasik P, van Asch M, Guo HF, Ferrari J, Godfray HCJ (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16: 214–218. [DOI] [PubMed] [Google Scholar]
- 48. Koch KA, Ragsdale DW (2011) Impacts of thiamethoxam seed treatment and host plant resistance on the soybean aphid fungal pathogen, Pandora neoaphidis . J Econ Entomol 104: 1824–1832. [DOI] [PubMed] [Google Scholar]
- 49. Oliver KM, Degnan PH, Hunter MS, Moran NA (2009) Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325: 992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Michel AP, Zhang W, Jung JK, Kang ST, Mian MAR (2009) Population genetic structure of Aphis glycines . Environ Entomol 38: 1301–1311. [DOI] [PubMed] [Google Scholar]
- 51. Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009) Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160: 387–398. [DOI] [PubMed] [Google Scholar]
- 52. Vorburger C, Sandrock C, Gouskov A, Castaneda LE, Ferrari J (2009) Genotypic variation and the role of defensive endosymbionts in an all-parthenogenetic host-parasitoid interaction. Evolution 63: 1439–1450. [DOI] [PubMed] [Google Scholar]
- 53. Engelstadter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40: 127–149. [Google Scholar]
- 54. Simon JC, Boutin S, Tsuchida T, Koga R, Le Gallic JF, et al. (2011) Facultative symbiont infections affect aphid reproduction. PLoS One 6: e21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, et al. (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332: 254–256. [DOI] [PubMed] [Google Scholar]
- 56. Moran NA, Dunbar HE (2006) Sexual acquisition of beneficial symbionts in aphids. Proc Natl Acad Sci U S A 103: 12803–12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Caspi-Fluger A, Inbar M, Mozes-Daube N, Katzir N, Portnoy V, et al. (2012) Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc Biol Sci 279: 1791–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoffmann AA, Hercus M, Dagher H (1998) Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster . Genetics 148: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, et al. (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dale C, Beeton M, Harbison C, Jones T, Pontes M (2006) Isolation, pure culture, and characterization of "Candidatus Arsenophonus arthropodicus," an intracellular secondary endosymbiont from the hippoboscid louse fly Pseudolynchia canariensis . Appl Environ Microbiol 72: 2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Russell JA, Weldon S, Smith AH, Kim KL, Hu Y, et al. (2013) Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol Ecol 22: 2045–59. [DOI] [PubMed] [Google Scholar]
- 62. Ruan YM, Xu J, Liu SS (2006) Effects of antibiotics on fitness of the B biotype and a non-B biotype of the whitefly Bemisia tabaci . Entomol Exp Appl 121: 159–166. [Google Scholar]
- 63. Griffiths GW, Beck SD (1974) Effects of antibiotics on intracellular symbiotes in the pea aphid, Acyrthosiphon pisum . Cell Tissue Res 148: 287–300. [DOI] [PubMed] [Google Scholar]
- 64. Wyckhuys KAG, Stone L, Desneux N, Hoelmer KA, Hopper KR, Heimpel GE (2008) Parasitism of the soybean aphid, Aphis glycines, by Binodoxys communis: the role of aphid defensive behaviour and parasitoid reproductive performance. Bull Entomol Res 98: 361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin LA, Ives AR (2003) The effect of parasitoid host-size preference on host population growth rates: an example of Aphidius colemani and Aphis glycines . Ecol Entomol 28: 542–550. [Google Scholar]
- 66.Samson RA, Evans HC, Latgé JP (1988) Atlas of entomopathogenic fungi. The Netherlands: Springer-Verlag.


