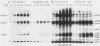Abstract
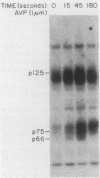
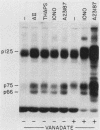
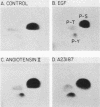
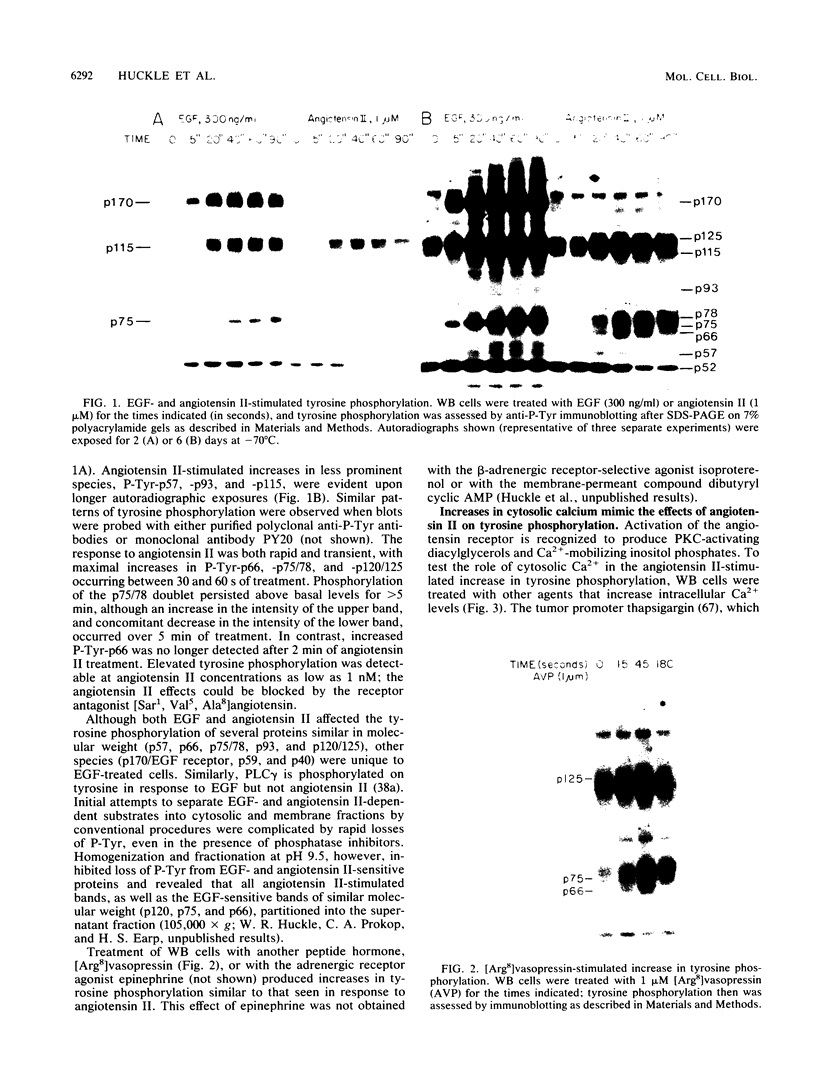
Cellular responses to epidermal growth factor (EGF) are dependent on the tyrosine-specific protein kinase activity of the cell-surface EGF receptor. Previous studies using WB rat liver epithelial cells have detected at least 10 proteins whose phosphotyrosine (P-Tyr) content is increased by EGF. In this study, we have examined alternate modes of activating tyrosine phosphorylation. Treatment of WB cells with hormones linked to Ca2+ mobilization and protein kinase C (PKC) activation, including angiotensin II, [Arg8]vasopressin, or epinephrine, stimulated rapid (less than or equal to 15-s) and transient increases in the P-Tyr content of several proteins (p120/125, p75/78, and p66). These proteins, detected by anti-P-Tyr immunoblotting, were similar in molecular weight to a subset of EGF-sensitive P-Tyr-containing proteins (P-Tyr-proteins). The increased P-Tyr content was confirmed by [32P]phosphoamino acid analysis of proteins recovered by anti-P-Tyr immunoprecipitation. Elevating intracellular [Ca2+] with the ionophore A23187 or ionomycin or with the tumor promoter thapsigargin mimicked the effects of hormones on tyrosine phosphorylation, whereas treatment with a PKC-activating phorbol ester did not. In addition, responses to angiotensin II were not diminished in PKC-depleted cells. Ca2+ mobilization, measured by fura-2 fluorescence, was coincident with the increase in tyrosine phosphorylation in response to angiotensin II or thapsigargin. Loading cells with the intracellular Ca2+ chelator bis-(o-aminophenoxy)ethane-N ,N ,N' , N'-tetraacetic acid (BAPTA) inhibited the appearance of all P-Tyr-proteins in response to angiotensin II, thapsigargin, or ionophores, as well as two EGF-stimulated P-Tyr-proteins. The majority of EGF-stimulated P-Tyr-proteins were not affected by BAPTA. These studies indicate that angiotensin II can alter protein-tyrosine phosphorylation in a manner that is secondary to, and apparently dependent on, Ca2+ mobilization. Thus, ligands such as EGF and angiotensin II, which act through distinct types of receptors, may activate secondary pathways involving tyrosine phosphorylation. These results also raise the possibility that certain growth-promoting effects of Ca2+ -mobilizing agents such as angiotensin II may be mediated via tyrosine phosphorylation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Ben-Ari E. T., Garrison J. C. Regulation of angiotensinogen mRNA accumulation in rat hepatocytes. Am J Physiol. 1988 Jul;255(1 Pt 1):E70–E79. doi: 10.1152/ajpendo.1988.255.1.E70. [DOI] [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Vekshtein V., Gordon H. M., Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989 Apr;13(4):305–314. doi: 10.1161/01.hyp.13.4.305. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Dodson R. W., Kraft A. S. Human neutrophils contain distinct cytosolic and particulate tyrosine kinase activities: possible role in neutrophil activation. Biochim Biophys Acta. 1989 Aug 31;997(3):292–301. doi: 10.1016/0167-4838(89)90200-8. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Bobik A., Grinpukel S., Little P. J., Grooms A., Jackman G. Angiotensin II and noradrenaline increase PDGF-BB receptors and potentiate PDGF-BB stimulated DNA synthesis in vascular smooth muscle. Biochem Biophys Res Commun. 1990 Jan 30;166(2):580–588. doi: 10.1016/0006-291x(90)90848-h. [DOI] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Stimulation and inhibition of growth by EGF in different A431 cell clones is accompanied by the rapid induction of c-fos and c-myc proto-oncogenes. EMBO J. 1985 May;4(5):1193–1197. doi: 10.1002/j.1460-2075.1985.tb03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Schwinn D. A., Randall R. R., Lefkowitz R. J., Caron M. G., Kobilka B. K. Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7159–7163. doi: 10.1073/pnas.85.19.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S., Ralston R., Alitalo K., Bishop J. M. Subcellular location of an abundant substrate (p36) for tyrosine-specific protein kinases. Mol Cell Biol. 1983 Mar;3(3):340–350. doi: 10.1128/mcb.3.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise J. L., Houck K. A., Michalopoulos G. K. Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science. 1985 Feb 15;227(4688):749–751. doi: 10.1126/science.2982212. [DOI] [PubMed] [Google Scholar]
- Cutry A. F., Kinniburgh A. J., Krabak M. J., Hui S. W., Wenner C. E. Induction of c-fos and c-myc proto-oncogene expression by epidermal growth factor and transforming growth factor alpha is calcium-independent. J Biol Chem. 1989 Nov 25;264(33):19700–19705. [PubMed] [Google Scholar]
- De B. K., Misono K. S., Lukas T. J., Mroczkowski B., Cohen S. A calcium-dependent 35-kilodalton substrate for epidermal growth factor receptor/kinase isolated from normal tissue. J Biol Chem. 1986 Oct 15;261(29):13784–13792. [PubMed] [Google Scholar]
- Earp H. S., Hepler J. R., Petch L. A., Miller A., Berry A. R., Harris J., Raymond V. W., McCune B. K., Lee L. W., Grisham J. W. Epidermal growth factor (EGF) and hormones stimulate phosphoinositide hydrolysis and increase EGF receptor protein synthesis and mRNA levels in rat liver epithelial cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1988 Sep 25;263(27):13868–13874. [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Wallace M. A., Wojcikiewicz R. J. Evidence for involvement of guanine nucleotide-binding regulatory proteins in the activation of phospholipases by hormones. FASEB J. 1988 Jul;2(10):2569–2574. doi: 10.1096/fasebj.2.10.2838362. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Platelet tyrosine-specific protein phosphorylation is regulated by thrombin. Mol Cell Biol. 1988 Sep;8(9):3603–3610. doi: 10.1128/mcb.8.9.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
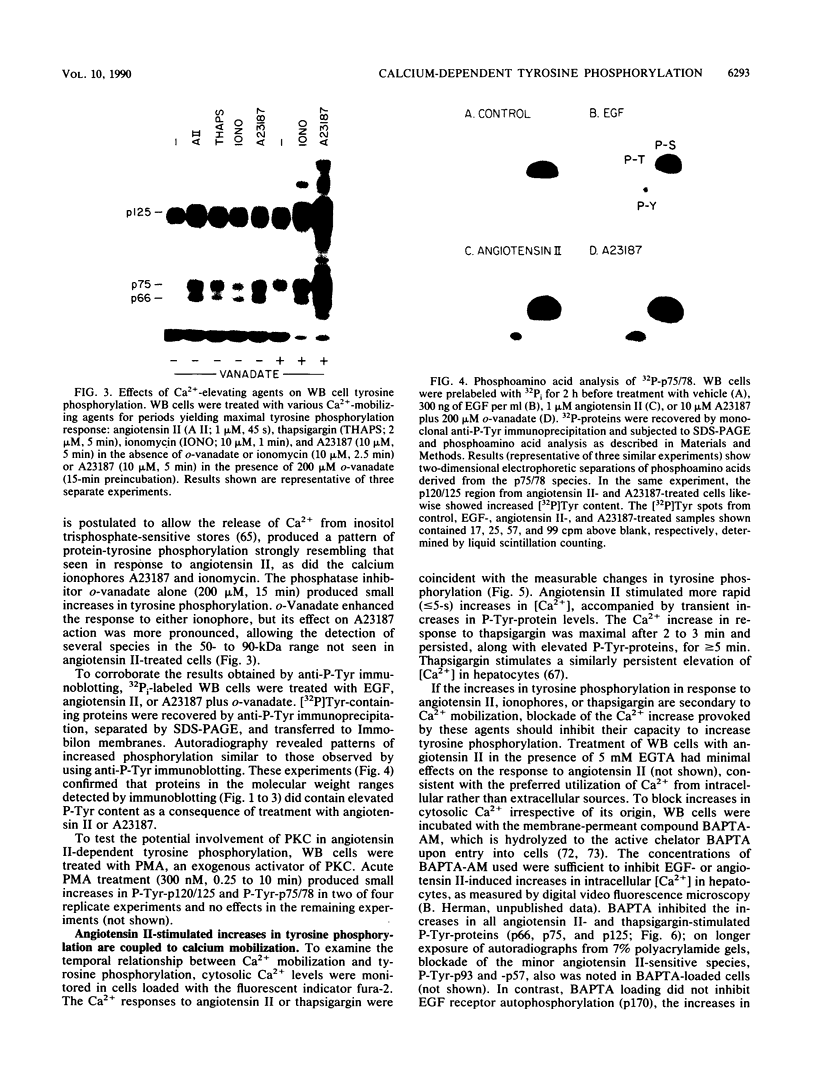
- Ferris D. K., Willette-Brown J., Martensen T., Farrar W. L. Interleukin 3 and phorbol ester stimulate tyrosine phosphorylation of overlapping substrate proteins. FEBS Lett. 1989 Mar 27;246(1-2):153–158. doi: 10.1016/0014-5793(89)80273-x. [DOI] [PubMed] [Google Scholar]
- Frohlich E. D., Iwata T., Sasaki O. Clinical and physiologic significance of local tissue renin-angiotensin systems. Am J Med. 1989 Dec 26;87(6B):19S–23S. doi: 10.1016/0002-9343(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Owens G. K. Arginine vasopressin-induced hypertrophy of cultured rat aortic smooth muscle cells. Hypertension. 1989 Oct;14(4):413–420. doi: 10.1161/01.hyp.14.4.413. [DOI] [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Ill C. R., Simonian M. H. Angiotensin stimulation of bovine adrenocortical cell growth. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5569–5573. doi: 10.1073/pnas.74.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. Two related but distinct forms of the Mr 36,000 tyrosine kinase substrate (calpactin) that interact with phospholipid and actin in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4258–4262. doi: 10.1073/pnas.83.12.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A., Brugge J. S. Thrombin treatment induces rapid changes in tyrosine phosphorylation in platelets. Proc Natl Acad Sci U S A. 1989 Feb;86(3):901–905. doi: 10.1073/pnas.86.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Huang C. K., Bonak V. A., Wang E., Casnellie J. E., Shiraishi T., Sha'afi R. I. Tyrosine phosphorylation in human neutrophil. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1478–1485. doi: 10.1016/0006-291x(89)90841-3. [DOI] [PubMed] [Google Scholar]
- Granot Y., Van Putten V., Schrier R. W. Vasopressin dependent tyrosine phosphorylation of a 38 kDa protein in human platelets. Biochem Biophys Res Commun. 1990 Apr 30;168(2):566–573. doi: 10.1016/0006-291x(90)92358-7. [DOI] [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Socorro L., Tsuda T., Delafontaine P., Alexander R. W. Secondary signalling mechanisms in angiotensin II-stimulated vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 1988 Feb;15(2):105–112. doi: 10.1111/j.1440-1681.1988.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hakii H., Fujiki H., Suganuma M., Nakayasu M., Tahira T., Sugimura T., Scheuer P. J., Christensen S. B. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111(3):177–181. doi: 10.1007/BF00389230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanley M. R. Growth factors. Neuropeptides as mitogens. Nature. 1985 May 2;315(6014):14–15. doi: 10.1038/315014a0. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Hepler J. R., Jeffs R. A., Huckle W. R., Outlaw H. E., Rhee S. G., Earp H. S., Harden T. K. Evidence that the epidermal growth factor receptor and non-tyrosine kinase hormone receptors stimulate phosphoinositide hydrolysis by independent pathways. Biochem J. 1990 Sep 1;270(2):337–344. doi: 10.1042/bj2700337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. K., Laramee G. R., Casnellie J. E. Chemotactic factor induced tyrosine phosphorylation of membrane associated proteins in rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1988 Mar 15;151(2):794–801. doi: 10.1016/s0006-291x(88)80351-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Blair L. A., Marshall J., Goedert M., Hanley M. R. The mas oncogene encodes an angiotensin receptor. Nature. 1988 Sep 29;335(6189):437–440. doi: 10.1038/335437a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Klett C., Hellmann W., Suzuki F., Nakanishi S., Ohkubo H., Ganten D., Hackenthal E. Induction of angiotensinogen mRNA in hepatocytes by angiotensin II and glucocorticoids. Clin Exp Hypertens A. 1988;10(6):1009–1022. doi: 10.1080/07300077.1988.11878797. [DOI] [PubMed] [Google Scholar]
- Kohno M., Pouysségur J. Alpha-thrombin-induced tyrosine phosphorylation of 43,000- and 41,000-Mr proteins is independent of cytoplasmic alkalinization in quiescent fibroblasts. Biochem J. 1986 Sep 1;238(2):451–457. doi: 10.1042/bj2380451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerea K. M., Tonks N. K., Krebs E. G., Fischer E. H., Glomset J. A. Vanadate and molybdate increase tyrosine phosphorylation in a 50-kilodalton protein and stimulate secretion in electropermeabilized platelets. Biochemistry. 1989 Nov 28;28(24):9286–9292. doi: 10.1021/bi00450a008. [DOI] [PubMed] [Google Scholar]
- Macara I. G. Activation of 45Ca2+ influx and 22Na+/H+ exchange by epidermal growth factor and vanadate in A431 cells is independent of phosphatidylinositol turnover and is inhibited by phorbol ester and diacylglycerol. J Biol Chem. 1986 Jul 15;261(20):9321–9327. [PubMed] [Google Scholar]
- McCune B. K., Earp H. S. The epidermal growth factor receptor tyrosine kinase in liver epithelial cells. The effect of ligand-dependent changes in cellular location. J Biol Chem. 1989 Sep 15;264(26):15501–15507. [PubMed] [Google Scholar]
- Nakamura S., Yamamura H. Thrombin and collagen induce rapid phosphorylation of a common set of cellular proteins on tyrosine in human platelets. J Biol Chem. 1989 May 5;264(13):7089–7091. [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddie K. M., Litz J. S., Balserak J. C., Payne D. M., Creutz C. E., Parsons S. J. Modulation of pp60c-src tyrosine kinase activity during secretion in stimulated bovine adrenal chromaffin cells. J Neurosci Res. 1989 Sep;24(1):38–48. doi: 10.1002/jnr.490240107. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Sefton B. M. Phosphorylation of the transforming protein of Rous sarcoma virus: direct demonstration of phosphorylation of serine 17 and identification of an additional site of tyrosine phosphorylation in p60v-src of Prague Rous sarcoma virus. J Virol. 1986 Jul;59(1):73–81. doi: 10.1128/jvi.59.1.73-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet N., Déziel Y., Lehoux J. G. Vasopressin: a potent growth factor in adrenal glomerulosa cells in culture. J Steroid Biochem. 1984 Jan;20(1):449–454. doi: 10.1016/0022-4731(84)90252-8. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schelling P., Ganten D., Speck G., Fischer H. Effects of angiotensin II and angiotensin II antagonist saralasin on cell growth and renin in 3T3 and SV3T3 cells. J Cell Physiol. 1979 Mar;98(3):503–513. doi: 10.1002/jcp.1040980309. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Baur U., Bürgin M., Bühler F. R. Amiloride sensitive activation of S6 kinase by angiotensin II in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Feb 29;151(1):583–589. doi: 10.1016/0006-291x(88)90634-1. [DOI] [PubMed] [Google Scholar]
- Sherline P., Mascardo R. Catecholamines are mitogenic in 3T3 and bovine aortic endothelial cells. J Clin Invest. 1984 Aug;74(2):483–487. doi: 10.1172/JCI111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Stachowiak M. K., Jiang H. K., Poisner A. M., Tuominen R. K., Hong J. S. Short and long term regulation of catecholamine biosynthetic enzymes by angiotensin in cultured adrenal medullary cells. Molecular mechanisms and nature of second messenger systems. J Biol Chem. 1990 Mar 15;265(8):4694–4702. [PubMed] [Google Scholar]
- Stratton K. R., Worley P. F., Huganir R. L., Baraban J. M. Muscarinic agonists and phorbol esters increase tyrosine phosphorylation of a 40-kilodalton protein in hippocampal slices. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Whipple J. H., Fujita-Yamaguchi Y., Dubler R. E., Cheng K., Larner J. A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem. 1984 May 25;259(10):6650–6658. [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Thom R. E., Casnellie J. E. Pertussis toxin activates protein kinase C and a tyrosine protein kinase in the human T cell line Jurkat. FEBS Lett. 1989 Feb 13;244(1):181–184. doi: 10.1016/0014-5793(89)81188-3. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Charbonneau H. Protein tyrosine dephosphorylation and signal transduction. Trends Biochem Sci. 1989 Dec;14(12):497–500. doi: 10.1016/0968-0004(89)90184-9. [DOI] [PubMed] [Google Scholar]
- Tsao M. S., Earp H. S., Grisham J. W. The effects of epidermal growth factor and the state of confluence on enzymatic activities of cultured rat liver epithelial cells. J Cell Physiol. 1986 Feb;126(2):167–173. doi: 10.1002/jcp.1041260204. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature. 1981 Apr 9;290(5806):527–528. doi: 10.1038/290527a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Hamamori Y., Fukumoto Y., Kaibuchi K., Takai Y. Epidermal growth factor increases c-myc mRNA without eliciting phosphoinositide turnover, protein kinase C activation, or calcium ion mobilization in Swiss 3T3 fibroblasts. J Biochem. 1986 Dec;100(6):1631–1635. doi: 10.1093/oxfordjournals.jbchem.a121871. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]
- Zagari M., Stephens M., Earp H. S., Herman B. Relationship of cytosolic ion fluxes and protein kinase C activation to platelet-derived growth factor induced competence and growth in BALB/c-3T3 cells. J Cell Physiol. 1989 Apr;139(1):167–174. doi: 10.1002/jcp.1041390123. [DOI] [PubMed] [Google Scholar]