Abstract
The CoPanFlu-France cohort of households was set up in 2009 to study the risk factors for infection by the pandemic influenza virus (H1N1pdm) in the French general population. The authors developed an integrative data-driven approach to identify individual, collective and environmental factors associated with the post-seasonal serological H1N1pdm geometric mean titer, and derived a nested case-control analysis to identify risk factors for infection during the first season. This analysis included 1377 subjects (601 households). The GMT for the general population was 47.1 (95% confidence interval (CI): 45.1, 49.2). According to a multivariable analysis, pandemic vaccination, seasonal vaccination in 2009, recent history of influenza-like illness, asthma, chronic obstructive pulmonary disease, social contacts at school and use of public transports by the local population were associated with a higher GMT, whereas history of smoking was associated with a lower GMT. Additionally, young age at inclusion and risk perception of exposure to the virus at work were identified as possible risk factors, whereas presence of an air humidifier in the living room was a possible protective factor. These findings will be interpreted in light of the longitudinal analyses of this ongoing cohort.
Introduction
Since the novel influenza A/H1N1 pandemic virus (H1N1pdm) started spreading in April 2009, several studies have identified risk factors for H1N1pdm infection in the community such as young age [1]–[3], ethnicity [2], [4], male gender [4], urban area [5], low pre-epidemic serologic titer [3]–[5], use of public transport [4], household size [6]–[9] and presence of an index case in the household [3], especially if it was a child [10].
The CoPanFlu-France cohort, which has previously been described elsewhere [11], aimed at studying the risk of influenza infection as a complex combination of biological characteristics (including immunity), individual or collective behaviors and environmental context. This integrative approach consists in comprehensively collecting and analyzing epidemiological data on subjects and their environment as well as biological samples [12], [13].
Inclusion of households started in December 2009, at the end of the first H1N1pdm season in metropolitan France. We studied factors associated with the post-pandemic H1N1pdm titer from blood samples collected at inclusion. Previous studies showed that post-pandemic titer was linked to age classes [2], [14]–[20] and to pandemic vaccination status [21]. Relying on the massive amount of data collected at entry in the cohort, we tried to find other independent associations with this titer. In a complementary study, we carried out a nested case-control analysis in these subjects to identify risk factors for probable infection during the first H1N1pdm season.
Materials and Methods
Study design
This study relies on 601 households (1450 subjects) included in the study between December 2009 and July 2010, according to a stratified geographical sampling scheme in the French general population. More details on this sampling procedure, the representativeness of the sample and the global study design are available in a previous publication [11]. A total of 575 households (96%) were included after the first pandemic season (September 7 to December 27, 2009 [22]).
During the inclusion visit, nurses collected detailed data from all subjects with questionnaires and blood samples for serological analyses. As 73 of these samples (5.0%) were either too difficult to obtain (young children especially) or of insufficient quality or quantity to be analyzed, the analyses presented here focused on the 1377 subjects for whom haemagglutination inhibition (HI) titer was measured.
Variables
HI assay
The outcome measure was the post-seasonal HI titer, measured from blood samples collected at inclusion. A standard HI technique was adapted to the detection and quantification of antibodies to H1N1pdm. HI assay was conducted in a Bio-Safety Level 3 laboratory using 5.33 haemagglutinating units of non-inactivated antigen [14]. The antigen used was made of a dilution of cell culture supernatant of a H1N1pdm strain (strain OPYFLU-1 isolated from a young patient returning from Mexico in early May 2009) [23]. A final volume of 75 µl was used, including 25 µl of serum dilution, 25 µl of virus suspension, and 25 µl of a 1% RBC suspension in PBS (v/v: 0.33%). The HI titer was determined as the highest dilution providing clear inhibition of haemagglutination in two independent readings [24]. All experiments were conducted using serial dilutions (1/10–1/1280) of heat-inactivated sera, group O human erythrocytes (French Blood Bank). All experiments were performed with same negative and positive controls [25] and with a serum agglutinating activity control. All steps of HI assay were performed on Eppendorf epMotion working stations.
Definition of infections (case-control analysis)
Though some authors previously carried out risk factors analyses after defining cases as subjects with HI titer ≥1/40 [2], [26], we chose in our main analysis a higher threshold for our definition as titers between 1/40 and 1/80 were likely to result from a cross-reaction. We therefore defined cases as subjects with HI titer ≥1/80 and all other subjects were considered as controls. In two sensitivity analyses, we additionally defined (i) controls as subjects with HI titer <1/40 and (ii) cases as subjects with HI titer ≥1/80 who reported an influenza-like illness (ILI) during the pandemic season and controls as subjects with HI titer <1/40 and no history of ILI. All pandemic vaccine recipients were excluded from these analyses.
Covariates
All covariates used in the analysis are listed elsewhere [11] and detailed in Tables S1–S6 in File S1. The relation with HI titer was studied for 310 covariates, gathered according to 6 main dimensions: 1) sociodemographic characteristics, smoking habits and medical history, 2) vaccination and preventive measures against the virus, 3) indoor housing, 4) attitudes, beliefs and risk perception, 5) nature of meetings with other people and characteristics of contacts and 6) ecological data regarding the surrounding environment. For dimensions 1 to 5, we used data collected from questionnaires completed by the household members, with the help of the visiting nurse. For geographic data, we geocoded the addresses of households and used information on the surrounding demographic and socio-economic context provided by the French Institut national de la statistique et des études économiques (Insee) regarding statistical block groups of about 2000 inhabitants (IRIS) [27].
Definitions and coding
Some quantitative covariates were either dichotomized or log-transformed to enhance log-linearity of the studied relation (see supplementary material for details). Age was studied as a quantitative covariate. Subjects reported medical history and vaccinations with the help of their health records. We defined history of ILI as fever ≥37.8°C and cough and/or sore throat without another known cause [28] between September 7, 2009 and the date of inclusion. This covariate was excluded from the case-control analysis, which focused on possible risk factors. Daily frequency of hand washing was reported for the day before inclusion. For covariates describing smoking habits and preventive measures against the virus, characteristics of other members of the household was studied as an individual explanatory covariate (as a mean for quantitative covariates and as a proportion for binary covariates). Covariates regarding attitudes, beliefs and risk perception were collected from all subjects aged over 15 years with a dedicated questionnaire. Subjects were proposed affirmative sentences and were asked for all of them if they totally agreed, partly agreed, partly disagreed or totally disagreed. These answers were dichotomized (agree/disagree).
A contact was defined as someone the subject either spoke with (at least 3 words) or had a physical contact with. All subjects reported meetings with their contacts during a 3-day period ending the day before inclusion. Duration and location of meetings were collected, as well as age of contacts. In order to study meetings as covariates likely to be associated with the HI titer, we summed the individual durations of daily meetings according to their location (home, work, transports and at school) and to the age of contacts respectively. Summed durations of meetings were log-transformed (with an imputed value of 0.01 minute for subjects reporting a null summed duration of meetings). No information was collected on simultaneity of meetings, and the total reported duration of meetings was additional (e.g., a 10-minute meeting with 3 contacts simultaneously accounted for 30 minutes of meeting).
Statistical methods
All collected covariates likely to be associated with post-seasonal elevated HI titer were studied. Comparison tests between subgroups were Fisher's exact test (for binomial covariates) and Kruskal-Wallis rank sum test (for continuous covariates).
Estimation of geometric mean titers (GMTs)
GMTs were estimated for HI assays with the use of regression models for interval-censored data [29], [30] accounting for the within-household correlation. Post-stratification was used to compute representative post-seasonal GMT in the French general population. Calculation and use of sampling weights were detailed elsewhere [11].
We defined the “GMT ratio” (GMTR) as the multiplicative factor between the GMT in exposed versus non-exposed (for a binary covariate) or for each unit increase (for a quantitative covariate).
Control for confounding
As age and pandemic vaccination status had an important impact on the serological titer, GMTR was systematically adjusted on these major confounders in all univariable analyses. For analyses regarding environmental characteristics of the bedroom and of the IRIS, correlations were considered at these two levels respectively. Analyses of contacts were adjusted on the proportion of weekend days in the 3-day period.
Case-control analysis
Risk factors for infection were studied with the use of alternating logistic regression to model the pairwise odds ratios (ORs) between responses of subjects living in the same room, the same household or the same IRIS [31]. All univariable analyses were adjusted on age and control for confounding was carried out with the same adjustment measures as those used for the GMT analysis.
Model selection
The same selection process was used for both analyses. GMTRs and ORs were estimated for all covariates individually. Since a large number of covariates were tested, we adjusted the p-value to control the alpha inflation associated with multiple hypothesis testing and to account for the false discovery rate (FDR) for all covariates [32].
All covariates with an adjusted P<0.30 in univariable analyses were included in a multivariable analysis. Thirty datasets were imputed via multiple imputations by chained equations (MICE) [33]. Covariates related to attitudes, beliefs and risk perception for children were sampled from subjects over 15 years in the same household.
The criterion for model selection was the mean residual sum of squares with 10-fold cross-validation – aimed at avoiding overfitting and controlling FDR – run over the 30 imputed datasets and considering only models with P<0.05 for all covariates.
Resulting coefficients and standard errors were combined to obtain the reported results [34]. Additional multivariable models were estimated separately stratified by pandemic vaccination status.
Statistical analyses were performed with R version 2.15. We estimated GMTRs with the function “survreg” (package “survival” version 2.36). Multiple imputation was done with the package “mice” and we ran alternating logistic regression with the package “orth”.
Results
Descriptive data
Characteristics of the 1377 subjects are given in Tables S1–S6 in File S1. The median age at inclusion was 43.1 years (interquartile range (IQR): 20.7, 59.9 years), 38 children were aged 2 to 5 years and 14 children were aged <2. A total of 561 subjects (40.1%) had at least one history of chronic disease. History of ILI since the beginning of the pandemic wave was reported in 99 subjects (7.5%). For the 3 previous seasons, the proportion of ILI ranged from 7.3% to 19.1%. History of smoking was reported in 544 subjects (39.5%).
The proportion of pandemic vaccine recipients was 12.2%. The median time since pandemic vaccination was 3.2 (IQR: 1.7, 5.2) months. Pandemic vaccine recipients were younger than seasonal vaccine recipients: 46.4 (17.1, 63.0) vs. 63.8 (IQR: 50.4, 72.0) years (P<0.0001). Nine hundred and thirteen subjects (66.3%) were living in a house and the median area per inhabitant was 36.7 (IQR: 25.0, 52.5) m2.
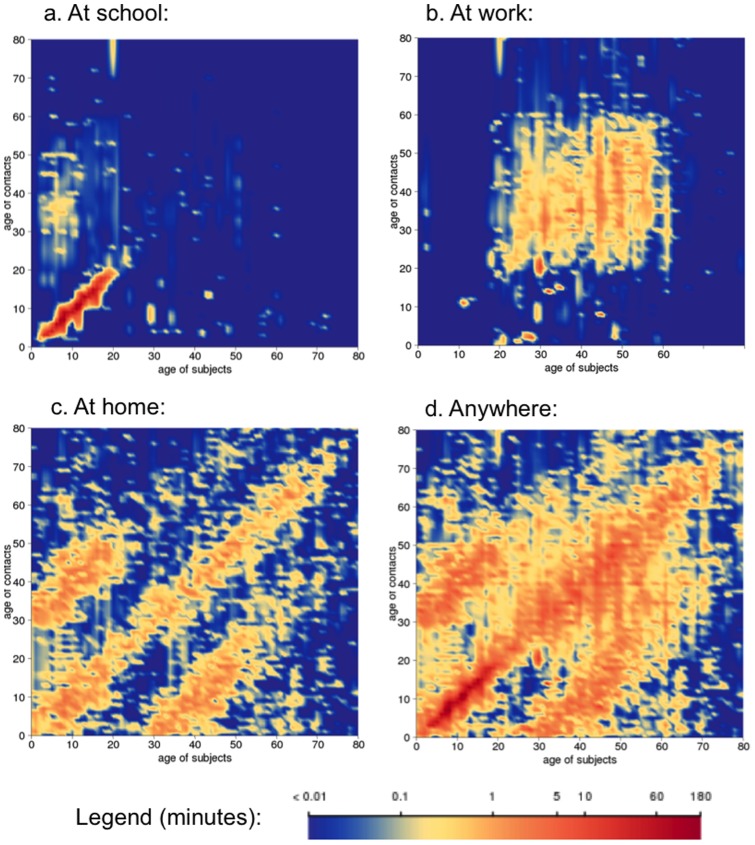
Detailed data on meetings was collected in 1360 out of the 1377 subjects. The median number of reported daily meetings was 6 (IQR: 3, 10) and the median summed duration was 963 (IQR: 503, 1646) mn/day, with significant differences according to age groups and locations (Figure 1). Subjects aged less than 15 years had a higher daily duration of meetings than older ones: 1847 (IQR: 1147, 2564) mn vs. 848 (IQR: 440, 1378) mn, P<0.0001. Children at school reported a large amount of meetings with children of the same age. Working adults aged 20 to 60 years had many meetings with persons of their age. At home, subjects had meetings with people of their age and with persons from the previous or next generation.
Figure 1. Mean duration of daily meetings (in minutes) of CoPanFlu subjects according to location, age of subjects (±6 months) and age of contacts (±6 months), with 3-year smoothing for both axes.
GMT estimates (post-stratified estimates)
Raw measured HI titer was ≥1/20 for 1319 subjects (95.8%), ≥1/40 for 832 subjects (60.4%), ≥1/80 for 259 subjects (18.8%) and ≥1/160 for 50 subjects (3.6%). After post-stratification the estimated proportions were 95.3% for ≥1/20, 59.0% for ≥1/40, 16.1% for ≥1/80 and 2.8% for ≥1/160.
The estimated GMT for the general population was 47.1 (95% confidence interval (CI): 45.1, 49.2]) It was higher in pandemic vaccine recipients (80.3 (95% CI: 69.8, 92.5) vs. 44.2 (95% CI: 42.4, 46.0) for unvaccinated subjects with no history of ILI and 58.7 (95% CI: 51.7, 66.6) for unvaccinated subjects with history of ILI) and in subjects aged ess than 5 years (55.2 (95% CI: 49.2, 62.0) vs. 45.7 (95% CI: 43.8, 47.6) for older ones). Figure 2 gives an overview of the estimated post-stratified GMT with respect to the general population structure, in relation to pandemic vaccination status, history of ILI and age of subjects.
Figure 2. Geometric mean titer (GMT) in relation to age and pandemic vaccination in the general population.
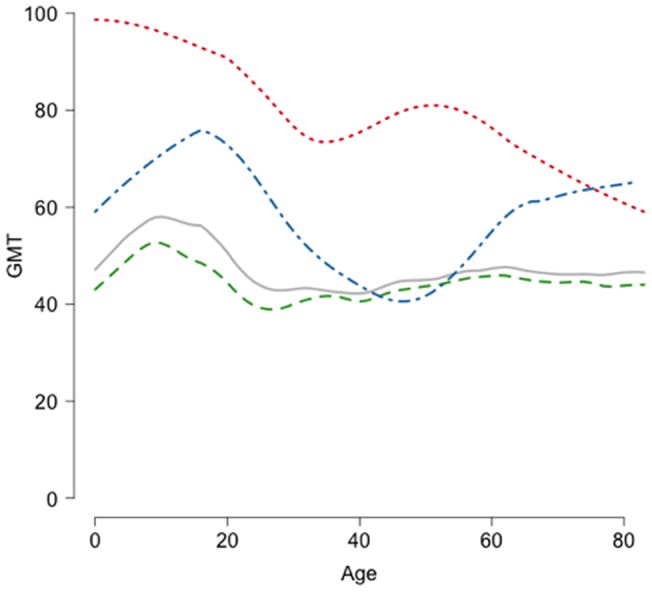
Red curve: pandemic vaccine recipients (N = 157); green curve: subjects with no pandemic vaccine and no history of influenza-like illness (ILI) (N = 1,067); blue curve: subjects with no pandemic vaccine and history of ILI (N = 95); gray curve: all subjects (N = 1,377). Smoothed GMTs are estimated for subjects aged between 5 years below and 5 years above the indicated age. GMTs are estimated for each interval based on all subjects in the interval and post-stratified with respect to the general population structure.
Factors associated with the GMT
All univariable GMTR estimates are listed in Tables S1–S6 in File S1. A total of 40 covariates with adjusted P<0.30 were retained in the multivariate analysis.
Selected multivariable models are listed in Table 1. Considering all the subjects (irrespective of the vaccination status), the final model retained (i) pandemic vaccination, 2009 seasonal vaccination, history of ILI for season 2009–2010, asthma, COPD, duration of meetings at school and IRIS proportion of workers using public transports as covariates associated with a higher GMT, and (ii) history of smoking as covariate associated with a lower GMT.
Table 1. Multivariable models for geometric mean titer ratio in CoPanFlu-France subjects at inclusion.
| All subjects (N = 1377) | |||
| Covariate | GMTR | 95% CI | P |
| Pandemic vaccine recipient (B) | 1.77 | 1.56, 2.01 | <0.0001 |
| Seasonal vaccine recipient (2009) (B) | 1.11 | 1.01, 1.21 | <0.03 |
| History of ILI for season 2009–2010 (B) | 1.31 | 1.15, 1.49 | <0.0001 |
| Asthma (B) | 1.17 | 1.01, 1.37 | <0.05 |
| Chronic obstructive pulmonary disease (B) | 1.28 | 1.05, 1.56 | <0.02 |
| History of smoking (B) | 0.93 | 0.88, 0.99 | <0.03 |
| Duration of meetings at school (L) | 1.03 | 1.01, 1.04 | <0.01 |
| Proportion of workers using public transports to go to work (Q) | 1.45 | 1.00, 2.10 | <0.05 |
(B): binary covariates; (Q): quantitative covariates; (L): log-transformed quantitative covariates; GMTR: geometric mean titer ratio; CI: confidence interval.
Considering the 1,207 subjects without pandemic vaccination, “asthma” was the only covariate that did not remain in the final model. Considering the 171 pandemic vaccine recipients, history of ILI remained in the model, while older age at inclusion and time since pandemic vaccination were associated with a lower GMT.
Case-control analysis
The 1,207 unvaccinated subjects were included in this analysis, 171 as cases and 1,036 as controls. The proportions of subjects with a history of ILI were 18.0% in cases and 6.5% in controls (P<0.0001). The final multivariable model retained (i) COPD, asthma, duration of meetings at school, proportion of workers using public transports and belief that not going to work protects against H1N1pdm as factors associated with a higher risk of probable infection, and (ii) older age and having an air humidifier in the living room as factors associated with a lower risk (see Table 2 for details). Though we estimated pairwise odds ratios between responses of subjects living in the same room, the same household or the same IRIS, only the household level was kept in the final model as the other ones were not significant, OR = 3.31 (95% CI: 1.82, 6.02). Multivariable models for the sensitivity analyses retained subsets of these factors with no additional factors (Table S7 and S8 in File S2).
Table 2. Multivariable models for the case-control analysis of risk factors for probable infection in CoPanFlu-France unvaccinated subjects.
| Subjects without pandemic vaccination, 171 cases with HI titer ≥1/80, 1,036 controls with HI titer <1/80 | |||
| Covariate | OR | 95% CI | P |
| Age at inclusion (per 10 years) (Q) | 0.87 | 0.77, 0.98 | <0.02 |
| Chronic obstructive pulmonary disease (B) | 2.89 | 1.41, 5.92 | <0.01 |
| Asthma (B) | 2.41 | 1.32, 4.42 | <0.01 |
| Duration of meetings at school (L) | 1.11 | 1.03, 1.19 | <0.01 |
| Air humidifier in the living room (B) | 0.64 | 0.41, 0.99 | <0.05 |
| Believes that not going to work protects against H1N1pdm (B) | 1.61 | 1.02, 2.53 | <0.05 |
| Proportion of workers using public transports to go to work (Q) | 11.2 | 2.08, 60.0 | <0.01 |
| Pairwise odds ratios between cases living in the same household | 3.31 | 1.82, 6.02 | <0.0001 |
(B): binary covariates; (Q): quantitative covariates; (L): log-transformed quantitative covariates; OR: odds ratio; CI: confidence interval.
Discussion
Covariates associated with HI titer
Post-pandemic elevated HI titer can be explained by a pre-pandemic elevated titer, a recent increase in titer due to an infection by the pandemic virus or to another antigenic stimulation (e.g., pandemic vaccination), or by any combination of these different factors. We review our findings in light of other studies on the same topic.
The global multivariate model including pandemic vaccination gave information on the association of this factor with the GMT. Adjustment on this factor in the same model allowed us to study factors that may have an impact on GMT increase after either vaccination or infection, whereas stratified analyses according to this vaccination intended to focus more specifically on factors associated with other causes of elevated GMT.
We found a lower anti-H1N1pdm GMT in older subjects in the univariable analysis and in the multivariable model run among pandemic vaccine recipients. This covariate did not remain in the other multivariate models mainly because of the adjustment on duration of contacts at school (age was significantly associated with the GMT in all models when we excluded this covariate). Older age was also associated with a lower risk of probable infection in the case-control analysis.
These results are consistent with other cross-sectional post-pandemic studies worldwide, including modeling [35] and serological [14] studies in France, which reported a much higher infection rate in children and young adults [16], [19], [20], [36]–[44].
As expected, a reported history of ILI was associated with an elevated GMT, which indicates that some of these ILIs were probably caused by H1N1pdm infection. Though this factor lacks sensitivity and specificity to be considered as a good correlate of infection, its coefficient in selected multivariable models gives more information on the relative role of infections among all causes leading to a GMT increase. Its association with the GMT in vaccinated subjects indicates that the GMT was also caused by H1N1pdm infections. Indeed, as most vaccinations occurred at the end of the pandemic course (Figure 3), we could not distinguish whether the increased GMT in vaccine recipients was caused by vaccination itself or by previous infection.
Figure 3. Weekly incidence of influenza-like illnesses (ILIs) in France (French General Practitioner Sentinel network [22]) and weekly pandemic vaccinations in CoPanFlu subjects, weeks 2009–26 to 2010–08.
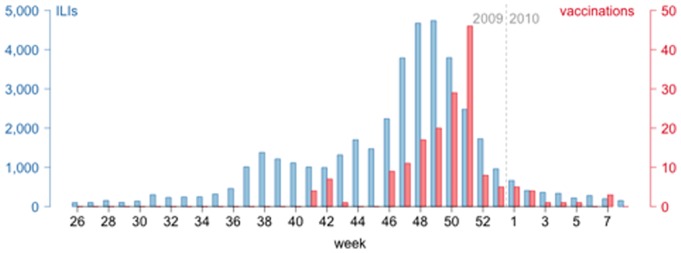
Blue bars (left scale): national weekly incidence of ILIs; red bars (right scale): number of weekly pandemic vaccinations in CoPanFlu subjects.
Asthma and COPD were associated with a higher GMT and possible risk factors in the case-control analysis. Asthmatics may have increased susceptibility for H1N1pdm infection [45], possibly because of alterations in the airway architecture [46], [47] and impairment of innate immunity [47]. Another hypothesis to explain a higher GMT in subjects with such medical conditions, regardless of their susceptibility to infection, would be a more severe illness [48] involving a greater immune response [49].
We found that smoking history was associated with a lower GMT. Although several studies already found an association between cigarette smoking and risk to contract influenza infection [50]–[52], smokers have a well-known diminished serological response to influenza infection or vaccination [52], the immunosuppressive mechanism is still unclear [53]–[56].
Seasonal vaccination for any season since 2006–2007 was associated with an increase in the GMT, maybe because of a cross-reactive immune response with seasonal vaccination H1N1 strains [57]though studies investigating this association were all inconclusive [3], [58]. Another hypothesis would consider that elevated post-seasonal titer might be a consequence of an increased risk of pandemic infection in seasonal vaccine recipients [59], though conflicting results were reported about this association [60]–[64].
In covariates related to the environmental characteristics of the housing, only the association between presence of an air humidifier in the living room and lower risk remained in the case-control multivariable model, which may be consistent with the possible impact of relative humidity on influenza aerosol transmission [65], [66].
The multivariable analysis retained no covariate related to attitudes, beliefs and risk perception, except the belief that not going to work may protect against H1N1pdm infection, associated with a higher risk in the case-control analysis. We have no clear interpretation for this finding, except that this covariate may be a correlate of more general characteristics of risk perception, which affect the transmission patterns of pandemic influenza.
Increasing GMT and a higher risk of probable infection associated with duration of meetings at school were not surprising since schools are identified as places with high meeting rates between influenza susceptible subjects [67]. Interestingly, we did not find a significant association of GMT with daily duration of meetings with children younger than 10 years old regardless of location, suggesting that school favors transmissions by a particular pattern of contacts or environmental characteristics [67], [68].
The multivariable analysis retained no covariate related to the characteristics of the surrounding area, except the proportion of workers using public transportation to go to work, which also appeared as a possible risk factor.
The important pairwise OR we found in the case-control analysis for subjects living in the same household suggests a common environmental exposure or susceptibility for these subjects who often belong to the same family, or more probably an elevated intra-household secondary attack rate (estimated 4 to 37% in previous household studies [10]).
Limitations
Though households were sampled in the general population, some households refused to participate, which may induce a selection bias. However, comparisons with French population census data suggest that this bias was controlled [11], and post-stratification of the GMT by age and vaccination status with respect to the French population structure did not modify the results significantly. We did not post-stratify our estimations of the GMTR, as the choice of the auxiliary covariates used to adjust the sampling weights could have induced important changes in the standard error of our estimates leading to spurious associations [69].
The timeline of inclusion may have induced recall or reporting biases. The cohort was designed to include households before the 2009 pandemic season and to follow-up subjects during the influenza season. As inclusions were delayed, data regarding ILIs were collected retrospectively and recall bias may be important in subjects with late inclusion. Moreover, we found a decreasing GMT according to time since vaccination in pandemic vaccine recipients, and we cannot exclude an antibody loss in the months following an infection, although we did not find any association between GMT and date of inclusion in unvaccinated subjects. Such limitations may have biased the association between GMT and other covariates.
In the case-control analysis, cases were defined serologically, yet we know that an elevated titer can sometimes be explained by cross-reactions, especially in the elderly [16], and that infected subjects can show a low titer a few months after infection [70]. This lack of specificity and sensitivity to identify infections must be considered in light of the sensitivity analyses results, which often showed similar results with different case definitions.
Another limitation may be linked to the amount of data collected. Though we controlled this FDR with the use of specific procedures, multiple testing of hundreds of covariates results in an important risk of finding spurious associations, due to the alpha inflation phenomenon.
Because of these limitations, our analysis must be understood as a hypothesis generating study aimed at identifying the possible role of many factors that would probably not have been studied otherwise. Further studies would be necessary to confirm the impact of these factors and their implications for the control of influenza.
Conclusion
We used a data-driven framework to carry out an exploratory analysis of potential relevant risk factors for infection. This hypothesis generating tool relying on an integrated approach allowed us to highlight the possible impact of previously unknown factors from several dimensions usually studied separately, such as presence of an air humidifier (indoor environment), duration of meetings at school (social contacts), characteristics of the local population or risk perception. Additional data is being collected and analyzed in this ongoing cohort. The longitudinal analysis of these households will permit integrative analyses of complex phenomena such as individual, collective and environmental risk factors for infection, routes of transmission, or determinants of the immune response to infection or vaccination.
Supporting Information
Tables S1–S6. Description and univariable analyses for all covariates.
(DOC)
Tables S7 and S8. Multivariable models for the case-control analysis of risk factors for probable infection in CoPanFlu-France unvaccinated subjects: sensitivity analysis.
(DOC)
Funding Statement
This study was supported by the Institut de Microbiologie et Maladies Infectieuses (IMMI-AVIESAN), the Institut de Santé Publique (ISP-AVIESAN), the French Ministry of Health and Assistance Publique Hôpitaux de Paris – PHRC 2010 #AOM10199, the French Ministry of research and the Institut de Recherche en Sante Publique (IReSP – TGIR 2009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lee DH, Kim CW, Kim J-H, Lee JS, Lee MK, et al. (2010) Risk factors for laboratory-confirmed household transmission of pandemic H1N1 2009 infection. Am J Infect Control 38: e43–45 doi:10.1016/j.ajic.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 2. Bandaranayake D, Huang QS, Bissielo A, Wood T, Mackereth G, et al. (2010) Risk factors and immunity in a nationally representative population following the 2009 influenza A(H1N1) pandemic. PLoS ONE 5: e13211 doi:10.1371/journal.pone.0013211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen MIC, Lee VJM, Lim W-Y, Barr IG, Lin RTP, et al. (2010) 2009 influenza A(H1N1) seroconversion rates and risk factors among distinct adult cohorts in Singapore. JAMA 303: 1383–1391 doi:10.1001/jama.2010.404. [DOI] [PubMed] [Google Scholar]
- 4. Lim W-Y, Chen CHJ, Ma Y, Chen MIC, Lee VJM, et al. (2011) Risk Factors for Pandemic (H1N1) 2009 Seroconversion among Adults, Singapore, 2009. Emerging Infect Dis 17: 1455–1462 doi:10.3201/eid1708.101270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao D-Y, Cheng K-F, Li T-C, Wu T-N, Chen C-Y, et al. (2011) Factors associated with infection by 2009 pandemic H1N1 influenza virus during different phases of the epidemic. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 15: e695–e701 doi:10.1016/j.ijid.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein E, Cowling BJ, O'Hagan JJ, Danon L, Fang VJ, et al. (2010) Oseltamivir for treatment and prevention of pandemic influenza A/H1N1 virus infection in households, Milwaukee, 2009. BMC Infect Dis 10: 211 doi:10.1186/1471-2334-10-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan OW, Parks S, Shim T, Blevins PA, Lucas PM, et al. (2010) Household transmission of pandemic (H1N1) 2009, San Antonio, Texas, USA, April-May 2009. Emerging Infect Dis 16: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papenburg J, Baz M, Hamelin M-È, Rhéaume C, Carbonneau J, et al. (2010) Household Transmission of the 2009 Pandemic A/H1N1 Influenza Virus: Elevated Laboratory-Confirmed Secondary Attack Rates and Evidence of Asymptomatic Infections. Clin Infect Dis. Available: http://www.ncbi.nlm.nih.gov/pubmed/20887206. Accessed 5 October 2010. [DOI] [PubMed]
- 9. Sikora C, Fan S, Golonka R, Sturtevant D, Gratrix J, et al. (2010) Transmission of pandemic influenza A (H1N1) 2009 within households: Edmonton, Canada. J Clin Virol 49: 90–93 doi:10.1016/j.jcv.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 10. Lau LLH, Nishiura H, Kelly H, Ip DKM, Leung GM, et al. (2012) Household Transmission of 2009 Pandemic Influenza A (H1N1): A Systematic Review and Meta-analysis. Epidemiology 23: 531–542 doi:10.1097/EDE.0b013e31825588b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lapidus N, De Lamballerie X, Salez N, Setbon M, Ferrari P, et al. (2012) Integrative study of pandemic A/H1N1 influenza infections: design and methods of the CoPanFlu-France cohort. BMC public health 12: 417 doi:10.1186/1471-2458-12-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bougnères P, Valleron A-J (2008) Causes of early-onset type 1 diabetes: toward data-driven environmental approaches. J Exp Med 205: 2953–2957 doi:10.1084/jem.20082622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel CJ, Bhattacharya J, Butte AJ (2010) An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS ONE 5: e10746 doi:10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delangue J, Salez N, Ninove L, Kieffer A, Zandotti C, et al. (2011) Serological study of the 2009 pandemic due to influenza A H1N1 in the metropolitan French population. Clin Microbiol Infect. Available: http://www.ncbi.nlm.nih.gov/pubmed/21635661. Accessed 8 June 2011. [DOI] [PubMed]
- 15. Gilbert GL, Cretikos MA, Hueston L, Doukas G, O'Toole B, et al. (2010) Influenza A (H1N1) 2009 antibodies in residents of New South Wales, Australia, after the first pandemic wave in the 2009 southern hemisphere winter. PLoS ONE 5: e12562 doi:10.1371/journal.pone.0012562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, et al. (2010) Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 375: 1100–1108 doi:10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 17. Tandale BV, Pawar SD, Gurav YK, Chadha MS, Koratkar SS, et al. (2010) Seroepidemiology of pandemic influenza A (H1N1) 2009 virus infections in Pune, India. BMC Infect Dis 10: 255 doi:10.1186/1471-2334-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tian L, Shi W, Ying-Deng, Pang X, Peng-Yang, et al (2011) Serologic survey of pandemic influenza A (H1N1 2009) in Beijing, China. Prev Med 52: 71–74 doi:10.1016/j.ypmed.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 19. Xu C, Bai T, Iuliano AD, Wang M, Yang L, et al. (2011) The Seroprevalence of Pandemic Influenza H1N1 (2009) Virus in China. PLoS ONE 6: e17919 doi:10.1371/journal.pone.0017919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zimmer SM, Crevar CJ, Carter DM, Stark JH, Giles BM, et al. (2010) Seroprevalence Following the Second Wave of Pandemic 2009 H1N1 Influenza in Pittsburgh, PA, USA. PLoS ONE 5: e11601 doi:10.1371/journal.pone.0011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vajo Z, Tamas F, Sinka L, Jankovics I (2010) Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009-10 influenza season: a multicentre, randomised controlled trial. Lancet 375: 49–55 doi:10.1016/S0140-6736(09)62039-0. [DOI] [PubMed] [Google Scholar]
- 22.Sentiweb (n.d.) Sentinelles network France – Weekly epidemiological record. Available: http://sentiweb.org/. Accessed 1 February 2011.
- 23. Nougairède A, Ninove L, Zandotti C, Salez N, Mantey K, et al. (2010) Novel Virus Influenza A (H1N1sw) in South-Eastern France, April-August 2009. PLoS ONE 5: e9214 doi:10.1371/journal.pone.0009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wood JM, Gaines-Das RE, Taylor J, Chakraverty P (1994) Comparison of influenza serological techniques by international collaborative study. Vaccine 12: 167–174. [DOI] [PubMed] [Google Scholar]
- 25. Dellagi K, Rollot O, Temmam S, Salez N, Guernier V, et al. (2011) Pandemic influenza due to pH1N1/2009 virus: estimation of infection burden in Reunion Island through a prospective serosurvey, austral winter 2009. PLoS ONE 6: e25738 doi:10.1371/journal.pone.0025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Achonu C, Rosella L, Gubbay JB, Deeks S, Rebbapragada A, et al. (2011) Seroprevalence of pandemic influenza H1N1 in Ontario from January 2009-May 2010. PLoS ONE 6: e26427 doi:10.1371/journal.pone.0026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Insee (2011) Definitions and methods – Statistical block groups (IRIS). Available: http://www.insee.fr/en/methodes/default.asp?page=definitions/ilots-regr-pour-inf-stat.htm. Accessed 12 January 2011.
- 28.CDC (n.d.) Flu activity and surveillance: reports and surveillance methods in the United States. Available: http://www.cdc.gov/flu/weekly/fluactivitysurv.htm. Accessed 5 October 2010.
- 29. Nauta JJP (2006) Eliminating bias in the estimation of the geometric mean of HI titres. Biologicals 34: 183–186 doi:10.1016/j.biologicals.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Siev D (2007) Reply to “Nauta JJP, Eliminating bias in the estimation of the geometric mean of HI titers” [Biologicals 2006;34(3): 83–6]. Biologicals 35: 149–151; author reply 153. doi:10.1016/j.biologicals.2006.06.002. [DOI] [PubMed]
- 31.Carey V, Zeger SL, Diggle P (1993) Modelling multivariate binary data with alternating logistic regressions. Biometrika 80: 517 –526. doi:10.1093/biomet/80.3.517.
- 32. Storey JD, Taylor JE, Siegmund D (2004) Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 66: 187–205 doi:10.1111/j.1467-9868.2004.00439.x. [Google Scholar]
- 33. Rubin DB, Schenker N (1991) Multiple imputation in health-care databases: an overview and some applications. Stat Med 10: 585–598. [DOI] [PubMed] [Google Scholar]
- 34.Schafer JL (1997) Analysis of incomplete multivariate data. Chapman & Hall. 448 p.
- 35. Carrat F, Pelat C, Levy-Bruhl D, Bonmarin I, Lapidus N (2010) Planning for the next influenza H1N1 season: a modelling study. BMC Infect Dis 10: 301 doi:10.1186/1471-2334-10-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adamson WE, McGregor EC, Kavanagh K, McMenamin J, McDonagh S, et al. (2011) Influenza A(H1N1)2009 antibody seroprevalence in Scotland following the 2010/11 influenza season. Euro Surveill 16: 19871. [PubMed] [Google Scholar]
- 37. Allwinn R, Geiler J, Berger A, Cinatl J, Doerr HW (2010) Determination of serum antibodies against swine-origin influenza A virus H1N1/09 by immunofluorescence, haemagglutination inhibition, and by neutralization tests: how is the prevalence rate of protecting antibodies in humans? Med Microbiol Immunol 199: 117–121 doi:10.1007/s00430-010-0143-4. [DOI] [PubMed] [Google Scholar]
- 38. Bansal S, Pourbohloul B, Hupert N, Grenfell B, Meyers LA (2010) The shifting demographic landscape of pandemic influenza. PLoS ONE 5: e9360 doi:10.1371/journal.pone.0009360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deng Y, Pang XH, Yang P, Shi WX, Tian LL, et al. (2011) Serological survey of 2009 H1N1 influenza in residents of Beijing, China. Epidemiol Infect 139: 52–58 doi:10.1017/S0950268810002189. [DOI] [PubMed] [Google Scholar]
- 40. Lerdsamran H, Pittayawonganon C, Pooruk P, Mungaomklang A, Iamsirithaworn S, et al. (2011) Serological Response to the 2009 Pandemic Influenza A (H1N1) Virus for Disease Diagnosis and Estimating the Infection Rate in Thai Population. PLoS ONE 6: e16164 doi:10.1371/journal.pone.0016164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McLeish NJ, Simmonds P, Robertson C, Handel I, McGilchrist M, et al. (2011) Sero-Prevalence and Incidence of A/H1N1 2009 Influenza Infection in Scotland in Winter 2009–2010. PLoS ONE 6: e20358 doi:10.1371/journal.pone.0020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skowronski DM, Hottes TS, Janjua NZ, Purych D, Sabaiduc S, et al. (2010) Prevalence of seroprotection against the pandemic (H1N1) virus after the 2009 pandemic. CMAJ 182: 1851–1856 doi:10.1503/cmaj.100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu JT, Ma ESK, Lee CK, Chu DKW, Ho P-L, et al. (2010) The Infection Attack Rate and Severity of 2009 Pandemic H1N1 Influenza in Hong Kong. Clin Infect Dis 51: 1184–1191 doi:10.1086/656740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Kerkhove MD, Hirve S, Koukounari A, Mounts AW (2013) Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respi Viruses. doi:10.1111/irv.12074. [DOI] [PMC free article] [PubMed]
- 45.Kloepfer KM, Olenec JP, Lee WM, Liu G, Vrtis RF, et al. (2012) Increased H1N1 Infection Rate in Children with Asthma. American Journal of Respiratory and Critical Care Medicine. Available: http://www.ncbi.nlm.nih.gov/pubmed/22366048. Accessed 2 March 2012. [DOI] [PMC free article] [PubMed]
- 46. Kesic MJ, Hernandez M, Jaspers I (2012) Airway protease/antiprotease imbalance in atopic asthmatics contributes to increased Influenza A virus cleavage and replication. Respir Res 13: 82 doi:10.1186/1465-9921-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Juhn YJ (2012) Influence of asthma epidemiology on the risk for other diseases. Allergy Asthma Immunol Res 4: 122–131 doi:10.4168/aair.2012.4.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsu AC-Y, See HV, Hansbro PM, Wark PAB (2012) Innate immunity to influenza in chronic airways diseases. Respirology 17: 1166–1175 doi:10.1111/j.1440-1843.2012.02200.x. [DOI] [PubMed] [Google Scholar]
- 49. Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, et al. (2011) Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med 17: 195–199 doi:10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kark JD, Lebiush M, Rannon L (1982) Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med 307: 1042–1046 doi:10.1056/NEJM198210213071702. [DOI] [PubMed] [Google Scholar]
- 51. Arcavi L, Benowitz NL (2004) Cigarette smoking and infection. Arch Intern Med 164: 2206–2216 doi:10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 52. MacKenzie JS, MacKenzie IH, Holt PG (1976) The effect of cigarette smoking on susceptibility to epidemic influenza and on serological responses to live attenuated and killed subunit influenza vaccines. J Hyg (Lond) 77: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horvath KM, Brighton LE, Zhang W, Carson JL, Jaspers I (2011) Epithelial cells from smokers modify dendritic cell responses in the context of influenza infection. Am J Respir Cell Mol Biol 45: 237–245 doi:10.1165/rcmb.2010-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mian MF, Stämpfli MR, Mossman KL, Ashkar AA (2009) Cigarette smoke attenuation of poly I:C-induced innate antiviral responses in human PBMC is mainly due to inhibition of IFN-beta production. Mol Immunol 46: 821–829 doi:10.1016/j.molimm.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Mian MF, Lauzon NM, Stämpfli MR, Mossman KL, Ashkar AA (2008) Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. Journal of Leukocyte Biology 83: 774 –784. doi:10.1189/jlb.0707481. [DOI] [PubMed]
- 56. Wu W, Patel KB, Booth JL, Zhang W, Metcalf JP (2011) Cigarette smoke extract suppresses the RIG-I-initiated innate immune response to influenza virus in the human lung. Am J Physiol Lung Cell Mol Physiol 300: L821–830 doi:10.1152/ajplung.00267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemaitre M, Leruez-Ville M, De Lamballerie XN, Salez N, Garrone P, et al. (2010) Seasonal H1N1 2007 influenza virus infection is associated with elevated pre-exposure antibody titers to the 2009 pandemic influenza A (H1N1) virus. Clin Microbiol Infect. Available: http://www.ncbi.nlm.nih.gov/pubmed/20731679. Accessed 25 August 2010. [DOI] [PubMed]
- 58. Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, et al. (2009) Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 361: 1945–1952 doi:10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 59. Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, et al. (2010) Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med 7: e1000258 doi:10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kelly HA, Grant KA, Fielding JE, Carville KS, Looker CO, et al. (2011) Pandemic influenza H1N1 2009 infection in Victoria, Australia: no evidence for harm or benefit following receipt of seasonal influenza vaccine in 2009. Vaccine 29: 6419–6426 doi:10.1016/j.vaccine.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 61. Pebody R, Andrews N, Waight P, Malkani R, McCartney C, et al. (2011) No effect of 2008/09 seasonal influenza vaccination on the risk of pandemic H1N1 2009 influenza infection in England. Vaccine 29: 2613–2618 doi:10.1016/j.vaccine.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 62. Puig-Barberà J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Pérez-Vilar S, et al. (2010) Effectiveness of seasonal 2008–2009, 2009–2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellón, Spain. A test-negative, hospital-based, case-control study. Vaccine 28: 7460–7467 doi:10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 63. Cowling BJ, Ng S, Ma ESK, Cheng CKY, Wai W, et al. (2010) Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis 51: 1370–1379 doi:10.1086/657311. [DOI] [PubMed] [Google Scholar]
- 64. Garcia-Garcia L, Valdespino-Gómez JL, Lazcano-Ponce E, Jimenez-Corona A, Higuera-Iglesias A, et al. (2009) Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-control study in Mexico City. BMJ 339: b3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shaman J, Kohn M (2009) Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA 106: 3243–3248 doi:10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Myatt TA, Kaufman MH, Allen JG, Macintosh DL, Fabian MP, et al. (2010) Modeling the airborne survival of influenza virus in a residential setting: the impacts of home humidification. Environ Health 9: 55 doi:10.1186/1476-069X-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cauchemez S, Bhattarai A, Marchbanks TL, Fagan RP, Ostroff S, et al. (2011) Role of social networks in shaping disease transmission during a community outbreak of 2009 H1N1 pandemic influenza. Proc Natl Acad Sci USA 108: 2825–2830 doi:10.1073/pnas.1008895108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Potter GE, Handcock MS, Longini IM Jr, Halloran ME (2012) Estimating within-school contact networks to understand influenza transmission. The annals of applied statistics 6: 1–26 doi:10.1214/11-AOAS505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lumley T (2010) Post-stratification, raking, and calibration. Complex Surveys: A Guide to Analysis Using R. John Wiley and Sons. p. 147.
- 70. Wang M, Yuan J, Li T, Liu Y, Wu J, et al. (2011) Antibody dynamics of 2009 influenza A (H1N1) virus in infected patients and vaccinated people in China. PLoS ONE 6: e16809 doi:10.1371/journal.pone.0016809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6. Description and univariable analyses for all covariates.
(DOC)
Tables S7 and S8. Multivariable models for the case-control analysis of risk factors for probable infection in CoPanFlu-France unvaccinated subjects: sensitivity analysis.
(DOC)