Abstract
The macrophage-derived cytokine interleukin-1 (IL-1) can provide a second signal with antigen to elicit production of interleukin-2 (IL-2) by helper T cells. The pathway(s) involved remains controversial, with protein kinase C and cyclic AMP (cAMP) invoked as possible second messengers. In the murine thymoma EL4.E1, IL-1 could synergize with the phosphoinositide pathway, because the cells made higher levels of IL-2 in the presence of IL-1 than could be induced by phorbol ester plus calcium ionophore alone. IL-1 is unlikely to act through a sustained increase in cAMP in these cells because it did not raise cAMP levels detectably and because IL-1 and forskolin had opposite effects on IL-2 gene expression. Inducible expression of a transfected reporter gene linked to a cloned fragment of the murine IL-2 gene promoter was initially increased by IL-1 costimulation, implying that IL-1 can increase the rate of transcription of IL-2. The minimal promoter elements required for iL-1 responsiveness were located within 321 bp of the IL-2 RNA cap site, and further upstream sequences to -2800 did not modify this response. IL-1 costimulation resulted in enhanced activity of both an inducible NF-kappa B-like factor and one of two distinct AP-1-like factors that bind to IL-2 regulatory sequences. Neither was induced, however, by IL-1 alone. Another AP-1-like factor and NFAT-1, while inducible in other cell types, were expressed constitutively in the EL4.E1 cells and were unaffected by IL-1. These results are discussed in terms of the combinatorial logic of IL-2 gene expression.
Full text
PDF




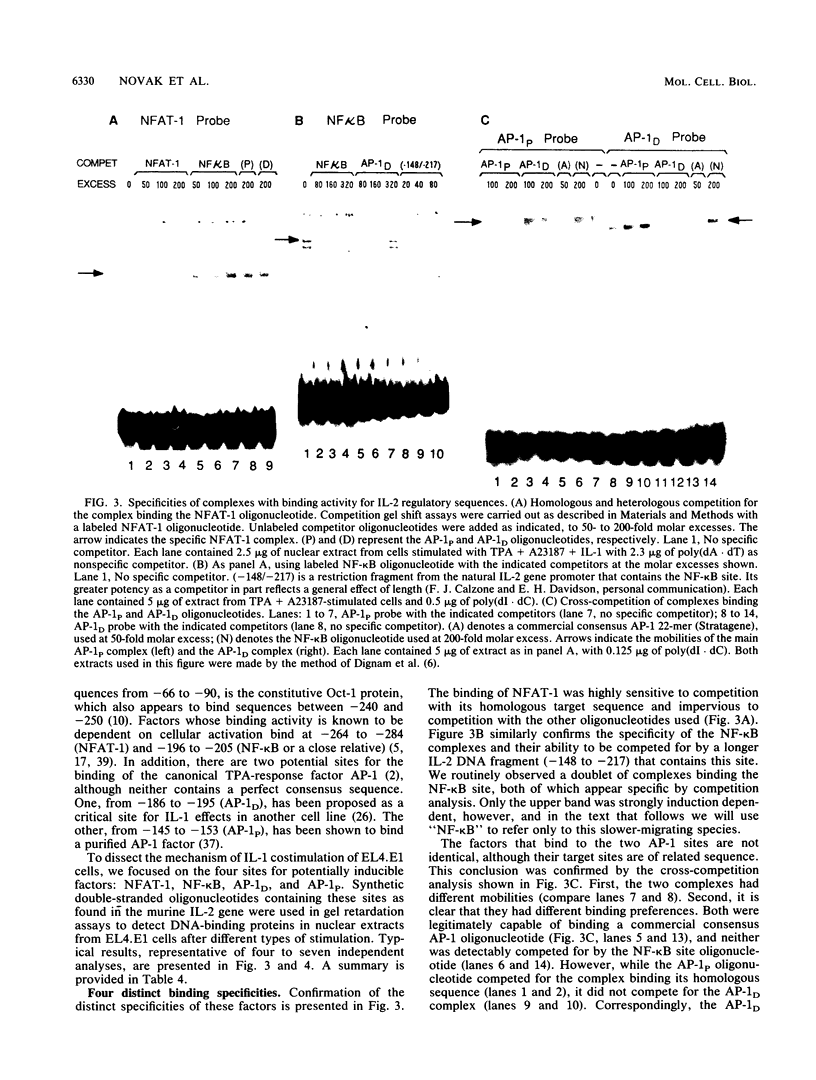




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. T., Ho S. N., Barna T. J., McKean D. J. Transmembrane signaling during interleukin 1-dependent T cell activation. Interactions of signal 1- and signal 2-type mediators with the phosphoinositide-dependent signal transduction mechanism. J Biol Chem. 1987 Feb 25;262(6):2719–2728. [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Thézé N., Thiebaud P., Hill R. L., Britten R. J., Davidson E. H. Developmental appearance of factors that bind specifically to cis-regulatory sequences of a gene expressed in the sea urchin embryo. Genes Dev. 1988 Sep;2(9):1074–1088. doi: 10.1101/gad.2.9.1074. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Didier M., Aussel C., Pelassy C., Fehlmann M. IL-1 signaling for IL-2 production in T cells involves a rise in phosphatidylserine synthesis. J Immunol. 1988 Nov 1;141(9):3078–3080. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Durand D. B., Shaw J. P., Bush M. R., Replogle R. E., Belagaje R., Crabtree G. R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol Cell Biol. 1988 Apr;8(4):1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Farrar J. J., Mizel S. B., Fuller-Farrar J., Farrar W. L., Hilfiker M. L. Macrophage-independent activation of helper T cells. I. Production of Interleukin 2. J Immunol. 1980 Aug;125(2):793–798. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Hackett R. J., Davis L. S., Lipsky P. E. Comparative effects of tumor necrosis factor-alpha and IL-1 beta on mitogen-induced T cell activation. J Immunol. 1988 Apr 15;140(8):2639–2644. [PubMed] [Google Scholar]
- Heckford S. E., Gelmann E. P., Agnor C. L., Jacobson S., Zinn S., Matis L. A. Distinct signals are required for proliferation and lymphokine gene expression in murine T cell clones. J Immunol. 1986 Dec 1;137(11):3652–3663. [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Koide S., Steinman R. M. Induction of murine interleukin 1: stimuli and responsive primary cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3802–3806. doi: 10.1073/pnas.84.11.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H., Chin J., Schmidt J. A., Abbas A. K. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstein T., June C. H., Ledbetter J. A., Stella G., Thompson C. B. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989 Apr 21;244(4902):339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- McGuire K. L., Yang J. A., Rothenberg E. V. Influence of activating stimulus on functional phenotype: interleukin 2 mRNA accumulation differentially induced by ionophore and receptor ligands in subsets of murine T cells. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6503–6507. doi: 10.1073/pnas.85.17.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Nau G. J., Kim D. K., Fitch F. W. Agents that mimic antigen receptor signaling inhibit proliferation of cloned murine T lymphocytes induced by IL-2. J Immunol. 1988 Nov 15;141(10):3557–3563. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Novak T. J., Rothenberg E. V. Differential transient and long-term expression of DNA sequences introduced into T-lymphocyte lines. DNA. 1986 Dec;5(6):439–451. doi: 10.1089/dna.1.1986.5.439. [DOI] [PubMed] [Google Scholar]
- Novak T. J., White P. M., Rothenberg E. V. Regulatory anatomy of the murine interleukin-2 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4523–4533. doi: 10.1093/nar/18.15.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten G., Herold K. C., Fitch F. W. Interleukin 2 inhibits antigen-stimulated lymphokine synthesis in helper T cells by inhibiting calcium-dependent signalling. J Immunol. 1987 Aug 15;139(4):1348–1353. [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Rothenberg E. V., Diamond R. A., Pepper K. A., Yang J. A. IL-2 gene inducibility in T cells before T cell receptor expression. Changes in signaling pathways and gene expression requirements during intrathymic maturation. J Immunol. 1990 Mar 1;144(5):1614–1624. [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P. L. Calcium mediates one of the signals required for interleukin 1 and 2 production by murine cell lines. Cell Immunol. 1984 Sep;87(2):720–726. doi: 10.1016/0008-8749(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gilbride K. J., Favata M. F. Lymphocyte activating factor promotes T-cell growth factor production by cloned murine lymphoma cells. Nature. 1980 Oct 30;287(5785):853–855. doi: 10.1038/287853a0. [DOI] [PubMed] [Google Scholar]
- Williams T. M., Eisenberg L., Burlein J. E., Norris C. A., Pancer S., Yao D., Burger S., Kamoun M., Kant J. A. Two regions within the human IL-2 gene promoter are important for inducible IL-2 expression. J Immunol. 1988 Jul 15;141(2):662–666. [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Daine B. Activation of IL 1-dependent and IL 1-independent T cell lines by calcium ionophore and phorbol ester. J Immunol. 1986 Feb 1;136(3):1033–1037. [PubMed] [Google Scholar]






