Abstract
Few studies have investigated how aging influences the neural basis of implicit associative learning and available evidence is inconclusive. One emerging behavioral pattern is that age differences increase with practice, perhaps reflecting the involvement of different brain regions with training. Many studies report hippocampal involvement early on with learning becoming increasingly dependent on the caudate with practice. We tested the hypothesis that the contribution of these regions to learning changes with age, due to differential age-related declines in the striatum and hippocampi. We assessed age-related differences in brain activation during implicit associative learning using the Triplets Learning Task. Over 3 event-related fMRI runs, 11 younger and 12 healthy older adults responded to only the third (target) stimulus in sequences of three stimuli (“triplets”) by corresponding key press. Unbeknownst to participants, the first stimulus’ location predicted one target location for 80% of trials and another target location for 20% of trials. Both age groups learned associative regularities, but differences in favor of the younger adults emerged with practice. The neural basis of learning (response to predictability) was examined by identifying regions that showed a greater response to triplets that occurred more frequently. Both age groups recruited the hippocampus early, but with training the younger adults recruited their caudate whereas the older adults continued to rely on their hippocampus. This pattern enables older adults to maintain near-young levels of performance early in training but not later, and adds to evidence that implicit associative learning is supported by different brain networks in younger and older adults.
Keywords: Implicit Learning, Implicit associative learning, Striatum, Hippocampus, Functional Neuroimaging
Implicit associative learning refers to the acquisition of information without intent or explicit knowledge of what has been learned (Frensch, 1998). Such learning is ubiquitous in daily life, underlying our sensitivity to routines and our ability to gain professional expertise (Cleeremans, 2002). Here, we examined the neural bases of implicit associative learning, and how these vary with adult age. Despite the importance of implicit learning in adapting to the world, it has been studied much less than its explicit counterpart in the context of aging.
This neglect is likely due to the dominant view that implicit learning is relatively unaffected by age (Dennis & Cabeza, 2008; Hedden & Gabrieli, 2004). Yet, this assumption is misleading; even though older adults can acquire new implicit associations, evidence from a range of tasks suggests that older adults rarely attain the level of performance of younger adults, and further, that the magnitude of age differences increases with training (Filoteo & Maddox, 2004; Maddox, Pacheco, Reeves, Zhu, & Schnyer, In press; Raz, Williamson, Gunning-Dixon, Head, & Acker, 2000). This finding is particularly true for probabilistic tasks, in which older adults’ learning asymptotes while younger adults continue to learn (Bennett, Howard, & Howard, 2007; Ciomek, Song, Howard, & Howard, 2007; Howard & Howard, 1997; Howard, Howard, Dennis, & Kelly, 2008; Simon, Howard, & Howard, 2010a). The current study asks why this is the case, by examining the neural bases of this form of learning in aging.
Research with younger adults suggests that implicit associative learning involves two interactive learning systems: one based on the medial temporal lobes (MTL) and the other based on the striatum. Specifically, studies show that the hippocampus is important for rapid association formation early in training, while the caudate is involved in integrating probabilistic information gradually over an extended temporal window (Poldrack & Packard, 2003). This pattern has been reported in the motor-based serial reaction time task (Nissen & Bullemer, 1987), in which people learn to make faster motor responses to repeating sequences vs. those that are randomly determined (Rose, Haider, Weiller, & Buchel, 2002; Schendan, Searl, Melrose, & Stern, 2003), as well as in the judgment-based weather prediction task (Knowlton, Squire, & Gluck, 1994), in which people learn to classify stimuli into two contrasting categories when given probabilistic feedback (Poldrack et al., 2001; Shohamy, Myers, Kalanithi, & Gluck, 2008).
Of note, healthy older adults have pronounced volume declines in the caudate (Gunning-Dixon, Head, McQuain, Acker, & Raz, 1998; Raz et al., 2003) while the hippocampus shows relatively little decline (Head, Snyder, Girton, Morris, & Buckner, 2005; Sullivan, Marsh, & Pfefferbaum, 2005; but see also Raz et al., 2004). Because studies show that Parkinson’s patients and animals with striatal damage have increased reliance on their intact hippocampus to support striatal-based learning (Moody, Bookheimer, Vanek, & Knowlton, 2004; Poldrack & Packard, 2003), we propose that age deficits in implicit associative learning appear with practice due to the relative aging of the hippocampus and caudate, and the functional reorganization of neural learning systems that occurs as a result. Early in training, older adults learn implicit associative regularities just as well as younger adults because the hippocampus, which is efficient at the rapid association formation needed early in training, is relatively spared with aging. However, age deficits emerge later because older adults continue to engage the hippocampus throughout training, and this structure is not as well-suited as the caudate for the gradual integration of complex, probabilistic associations (Ashby, Turner, & Horvitz, 2010).
To our knowledge, only five functional imaging studies have examined the effects of aging on the neural bases of implicit associative learning. One study found no age group differences in behavioral performance or in task-related brain activations, including striatal regions (Daselaar, Rombouts, Veltman, Raaijmakers, & Jonker, 2003). This age equivalence was likely due to the use of a “young” adult sample (aged 30-35) that is older than the 20-year-old groups used in other studies, which is important since the striatum shows linear declines starting in early adulthood (e.g., Raz et al., 2003). Of the remaining studies, each reported that older people had reduced striatal activation and increased reliance on regions elsewhere in the brain during learning relative to younger adults. In two studies, increased MTL activation was observed in older adults consistent with our hypothesis here (Dennis & Cabeza, In press; Rieckmann, Fischer, & Backman, 2010), whereas in two other studies, older adults recruited frontal or parietal cortices (Aizenstein et al., 2006; Fera et al., 2005). However, none of these studies yielded age-related deficits in behavioral learning. This may be a result of brief training, and/or that event timing was slowed down to fit the imaging protocol, which resulted in minimal learning effects in the younger adults that made it hard to detect age deficits. Likewise, some of the above studies used deterministic regularities, which may not produce age differences in learning (Howard & Howard, In press). Thus, it is not yet known which neural regions contribute to observed age differences in learning implicit probabilistic associations with practice.
Here we used the Triplets Learning Task (TLT; Howard et al., 2008), which uses event timing that is more conducive to learning and provides ample training to examine the time course of behavioral learning and of brain activations in younger and older adults. Importantly, studies using this task have shown that both age groups learn equally well early in training, but that age deficits appear over time (Howard et al., 2008; Simon et al., 2010a). Moreover, the TLT has been found to be sensitive to individual differences in striatal function in healthy younger adults (Simon et al., 2010b). In this task, participants view open circles that become red or green in discrete three-event sequences or “triplets.” On each trial, participants observe two red cues and respond only to a third green target via corresponding button, providing a continuous, performance-based learning measure. Unbeknownst to participants, triplets have a probabilistic second-order structure, in that the first cue’s location predicts one target location for a majority of the trials and another location for the remaining trials. Such complex probabilistic sequences minimize spontaneous explicit awareness (Reber, 1976), rendering it possible to study the evolution of implicit associative learning with training. In fact, people reveal such learning with practice by faster responses on more predictable trials despite having no explicit knowledge of the embedded regularities, even after 3 hours of training (Howard et al., 2008).
The present study used event-related functional imaging of the TLT to investigate the neural bases of implicit associative learning in healthy younger and older adults to examine why older adults rarely attain the level of learning of practiced younger adults. Our hypotheses focused on the hippocampus and caudate, and predicted that younger and older adults rely on these brain regions differently. Early on, hippocampal activation will be common to both age groups but older adults will reveal reduced caudate activity compared to younger adults. With more training, younger adults will continue to recruit the caudate whereas older adults will not, but will instead continue to recruit the hippocampus due to age-related striatal declines. We expect that this pattern of brain activity will result in age equivalent behavioral learning early on, but not later.
To ensure learning was implicit, we performed a separate behavioral experiment in younger adults who completed this identical TLT task outside of the scanner, followed by two sensitive measures of explicit knowledge.
Methods
Participants
For the fMRI study, 11 younger adults (18.8 ± .60 years old, 6 female) and 12 healthy, older adults (67.5 ± 3.2 years old, 9 female) received either course credit or monetary compensation for their participation. Younger adults were all students at Georgetown University and older adults responded to advertisements in the Washington Post Health Section. All participants, but one, were right-handed. Due to scanner malfunction, data were lost for 1 younger and 1 older adult in the final scan run (late training). The Georgetown University Institutional Review Board approved the experimental procedures, and all participants gave informed consent. Prior to participation, adults were screened for conditions that would prevent them from being able to enter the MRI environment. Participants were also screened for neurological disease or disorder, drugs known to influence cognition and/or meeting criteria for dementia (i.e. score below 27 on the Mini-Mental Status Exam) or abnormal intelligence status (i.e. scores outside the expected age-range on neuropsychological measures of processing speed, cued recall, free recall, verbal memory, vocabulary and reading ability) (see Table 1 for results).
Table 1.
Neuropsychological test results
| Cognitive Processing | Younger adults | Older adults | t | |
|---|---|---|---|---|
| MMSE | Screen for dementia | 30 (0.0) | 29.3 (.8) | −2.84*† |
| WAIS-III vocabulary | Vocabulary | 62.4 (6.7) | 67.9 (5.7) | 2.14* |
| WAIS-III digit symbol coding | Processing speed | 93.6 (14.8) | 60.7 (13.7) | −5.55** |
| WAIS-III digit symbol pairing | Cued recall | 15.5 (3.5) | 10.4 (5.0) | −2.77* |
| WAIS-III digit symbol recall | Free recall | 8.0 (1.3) | 7.0 (1.4) | NS |
| WAIS-III logical memory (unit) | Verbal memory | 49.6 (11.0) | 45.8 (6.4) | NS |
| WAIS-III logical memory (thematic) | Verbal memory | 18.7 (2.8) | 19.0 (2.0) | NS |
| WAIS-III digit span forward | Working memory | 12.4 (2.5) | 11.8 (2.0) | NS |
| WAIS-III digit span backward | Working memory | 7.4 (2.6) | 9.0 (2.6) | NS |
| COWAT-FAS Sum | Verbal fluency | 47.6 (12.2) | 46.0 (12.5) | NS |
| USC-REMT free recall correct | Free recall | 33.1 (4.9) | 24.9 (5.1) | −3.68* |
| USC-REMT free recall repetitions | Free recall | 2.6 (2.6) | 2.7 (3.6) | NS |
| USC-REMT free recall intrusions | Free recall | .6 (1.0) | 1.0 (.9) | NS |
| WJ-III Word Attack SS | Reading ability | 27.8 (2.4) | 27.5 (1.4) | NS |
| WJ-III Word Identification SS | Reading ability | 72.7 (2.8) | 74.2 (1.7) | NS |
Notes. All scores are given as mean (SD), with neuropsychological test scores based on raw data except where standard scores (SS = age-adjusted standard scores with a mean of 100 and SD of 15) are noted. Independent sample t-tests sow group effects (
p < .05,
p < .001, NS = not significant). Two younger participants did not complete the WAIS-III logical memory, COWAT-FAS and USC-REMT tests. MMSE = Mini Mental State Examination; WAIS-III = Wechsler Adult Intelligence Scale, 3rd Ed; COWAT-FAS = Controlled Oral Word Association Test-FAS; USC-REMT = University of Southern California- Repeatable Episodic Memory Test; and WJ-III = Woodcock-Johnson, 3rd Ed.
In the MMSE, all younger adults scored a perfect score (30) whereas older adults’ scores ranged from 28 to 30.
Participants in this imaging study completed three days of testing, although data from only the first day are reported here. On the first day, they completed the MRI scanning protocol, while the second and third testing days included two additional implicit learning tasks as well as a comprehensive neuropsychological test battery.
We did not include any probes of explicit awareness after the TLT in the fMRI study so as to keep learning implicit in the subsequent two implicit learning tasks. This decision was based on earlier evidence that neither younger nor older adults develop explicit knowledge in the TLT with more training than in the present study (Bennett, Romano, Howard, & Howard, 2008; Howard et al., 2008). However, to ensure that there was no explicit knowledge of the statistical regularities in the modified version of the TLT used here, we performed a separate behavioral experiment in younger adults which included both a post-experimental interview and a recognition task. For this separate behavioral study, 11 younger adults (20.1 ± 1.0 years old 6 female) received either monetary compensation or course credit for their participation. None had participated in the fMRI study.
Experimental paradigm
The TLT was a shortened and simplified version from that reported in Howard et al. (2008). Participants viewed three open circles on a computer screen, displayed against a gray background (Figure 1). Each trial or “triplet” consisted of the sequential presentation of two cue events (circles filling in red) followed by the target (a circle filling in green). Each red event was displayed for 200 ms and the green target remained in view for 850ms, with 250 ms separating events (total of 2000 msec/trial). Participants observed the first two red events and responded to only the third, green target event location as quickly and as accurately as possible via corresponding button (one of three buttons on a button box held in the right hand). Unbeknownst to participants, the first cue’s location probabilistically predicted the target event’s location as described below and the second cue’s location was unrelated, thereby creating second-order structure.
Figure 1.
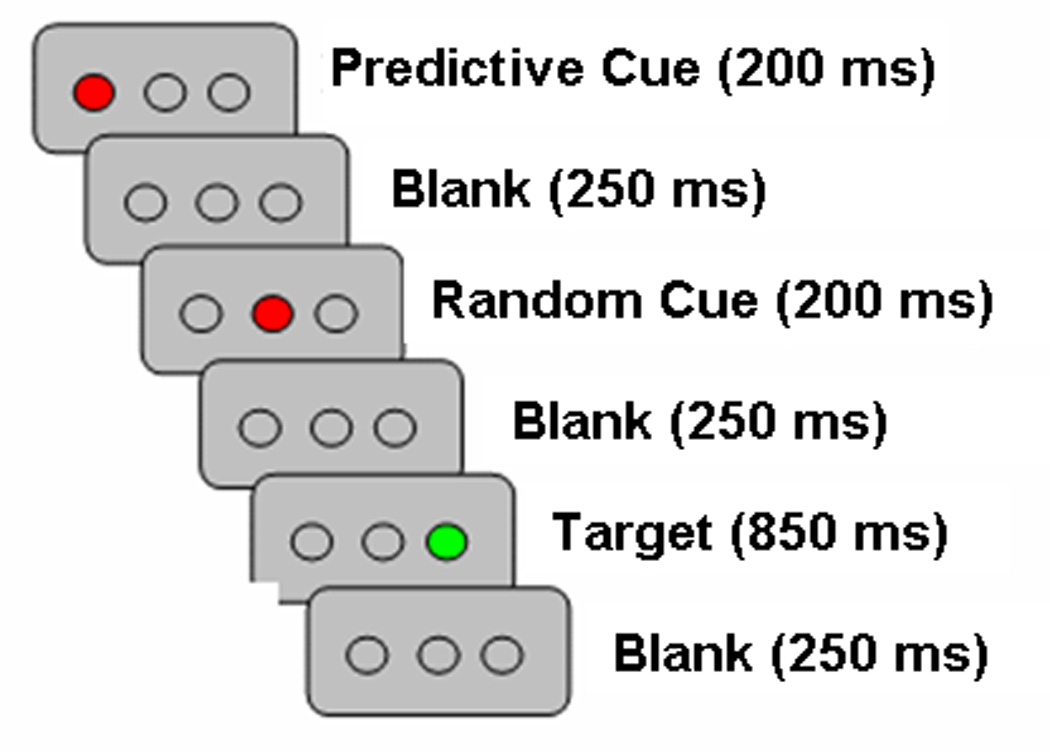
Sample presentation of one triplet in Triplets Learning Task.
Eighteen triplets (out of a possible 27) were presented: 9 occurred with high probability (HP) and 9 with low probability (LP). The frequency of HP to LP triplets was 4:1. Event locations were counterbalanced, such that cue and target events occurred equally often in each location. Trial order was presented in fixed, pseudorandomized sequence, optimized using OptSeq2 (Dale, 1999). Participants completed three runs, with breaks between each run. Each 6 minute and 30 second run included 108 HP and 27 LP trials that were presented in a rapid event-related design with a temporally jittered inter-trial interval (.5 – 6 sec, mean = 1.36 sec).
The behavioral sample performed the identical task as above. Again, participants were instructed to make behavioral responses by pressing one of three buttons in the right hand (using a keypad) that corresponded to the location of the target as quickly and accurately as possible. After testing, explicit knowledge was probed immediately in two ways. First, participants completed a post-experimental recognition task in which they observed each of the 27 possible triplets (only 18 of which had actually occurred during the TLT task), and they were instructed to judge if each triplet had occurred frequently, infrequently or never during training by responding 2, 1 or 0, respectively. Second, participants were interviewed about strategy and their declarative knowledge of triplet structure. Questions ranged from general inquiries about strategies used to improve performance to more specific questions that asked participants to describe any patterns or relationships between red and green events that they might have noticed.
fMRI acquisition
Imaging data were acquired using a 3.0 Tesla MRI system (Siemens Magnetom Trio, Erlangen, Germany). A technician positioned participants in the supine position with a circularly polarized head coil. A mirror mounted on the head coil allowed participants to view stimuli during scanning. Fitted padding minimized head movement.
A high resolution T1-weighted structural scan (MPRAGE) was acquired first, using a 3D MPRAGE sequence with a scan time of 7:23 minutes and the following parameters: TR/TE = 2300/2.94 ms, TI = 900 ms, 90° flip angle, 1 slab, 160 sagittal slices with a 1.0 mm thickness, FOV = 256×256 mm2, matrix = 256×256, resulting in an effective resolution of 1.0 mm3 isotropic voxels. A neurologist later reviewed these images and identified no clinically significant structural abnormalities (e.g., lesions or abnormal growths). Functional imaging was acquired on the same equipment while participants completed the TLT. Functional data were acquired along the AC-PC line using T2*-sensitive gradient Echo Planar Imaging pulse sequence with the following parameters: TR = 2500ms, TE = 30ms, 256×256 mm FOV, 64×64 acquisition matrix, 90° flip angle and a 0.3mm gap for an effective resolution of 4.0×4.0×4.0 mm3. Forty-two contiguous 3.7mm thick axial slices were acquired descending in the transverse plane for 154 time points for each run.
Behavioral analysis
To determine if participants showed implicit associative learning, we compared performance on HP vs. LP triplets. Repetitions (e.g. 111, 222) and trills (e.g. 121, 232) were excluded from the analyses reported below because they reflect pre-existing response tendencies (Boyer, Destrebecqz, & Cleeremans, 2005). Median reaction times (RTs) were determined for correct responses on each triplet type in each block of 27 trials. These medians were averaged to obtain a single mean RT for each individual and the two triplet types. A similar procedure was used to calculate the mean accuracy for each person for the two triplet types.
fMRI processing and data analysis
We focused our neuroimaging results only on Run 1, which will be referred to as early training, and Run 3, which will be referred to as late training. Our choice to analyze Runs 1 and 3 was driven by cognitive theories that have proposed distinct stages in learning, characterized as early training (i.e., fast encoding and rapid improvements) and late training (i.e., when performance reaches asymptote after associations are relatively well-learned) (e.g., Doyon, 2008; Karni & Bertini, 1997). Research has shown that different brain regions are involved for these distinct training stages, with early training involving the hippocampus and later learning involving the caudate (e.g., Poldrack et al., 2001). This choice was also based on previous work that has shown that older adults can acquire new implicit associations quickly and as well as younger adults (i.e., Run 1), but that age differences emerge with practice in favor of younger adults (i.e., Run 3) (e.g., Simon et al., 2010a). Thus, based on this previous literature, we did not have specific predictions for Run 2, nor did we know whether it should be considered early or late training1.
Functional images were analyzed in SPM5 (www.fil.ion.ucl.ac.uk/spm). The first 2 TRs were discarded from the analysis as they had been included for signal stabilization. All participants displayed less than 3mm of motion in any direction within each run, so no data were eliminated due to motion artifact. Images were slice-timed, motion corrected and spatially normalized to the MNI template using each subject’s high resolution structural MPRAGE. Normalized image volumes were then smoothed (8mm full width at half maximum Gaussian kernel) and temporally filtered (128 second high-pass filter). fMRI responses for correct responses on HP and LP triplets were modeled by a canonical hemodynamic response function, and autocorrelations removed signal related to biorhythms. The remaining trial types (trills, repetitions and incorrect trials) were excluded from analysis.
For each participant, an activation map was generated using a linear contrast identifying regions that showed greater response to predictable events (HP > LP) in the first run (early training) and in the third run (late training). Individual-level contrasts were entered into second-level analysis with subjects as a random factor. Given the study’s primary focus on the hippocampus and caudate, these areas were targeted as regions of interest (ROI) using one mask including both regions in both hemispheres, defined anatomically by the automated anatomic labeling (AAL) library (Tzourio-Mazoyer et al., 2002). Correction for multiple comparisons was performed using 3dClustsim (a function from AFNI), based upon Monte Carlo simulation of random correlated noise distribution to estimate the probability of false positive detection at p < .05 (p < .005 with a cluster extent of 19 voxels for the gray matter mask of the bilateral hippocampus and caudate combined (Ward, 2000: http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). Two second-level analyses were performed separately for early and late training: (1) To test our hypothesis of age group differences in the caudate and not the hippocampus, we compared the response to predictability between younger and older adults using a two-sample t-test. (2) To examine whether the hippocampus and caudate engagement varied with the magnitude of behavioral learning, we ran correlations between individual implicit associative learning scores and the response to predictability (activation associated with the HP > LP contrast) within each age group in a voxel-wise manner with the combined anatomical mask. Learning scores were calculated as the difference in performance between HP and LP triplets at both early and late training (i.e., LP triplet RT minus HP triplet RT).
Finally, as an exploratory analysis, we examined whether any regions in the rest of the brain differed by age group, using a two-sample t-test similar to that in (1) above, but without any mask, using an uncorrected threshold, p < .001.
All reported coordinates were converted from MNI to Talairach space using the algorithm mni2tal (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
Results
Behavioral results
fMRI Study
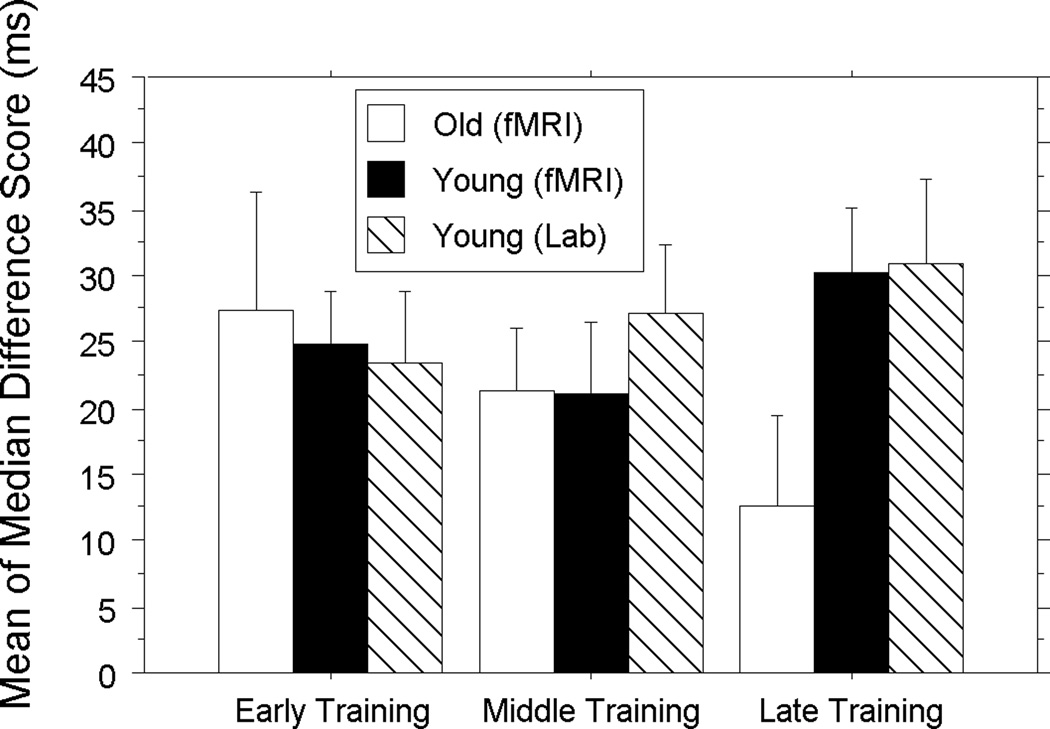
Data were analyzed using a Group (fMRI Young, fMRI Old)×Triplet (HP, LP)×Training Stage (Early, Middle, Late) mixed-design ANOVA. Group varied between-subjects, and triplet type and training stage varied within-subjects. Overall accuracy was high (Younger: M: 99.0%, SD: .03; Older: M: 98.4%, SD: .04), and the ANOVA for accuracy revealed no significant main effects or interactions, p’s > .24. This aids interpretation in that there were no age-related speed-accuracy trade-offs. As expected, the ANOVA for RT produced a main effect of group, F (1, 19) = 36. 22, p < .0001, revealing that younger adults responded significantly faster than older adults (Younger: 368.31 ± 49.96; Older: 482.72 ± 41.76). A main effect of training stage, F (1, 19) = 9.39, p = .0005, revealed significantly faster responses over time (Early: 439.64 ± 71.71; Middle: 423.59 ± 75.68, Late: 420.34 ± 72.66), and a main effect of triplet type, F (1, 19) = 47.19, p < .0001, revealed associative learning or significantly faster responses to HP than LP triplets (HP: 416.66 ± 72.08; LP: 439.50 ± 73.33). The triplet type×training stage interaction was not significant, F (1, 19) = 1.43, p = .252; however, the critical group×triplet type×training stage interaction was significant, F (1, 19) = 3.95, p < .05, showing age group differences in associative learning over time. RT difference scores, shown in Figure 2, revealed that younger adults had significantly higher learning scores than older adults only late in training (i.e., Run 3), t (19) = 2.05, p = .05.
Figure 2.
Difference of mean of median reaction times (RT) for Low – High Probability triplets in milliseconds over early, middle and late learning for younger (black) and older (white) adults in the fMRI study and younger adults in the separate behavioral study (cross-hatch). Error bars represent the standard error of the mean.
We further evaluated this age deficit in late learning by examining each individual’s implicit associative learning scores in Run 3, i.e., LP triplet RT minus HP triplet RT. Using a median split, we classified any subject with a difference score ≥ 20.2 ms as being a high learner and any other subject as a low learner. Of the high learners, 8 were younger adults and 3 were older adults whereas of the low learners 2 were younger adults and 8 were older adults, χ2 (2) = 5.8, p = .02. Thus, late in training, most younger adults were classified as high learners and most older adults as low learners.
Though neuropsychological measures had been collected to characterize our sample as normal, we also correlated these scores with individual implicit associative learning scores from early and late training to determine whether age-related differences in processing speed, free recall, or cued recall were related to age differences observed in late associative learning. There were no significant correlations between individual RT difference scores at early or late training and any of the neuropsychological measures, either within or across groups (p’s > .21).
Behavioral Study
Participants were highly accurate for both the HP and LP triplets across training stages (M: 99.2%, SE: 002). To assess potential differences in implicit associative learning inside and outside of the scanner, mixed design ANOVAs were conducted separately for RT and accuracy: Group (fMRI Young, Lab Young)×Triplet Type (HP, LP)×Training Stage (Early, Late). As expected, there were no significant main effects of experiment or interactions between the studies for either behavioral measure (p’s > .51), indicating similar learning between the two experiments (see Figure 2).
Measures of explicit knowledge
Younger adults in the separate behavioral study also completed two sensitive measures of explicit knowledge after testing. Post-experimental interviews revealed no strategies related to learning and no evidence of explicit knowledge, in that no one reported that the first cue predicted the target or that some triplets occurred more often than others. To assess explicit judgments of triplet frequencies on the recognition task, a one-way repeated measures ANOVA was conducted on the mean recognition ratings for each triplet type (HP, LP and those that never occurred). Data were lost for 1 participant, leaving 10 participants in this analysis. There was no relationship between judgment and triplet category, F(2,18) = .46, p > .05, such that all triplet types were rated as having occurred equally often during training (HP: 1.19 ± .17, LP1.14 ± .20, never occurred: 1.21 ± .25).
To examine individuals, 3 × 3 chi-square analyses for each person compared judgments (more often, less often, never) for the three triplet categories (HP, LP, never occurred). As expected, no individual had explicit knowledge of triplet frequencies, in that ratings did not vary with triplet type (p’s > .09). Moreover, associative learning was independent of recognition task judgments; triplet ratings on the recognition task (i.e., HP-LP ratings) did not correlate with individual RT difference scores in early or late training (p’s > .12). In sum, participants unknowingly learn regularities of the sort studied here.
fMRI results
Age group differences in the hippocampus and caudate
Consistent with our predictions and as seen in Figure 3, in early training we observed two clusters of activity in the left caudate that were significantly greater in younger versus older adults (x, y, z = −14, −11, 25; z = 3.31, k = 33 voxels; x, y, z = −8, 15, 6; z = 3.21, k = 32). Of note, and also as predicted, there were no significant age-related differences in the hippocampus in early training.
Figure 3.
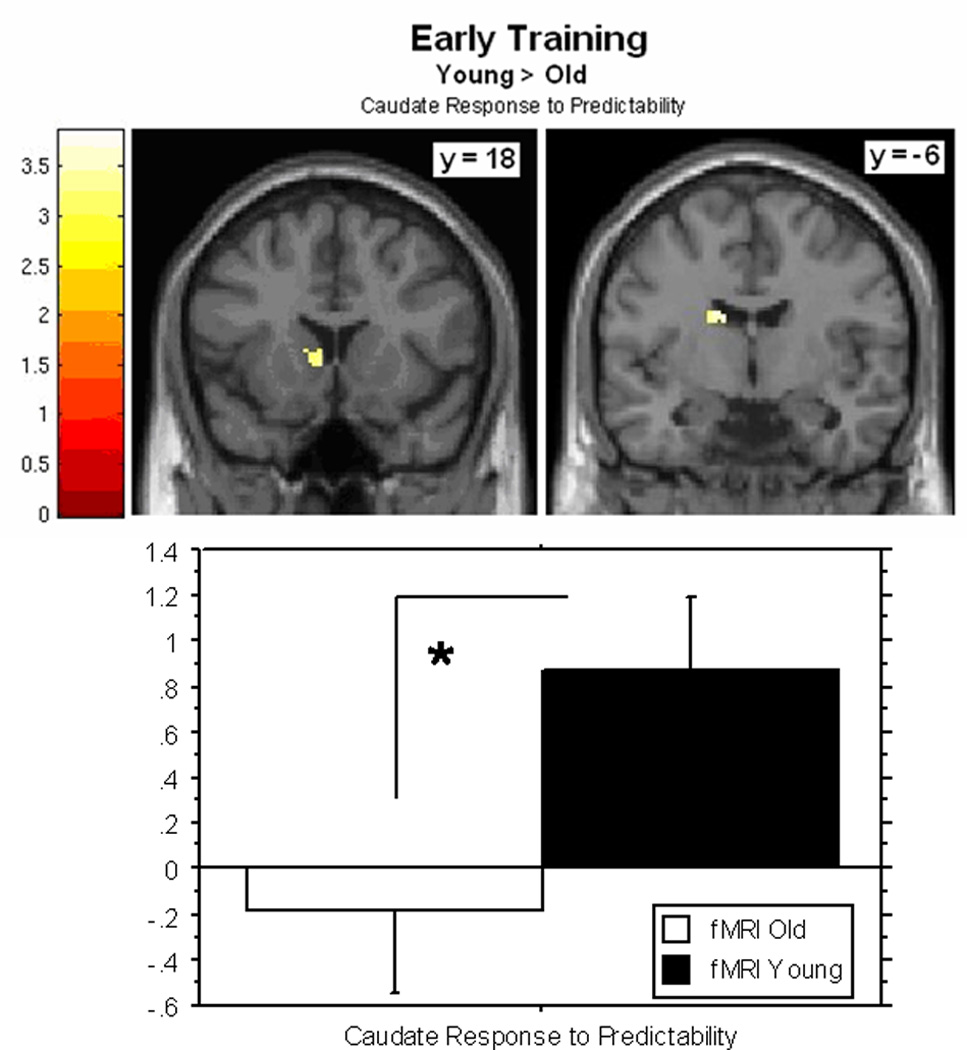
Clusters of activity in the left caudate in which younger adults show a greater response to predictability than older adults in early training. Graph shows contrast estimates, extracted from the mean of activated clusters using MARSBAR (±standard error) in younger and older adults (*p < 0.05).
The previous analysis does not reveal whether the hippocampus showed a response to predictability in each group, so we examined this using one-sample t-tests separately for younger and older adults using the same corrected threshold as above. As predicted, at early training hippocampal involvement was observed, in the left hemisphere in younger adults (x, y, z = −19, −38, 9; z = 3.50, k = 24 voxels) and bilaterally in older adults (x, y, z = 32, −16, −6; z = 3.90, k = 47 voxels; x, y, z = 25, −34, 7; z = 3.60, k = 44 voxels).
In late training, the response to predictability in the hippocampus and caudate did not differ significantly for younger and older adults.
Correlations with implicit associative learning
Using the aforementioned anatomical mask of the bilateral hippocampus and caudate, in early training no significant correlations were observed between the neural response to predictability and individual implicit associative learning scores for either age group.
Late training, however, revealed the predicted dissociation, such that individual differences in learning were related to individual differences in caudate response for younger adults and in hippocampal response for older adults. That is, as shown in Figure 4a for younger adults two clusters were observed in the bilateral caudate (x, y, z = 12, 10, −4; z = 4.44, k = 37 voxels; x, y, z = −16, 2, 20 ; z = 3.17, k = 43 voxels), both showing positive correlations with learning, whereas for older adults no significant clusters were observed in the caudate. In contrast, older adults revealed a positive correlation between learning scores and the hippocampus (x, y, z = −27, −37, 3; z = 3.23, k = 19 voxels) as shown in Figure 4b, that was not observed in the younger adults. Contrast estimates were then extracted from the mean of activated clusters using MARSBAR (Brett et al., 2002) in younger and older adults (see Figures 4c and 4d) to examine whether these correlations differed significantly between the age groups; the correlation between learning and the response to predictability was significantly larger in the younger than the older adults for the caudate, z = 1.98, p < .05, but the age group difference was not significant for the hippocampus, z = −.64, p > .05.
Figure 4.
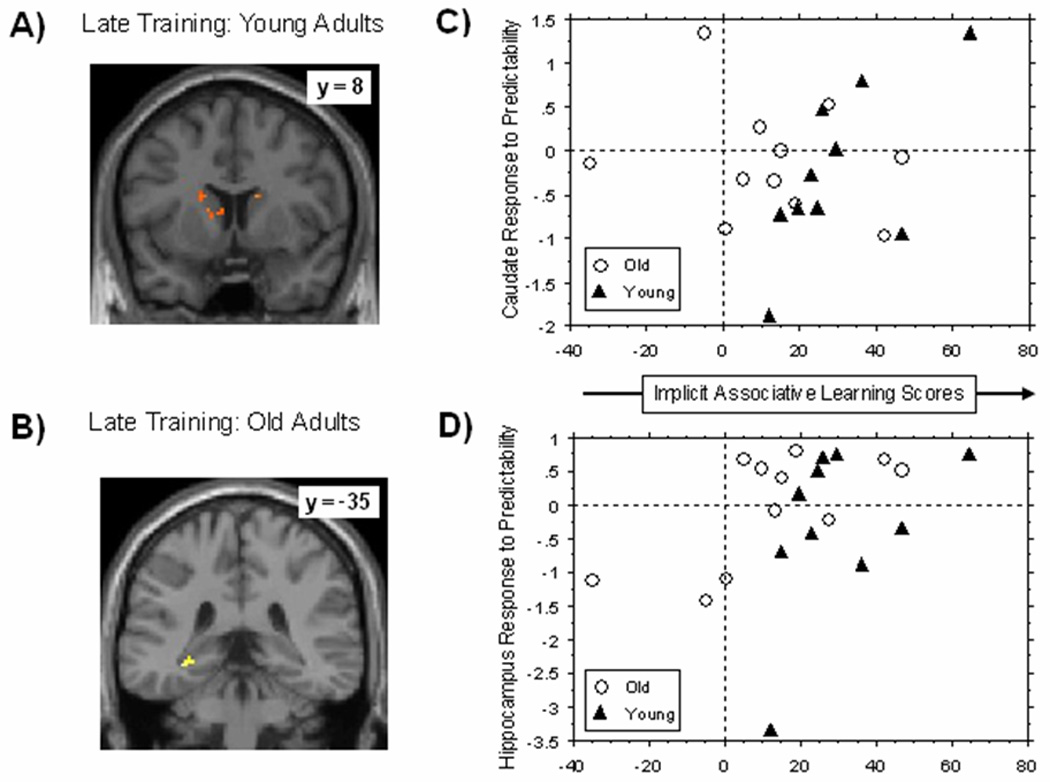
Figure 4. Regions showing significant correlations between implicit associative learning scores and neural response to predictability in (A) younger adults and (B) older adults at late training, using a combined mask of the bilateral caudate and bilateral hippocampus (anatomically-defined). The scatter plot is presented to visualize how individual differences in the (C) caudate and (D) hippocampus relate to individual differences in late implicit associative learning within each age group. Contrast estimates were extracted from the mean of activated clusters using MARSBAR (Brett et al., 2002) in younger and older adults.
Whole-brain age group differences
Early in training, younger adults showed a greater response to predictability than older adults in regions often observed during associative learning, including the bilateral caudate, the left dorsolateral prefrontal cortex and the bilateral cerebellum (see Table 4). In contrast, older adults showed a greater response to predictability than younger adults in parietal regions, including the inferior parietal lobule and the pre- and post-central gyri (Table 2). This is consistent with a previous study of aging and associative learning that showed compensatory parietal activation in healthy older adults that was related to deficient neural responses in the prefrontal cortex and caudate (Fera et al., 2005).
Table 2.
Age group differences in the neural response to predictability (High > Low Probability contrast) at early training
| Region | Hemisphere | BA | Talairach (mm) |
Peak z | Volume (mm3) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Younger > Older | |||||||
| Dorsolateral Prefrontal | |||||||
| Cortex | Left | 46 | −29 | 29 | 20 | 3.74 | 528 |
| Caudate | Left | −14 | −11 | 25 | 3.26 | 344 | |
| Caudate | Left | −8 | 15 | 6 | 3.19 | 232 | |
| Cerebellum | Right | 11 | −46 | −45 | 3.17 | 192 | |
| Cingulate | Left | 24 | −16 | 35 | 13 | 3.06 | 176 |
| Caudate | Right | 10 | 20 | 10 | 3.05 | 96 | |
| Cerebellum | Left | 19 | −20 | −58 | −14 | 2.98 | 88 |
| Older > Younger | |||||||
| Precentral Gyrus | Right | 6 | 47 | 5 | 19 | 3.85 | 232 |
| Inferior Parietal Lobule, Postcentral Gyrus | Right | 1/2/40 | 50 | −26 | 37 | 3.54 | 600 |
| Right | 39 | −21 | 30 | 3.24 | |||
| Cingulate/Precuneus | Right | 7/23/31 | 21 | −39 | 33 | 3.24 | 136 |
| Right | 13 | −38 | 31 | 2.88 | |||
| Precentral Gyrus | Right | 1/2 | 30 | −14 | 29 | 3.05 | 88 |
In late training, younger adults showed a greater response to predictability than older adults in the occipitotemporal cortices, whereas older adults showed a greater response than younger adults in bilateral frontal regions (see Table 3). This is consistent with findings that older adults show an age-related reduction in occipitotemporal activity coupled with an age-related increase in frontal activity (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008).
Table 3.
Age group differences in the neural response to predictability (High > Low Probability contrast) at late training
| Region | Hemisphere | BA | Talairach (mm) |
Peak z | Volume (mm3) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Younger > Older | |||||||
| Cingulate | Right | 24/32 | 17 | 35 | 17 | 3.95 | 264 |
| Occipital Gyrus | Left | 17/18 | −14 | −80 | 20 | 3.93 | 392 |
| Lingual Gyrus | Right | 36 | 29 | −42 | −10 | 3.69 | 872 |
| Inferior Temporal Gyrus, Middle Occipital Gyrus | Right | 36/37 | 36 | −71 | 2 | 3.45 | 992 |
| 19 | 36 | −74 | 14 | 3.43 | |||
| Lingual Gyrus | Left | 18 | −25 | −86 | −2 | 3.28 | 248 |
| Middle Temporal Gyrus | Right | 37 | 43 | −59 | 12 | 2.97 | 88 |
| Superior Frontal Gyrus | Right | 6 | 2 | −4 | 67 | 2.93 | 104 |
| Older > Younger | |||||||
| Inferior Frontal Gyrus | Right | 45/47 | 36 | 32 | 3 | 3.86 | 1304 |
| Right | 42 | 21 | 5 | 3.05 | |||
| Right | 47 | 26 | 2 | 2.94 | |||
| Cingulate | Left | 23 | −14 | −39 | 24 | 3.77 | 608 |
| Inferior Frontal Gyrus | Left | 44 | −34 | 17 | 22 | 3.07 | 168 |
Discussion
This study is the first to examine age differences in hippocampal and caudate involvement during early and late phases of learning of implicit associations. Results revealed three main findings. First, implicit associative learning is not spared in healthy aging but rather is characterized by age-related deficits evident later with practice. Second, this type of learning is supported by different neural regions in younger and older adults. Early in training, both age groups showed a similar response to predictability in the hippocampus, but younger adults showed a greater response to predictability than older adults in the caudate. Later in training, we observed systematic individual differences in the neural response to predictability that related to variation in the amount of learning within each age group; younger adults who showed greater implicit associative learning showed a greater caudate response to predictability, whereas for older adults, the hippocampus showed this relationship. The hippocampus is not as well suited as the caudate to the gradual acquisition of probabilistic associations over time (Ashby et al., 2010), so we argue that age deficits in learning emerge with practice due to age-related declines in the striatal learning system. To our knowledge, this is the first functional imaging study to examine the neural bases of implicit associative learning in aging when behavior is both spared (early) and impaired (late).
Age differences in behavioral learning
Younger and older adults both demonstrated learning of probabilistic associations in the TLT (i.e., faster responses to triplet events that occur more often), but age differences in learning emerged with practice in favor of the younger adults despite no changes in task demands (Howard et al., 2008; Simon et al., 2010a). Based on previous research from our lab we originally expected older adults’ learning to asymptote while younger adults’ would continue to increase across runs, but in the present study older adults’ learning scores appeared to decline from early to late training (p = .05). The underlying cause for this drop in performance is a matter for further investigation. However, from the present study, we know that this age-related learning deficit cannot be attributed to age differences in event timing within a triplet, since this did not depend on participant response rate and therefore could not be influenced by older adults’ longer and more variable response times (Howard, Howard, Dennis, & Yankovich, 2007). Nor could it be due to age-deficits in explicit associative learning (Old & Naveh-Benjamin, 2008) since no explicit knowledge was revealed on separate recognition tests. This age-related learning deficit also cannot be attributed to age differences on neuropsychological measures, as these scores did not correlate with learning in either age group. Finally, age deficits in learning are not a result of fatigue arising from longer training because training was relatively short in comparison to behavioral studies and further, age deficits have been observed in studies that had extended training via short sessions occurring over a number of days (Howard et al., 2004a). Instead, from our neuroimaging results, we argue that age differences in learning result from a fundamental deficit in the striatal learning system in older adults, a deficit which impairs learning of probabilistic associations of the sort embedded in the triplets task studied here.
Age differences in neural bases of learning
Early in training, older adults learn implicit associative regularities just as well as younger adults and the hippocampus reveals a similar response to predictable events in both age groups. The hippocampus is efficient at forming new stimulus representations that compress redundant information while differentiating predictive information (Gluck & Myers, 2001), and is likely involved early on as sequences are initially acquired, to flexibly bind the three events that occur within each triplet (Schendan et al., 2003). Early in training, we also observed a greater response to predictability in the caudate in younger adults as compared to older adults, even though the groups did not differ behaviorally. This result replicates reports of reduced striatal activation in older adults in associative learning tasks when behavioral learning was age equivalent (Aizenstein et al., 2006; Dennis & Cabeza, In press; Fera et al., 2005; Rieckmann et al., 2010). Although there is this initial learning period when age groups perform similarly behaviorally but not neurofunctionally, our study extends previous work by providing additional training that reveals age-related differences in both learning-related brain activity and behavior. This suggests that a caudate response to predictable events is necessary for the gradual acquisition and integration of triplet frequencies over time, or more generally, for learning implicit probabilistic associative events that occur more often with practice (Ashby et al., 2010).
Yet, when older adults showed impaired implicit associative learning later in training, we surprisingly did not see group-level age differences in the hippocampus or the caudate. The fact that we could only observe learning-related correlations late in training, but not group activations, is likely due to the variability in the amount of learning between individuals as training progresses. In late training, the caudate response to predictability was related to learning in younger adults only. Across subjects, greater learning scores were associated with greater caudate activation to more predictable events. Unlike younger adults, the hippocampus was associated with later learning in older adults only, such that older individuals who demonstrated greater learning in late training also showed a greater hippocampal activity in response to triplets that occurred more frequently. Reduced involvement of the caudate, but not the hippocampus, in older adults confirmed our predictions, which were based on evidence that the hippocampus exhibits less age-related morphological change than the caudate (Raz et al., 2005). Indeed, this claim is further supported by a diffusion tensor imaging study from our lab, which used the same sample of participants studied here. Importantly, Bennett, Madden, Vaidya, Howard and Howard (2010) found age group differences in the integrity of white matter connections (assessed by fractional anisotropy) for bilateral caudate-DLPFC tracts, but not for bilateral hippocampus-DLPFC tracts.
Taken all together, our results reveal age-related preservation of hippocampal function (e.g., Johnson, Schmitz, Asthanaab, Gluck, & Myers, 2008; Rand-Giovannetti et al., 2006) that accompanies striatal underactivation (Dennis & Cabeza, In press). This pattern is akin to how Parkinson’s patients and brain-lesion animals compensate for their striatal deficits (Moody et al., 2004; Poldrack & Packard, 2003). In fact, Rieckmann and Backman (2009) have argued that increased MTL activity in older adults might explain why some behavioral studies have observed no age deficits in implicit learning (e.g., Salthouse, McGuthry, & Hambrick, 1999). It is likely that older adults were able to maintain striatal-dependant behavioral learning via the hippocampus in these cases because the embedded regularities were deterministic, rather than probabilistic. Consistent with this view, behavioral age deficits were observed in the present study and in an implicit motor-based task that each had a complex second-order probabilistic structure (e.g., Howard & Howard, 1997), but not in a similar task that contained a complex second-order deterministic structure (Gaillard, Destrebecqz, Michiels, & Cleeremans, 2009). Because the striatum is better suited for learning complex, probabilistic associations over time than the hippocampus (Hartley & Burgess, 2005), age-related functional reorganization of learning systems may be unable to adequately compensate for striatal losses when learning probabilistic regularities, thereby producing age deficits in some forms of implicit associative learning. Future work should address this possibility empirically, by directly comparing the learning of deterministic vs. probabilistic regularities in younger and older adults.
No explicit knowledge
Despite having responded faster to high than low probability triplets during training, younger adults in the separate behavioral study were unable to discriminate between triplet frequencies in a post-experimental recognition task, consistent with work that had trained younger and older participants on up to 6,000 trials (Bennett et al., 2008; Howard et al., 2008; Simon et al., 2010a). This indicates that it is possible to test implicit learning in the TLT without use of a dual-task, such as counting tones simultaneously, which is often used to foster implicit learning in other studies (e.g., Grafton, Hazeltine, & Ivry, 1995). Training without a secondary task is better suited for isolating neural regions associated with implicit associative learning as opposed to those imposed by dual task demands, in addition to being less taxing and more practical for older adults.
Moreover, a post-training interview from this separate behavioral study revealed that people did not adopt conscious, deliberate strategies for stimulus selection in the TLT. This stands in contrast to other probabilistic associative learning tasks, like weather prediction, in which participants often use hypothesis-testing strategies that make it difficult to dissociate implicit from explicit learning (Meeter, Myers, Shohamy, Hopkins, & Gluck, 2006). Because age differences in strategy are not likely in the TLT, our results suggest that neural compensation with age can be non-strategic and does not always reflect deliberate changes in learning strategy (Reuter-Lorenz & Cappell, 2008).
Nonetheless, we cannot definitively establish that no explicit knowledge of triplet structure developed in our fMRI sample, even though our separate behavioral findings from younger adults suggest that it was unlikely. In fact, one possible interpretation of the finding that hippocampal activity is associated with better performance in older adults, is that older adults were explicitly aware of the triplet regularities. However, this is not likely for three reasons. First, as stated above, learning in the TLT has consistently been reported to be implicit for both younger and older adults, even with extended training (Bennett et al., 2008; Howard et al., 2008; Simon et al., 2010a). Second, younger adults are more likely to gain explicit knowledge than older adults in a range of learning/memory tasks, and typically, if younger adults do not become aware then older adults are not likely to either (e.g., Dennis, Howard, & Howard, 2006; Gaillard et al., 2009). Third, the hippocampus does not necessarily align with declarative awareness; recent work has divided memory systems based on processing modes rather than explicit knowledge (e.g., Henke, 2010), such that the same task, whether implicit or explicit, can involve either the medial temporal lobes or the striatum (e.g., Sadeh, Shohamy, Levy, Reggev, & Maril, 2011; Turk-Browne, Scholl, Chun, & Johnson, 2009). Thus, the most probable explanation is that the hippocampus is supporting learning of implicit associative contingencies throughout training in older adults, consistent with previous work in patients and younger adults (Chun & Phelps, 1999; Moody et al., 2004; Rose et al., 2002; Schendan et al., 2003).
Conclusions
In sum, older adults show declines in implicit associative learning, contrary to popular belief, and these deficits likely reflect age differences in the contribution of the hippocampus and caudate to implicit associative learning. Early on, when responding involves the relatively preserved hippocampus, there is age invariance in behavior, but when responding is dominated by the age-impaired striatum later in training, age differences emerge. Because the ability to acquire new implicit associations is essential throughout the lifespan, especially later in adulthood when little time is spent in formal schooling, our findings suggest that older adults may face challenges in acquiring new skills or adapting to new environments (e.g., graduated assisted living facilities and internet) even after extended exposure. Thus, future research should focus on understanding of the cognitive and neural bases of implicit learning in order to foster independent and successful aging.
Acknowledgments
This research was supported by grants R01AG036863, R37AG15450, F31AG034691 from the National Institute on Aging/National Institutes of Health; grant M01 RR023942-01 from the Georgetown Clinical Research Center and a dissertation grant to J.R.S. from the American Psychological Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. The authors want to thank Dr. Kelly Anne Barnes for help in designing the Triplets Learning Task for fMRI, Dr. William Gaillard for screening the high-resolution structural scans for abnormalities, Dr. Ilana Bennett, Meghan Shapiro and Stephanie Szary for help with data collection, and Dr. Xiong Jiang, Dr. John Van Meter, Eric Murphy and Evan Gordon for technical support and helpful comments and discussion. Preliminary findings from this project were presented at the Cognitive Neuroscience Society conference in San Francisco, CA in April of 2008 and 2011 and the Society for Neuroscience conference in Washington, DC, in November 2009.
Footnotes
We did examine the neural response to predictability from Run 2, and the pattern of results was consistent with what is reported for Run 3, only at an uncorrected threshold.
Due to the lack of triplet type×training stage interaction, we conducted a separate ANOVA across epochs (1–3) within the first session only to ensure that the observed main effect of triplet type was reflecting learning. This additional test revealed a significant triplet type×epoch interaction, F(2, 42) = 7.42, p < .0005, and no interactions with group (p’s > .08), indicating that associative learning occurred quickly for both age groups.
References
- Aizenstein HJ, Butters MA, Clark KA, Figurski JL, Andrew Stenger V, Nebes RD, et al. Prefrontal and striatal activation in elderly subjects during concurrent implicit and explicit sequence learning. Neurobiol Aging. 2006;27:741–751. doi: 10.1016/j.neurobiolaging.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Howard JH, Jr, Howard DV. Age-related differences in implicit learning of subtle third-order sequential structure. J Gerontol B Psychol Sci Soc Sci. 2007;62:P98–P103. doi: 10.1093/geronb/62.2.p98. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Jr, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Romano JC, Howard JH, Jr, Howard DV. Two forms of implicit learning in young adults with dyslexia. Ann N Y Acad Sci. 2008;1145:184–198. doi: 10.1196/annals.1416.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer M, Destrebecqz A, Cleeremans A. Processing abstract sequence structure: learning without knowing, or knowing without learning? Psychological Research. 2005;69:383–398. doi: 10.1007/s00426-004-0207-4. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox; Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan: 2002. [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Ciomek N, Song SS, Howard JHJ, Howard DV. Age deficits in probabilistic category learning. Washington, DC: Paper presented at the Association for Psychological Science; 2007. [Google Scholar]
- Cleeremans A. Encyclopedia of Cognitive Science. Vol. 2. New York: Macmillan Publishers; 2002. Models of implicit learning; pp. 491–499. [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Jonker C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Aging. 2003;24:1013–1019. doi: 10.1016/s0197-4580(03)00030-7. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging in Cognitive Aging. In: Craik FI, Salthouse TA, editors. Handbook of Aging and Cognition. 3rd Edition. Mahwah, NJ: Erlbaum; 2008. pp. 1–54. [Google Scholar]
- Dennis NA, Cabeza R. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.04.004. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Howard JH, Jr, Howard DV. Implicit sequence learning without motor sequencing in young and old adults. Exp Brain Res. 2006;175:153–164. doi: 10.1007/s00221-006-0534-3. [DOI] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–483. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, et al. Neural mechanisms underlying probabilistic category learning in normal aging. J Neurosci. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT. A quantitative model-based approach to examining aging effects on information-integration category learning. Psychology and Aging. 2004;19:171–182. doi: 10.1037/0882-7974.19.1.171. [DOI] [PubMed] [Google Scholar]
- Frensch PA. One concept, multiple meanings: On how to deine the concept of implicit learning. In: Stadler MA, Frensch PA, editors. Handbook of implicit learning. Thousand Oaks, CA: Sage Publications, Inc.; 1998. pp. 47–104. [Google Scholar]
- Gaillard V, Destrebecqz A, Michiels S, Cleeremans A. Effects of age and practice in sequence learning: A graded account of aging, learning and control. European Journal of Cognitive Psychology. 2009;21:255–282. [Google Scholar]
- Gluck MA, Myers CE. Gateway to Memory: An Introduction to Neural Network Models of the Hippocampus and Learning. (Vol.) Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Grafton ST, Hazeltine E, Ivry Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. AJNR Am J Neuroradiol. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Burgess N. Complementary memory systems: competition, cooperation and compensation. Trends Neurosci. 2005;28:169–170. doi: 10.1016/j.tins.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15:732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- Howard DV, Howard JH., Jr . Dissociable forms of implicit learning in aging. In: Naveh-Benjamin M, Ohta N, editors. Perspectives on human memory and aging. Psychology Press; (In press) [Google Scholar]
- Howard DV, Howard JH, Jr, Japikse K, DiYanni C, Thompson A, Somberg R. Implicit sequence learning: effects of level of structure, adult age, and extended practice. Psychol Aging. 2004a;19:79–92. doi: 10.1037/0882-7974.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV. Age differences in implicit learning of higher order dependencies in serial patterns. Psychol Aging. 1997;12:634–656. doi: 10.1037//0882-7974.12.4.634. [DOI] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV, Dennis NA, Kelly AJ. Implicit learning of predictive relationships in three-element visual sequences by young and old adults. Journal of Experimental Psychology: Learning, Memory and Cognition. 2008;34:1139–1157. doi: 10.1037/a0012797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JH, Jr, Howard DV, Dennis NA, Yankovich H. Event Timing and age deficits in higher-order sequence learning. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:647–668. doi: 10.1080/13825580601186635. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Asthanaab S, Gluck MA, Myers C. Associative Learning Over Trials Activates the Hippocampus in Healthy Elderly but not Mild Cognitive Impairment. Aging, Neuropsychology, and Cognition. 2008;15:129–145. doi: 10.1080/13825580601139444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Bertini G. Learning perceptual skills: behavioral probes into adult cortical plasticity. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learn Mem. 1994;1:106–120. [PubMed] [Google Scholar]
- Maddox WT, Pacheco J, Reeves M, Zhu B, Schnyer DM. Rule-based and information-integration category learning in normal aging. Neuropsychologia. 48:2998–3008. doi: 10.1016/j.neuropsychologia.2010.06.008. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeter M, Myers CE, Shohamy D, Hopkins RO, Gluck MA. Strategies in probabilistic categorization: results from a new way of analyzing performance. Learn Mem. 2006;13:230–239. doi: 10.1101/lm.43006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ. An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci. 2004;118:438–442. doi: 10.1037/0735-7044.118.2.438. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, et al. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62:433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, Acker JD. Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol. 2003;24:1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Reber AS. Implicit learning of synthetic languages: The role of instructional set. Journal of Experimental Psychology: Human Learning & Memory. 1976;2:88–94. [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Rieckmann A, Backman L. Implicit learning in aging: extant patterns and new directions. Neuropsychology Review. 2009;19:490–503. doi: 10.1007/s11065-009-9117-y. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, Backman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: Relations to performance. Neuroimage. 2010;50:1303–1312. doi: 10.1016/j.neuroimage.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Rose M, Haider H, Weiller C, Buchel C. The role of medial temporal lobe structures in implicit learning: an event-related FMRI study. Neuron. 2002;36:1221–1231. doi: 10.1016/s0896-6273(02)01105-4. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A. Cooperation between the Hippocampus and the Striatum during Episodic Encoding. J Cogn Neurosci. 2011;23:1597–1608. doi: 10.1162/jocn.2010.21549. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, McGuthry KE, Hambrick DZ. A framework for analyzing and interpreting differential aging patterns: Application to three measures of implicit learning. Aging, Neuropsychology and Cognition. 1999;6:1–18. [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Howard JH, Howard DV. Age Differences in Implicit Learning of Probabilistic, Unstructured Sequences. J Gerontol B Psychol Sci Soc Sci. 2010a doi: 10.1093/geronb/gbq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Stollstorff M, Westbay LC, Vaidya CJ, Howard JH, Howard DV. Dopamine transporter genotype predicts implicit sequence learning. Behav Brain Res. 2010b doi: 10.1016/j.bbr.2010.08.043. doi:10.1016/j.bbr.2010.1008.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiol Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Scholl BJ, Chun MM, Johnson MK. Neural evidence of statistical learning: Efficient detection of visual regularities without awareness. Journal of Cognitive Neuroscience. 2009;21:1934–1945. doi: 10.1162/jocn.2009.21131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]