Abstract
Spreading depression (SD), the likely cause of migraine aura and perhaps migraine, is triggered by widespread and unfettered neuronal hyperexcitability. Migraine and the initiating hyperexcitability of seizure, which involve oxidative stress (OS), are likely interrelated. Environmental enrichment (EE) decreases seizure and can reduce migraine. EE's well-characterized neuroprotective effect involves insulin-like growth factor-1 (IGF-1). Accordingly, we asked if IGF-1 could mitigate the hyperexcitability that initiates SD using rat hippocampal slice cultures. We demonstrate that IGF-1 significantly decreased SD susceptibility and related OS. We mimicked OS of SD and observed that IGF-1 abolished hyperexcitability from OS. Application of an antioxidant significantly decreased SD susceptibility and co-administration of an antioxidant with IGF-1 produced no additive effect, whereas an oxidizer significantly increased SD, and this effect was abrogated by IGF-1. Moreover, IGF-1 significantly decreased baseline OS, despite seemingly paradoxically increasing CA3 bursting. These results suggest that IGF-1 increased endogenous antioxidants to levels sufficient to buffer against the OS of SD. Insulin similarly mitigated SD susceptibility, but required a far greater dose. Since brain IGF-1 increases with EE, and, like insulin, independently functions as an EE mimetic, we suggest that EE mimetics are a novel source of therapeutics for SD, and by extension, migraine.
Keywords: hippocampus, IGF-1, slice culture, antioxidants, reactive oxygen species, migraine
Introduction
Migraine and its maladaptive transformation to high frequency and chronic migraine (HFCM) are immense health care burdens affecting 11% of the adult population worldwide, with 3% experiencing chronic daily headache (Rasmussen et al., 1991; Stovner et al., 2007). In the United States, these maladies result in annual medical and lost work-time costs of $30 billion (Goldberg, 2005; Hu et al., 1999), yet existing therapies, largely centering on use of anticonvulsants, are only modestly effective for treatment of HFCM (Mack, 2011).
Spreading depression (SD) is a slowly propagating loss of neuronal activity that is the most likely cause of migraine aura and perhaps migraine pain (Lauritzen and Kraig, 2005; Moskowitz et al., 1993). SD is associated with increased brain hydrogen peroxide concentration, likely due to the increased metabolic demands associated with this phenomenon (Viggiano et al., 2011). SD is preceded (Bureš et al., 1974; Somjen, 2001), and importantly, followed by increased synaptic activity, as recently shown (Grinberg et al., 2011). Synchronous and excessively increased brain excitability in a sufficient brain volume is necessary to trigger SD (Bureš et al., 1974; Somjen, 2001; Kunkler et al., 2005). Furthermore, without sufficient time for compensatory adaptation, recurrent epochs of excessively increased synaptic activity from repeated SDs may lower SD initiation threshold, and therefore be a determinant of HFCM (Kraig et al., 2010). Experiments performed using hippocampal slice cultures support this suggestion (Mitchell et al., 2010a).
In contrast, physiologically increased neuronal activity from environmental enrichment [(EE); i.e., increased physical, intellectual and social volitional opportunities], which necessarily occurs with sufficient time for adaptive change, has the opposite effect. EE is a well-recognized preconditioning stimulus that induces neuroprotection (Will et al., 2004). Notably, EE occurs with physiologically increased neuronal activity and increased hippocampus-based learning and memory (van Praag et al., 2000; Kraig et al., 2010) and EE reduces excessive hyperexcitability from seizures (Young et al., 1999; Kraig et al., 2010). Furthermore, there is evidence that EE reduces SD (Guedes et al., 1996) and improves migraine (Darabaneanu et al., 2011).
Since insulin-like growth factor-1 (IGF-1) is a primary determinant of neuroprotection from EE (Carro et al., 2001), we hypothesized that IGF-1 might act as an EE-mimetic that would reduce SD susceptibility. Our results here show that exposure to IGF-1 triggered a significant reduction in SD susceptibility, a protective effect that involves reduced oxidative stress (OS) and reduced hyperexcitability. Furthermore, insulin, which enhances hippocampus-based memory when delivered to brain via nasal administration (Stockhorst et al., 2004; Craft et al., 2011) had a similar protective effect against SD, but at a dose orders of magnitude greater than required for IGF-1. Moreover, while IGF-1 decreased the hyperexcitability for SD, it also increased spontaneous CA3 area bursting activity, consistent with the electrophysiological changes of hippocampus-based learning (Yanovsky et al., 1995). Our results provide the first evidence that EE-based signaling (i.e., involving IGF-1) can lead to development of novel therapeutics to prevent SD, and by extension, perhaps recurrent and HFCM. This work has appeared in preliminary form (Grinberg and Kraig, 2011).
Materials and Methods
Culture preparation, maintenance, and electrophysiology
Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago Medical Center and followed ARRIVE guidelines.
Slice cultures were prepared (Mitchell et al., 2010b) from nine day old male or female pups of Wistar rats (Charles River, Wilmington, MA, USA) and SD induced as previously described (Grinberg, et al., 2010; Pusic et al., 2011). All electrophysiological procedures were performed in serum-free media, containing (per 100 mL): Neurobosal medium (97 mL; #21103, Invitrogen, Carlsbad, CA, USA); Gem-21, (2.0 mL; #400-160-010; Gemini Bioproducts, Sacramento, CA, USA); Glutamax (1 mM; #35050, Invitrogen); Gentamicin (1 μg/mL; #15710-064, Invitrogen); D-glucose [(45%), 680 μL; #G8769, Sigma, St. Louis, MO, USA]; ascorbic acid (0.5 mM; #A4544, Sigma); Fungizone, (1 mg/mL; #15295, Invitrogen); NaCl (41 mM; #S6546, Sigma); Mg2Cl2 (0.8 mM; #M1028, Sigma); CaCl2 (1.6 – 2.4 mM; #21115, Sigma). The normalcy of slice culture electrophysiological function was verified by recording CA3 area field potentials evoked from bipolar electrical stimuli applied to the dentate gyrus (100 μs pulses, ≤ 0.2 Hz). The recording microelectrode was moved along the long axis of pyramidal neurons at the genu of CA3 until field potential excitatory post-synaptic responses were maximal (Fig. 1). Slices with CA3 field post-synaptic potential responses ≥ 3 mV (with applied currents of 10 – 20 μA) were used for experiments. The Matlab commands ‘filter’ and ‘butter’ (Matlab 7.1, Mathworks, Natick, MA, USA) were used to filter the digitized data with a second order high-pass Butterworth filter with a 1 Hz cutoff frequency for the signals shown in Figure 5.
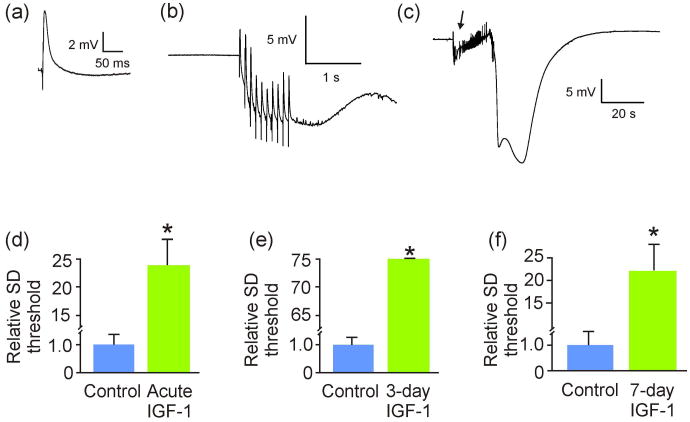
Figure 1. IGF-1 reduced spreading depression susceptibility in hippocampal slice cultures.
(a) Exemplary CA3 area evoked field potential. Experiments begin with establishment of the current intensity needed to evoke maximal field potential responses; stimuli of half-maximal intensity were then used to elicit subsequent field potentials. Only those cultures with CA3 pyramidal neuron post-synaptic responses of at least 3 mV were used for experiments. (b) CA3 response to dentate gyrus bipolar stimulation (10 pulses at 10 Hz, 500 μA) was used to elicit a spreading depression (SD), as shown in (c). (c) The spreading depression shown here was induced by the stimulation/response (arrow) shown in (b). (d) Average current necessary to induce SD (SD threshold) was significantly (*p = 0.001) higher when slice cultures were exposed to 40 ng/mL IGF-1 acutely (n = 6 and 7 for control and experimental slices, respectively). (e) Similarly, average SD threshold was also significantly (*p < 0.001) increased when slice cultures were exposed to IGF-1 for 3 days prior to SD (n = 8 and 7 for control and experimental slices, respectively). (f) Finally, average SD threshold was significantly (*p < 0.001) increased when slice cultures were exposed to IGF-1 for 7 days prior to SD (n = 11 and 6 for control and experimental slices, respectively.) Comparisons between groups made via Student's t-test.
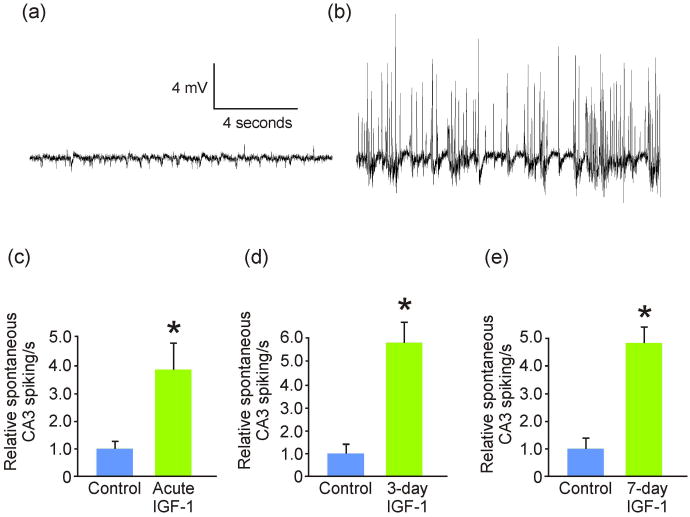
Figure 5. IGF-1 increased spontaneous neuronal spiking activity.
(a) Exemplary recording of unstimulated control CA3 pyramidal layer spontaneous electrophysiological activity. (b) Exemplary recording of 3-day IGF-1-exposed CA3 pyramidal layer spontaneous electrophysiological activity. (c) Acute IGF-1 treatment (n = 9) triggered a significant (*p = 0.03) increase in spontaneous CA3 pyramidal neuron spiking compared to control (n = 6). (d) 3-day IGF-1 treatment also (n = 7) triggered a significant (*p = 0.001) increase in spontaneous CA3 pyramidal neuron bursting compared to control (n = 6). (e) Similarly, 7-day IGF-1 treatment also (n = 6) triggered a significant (*p < 0.001) increase in spontaneous CA3 pyramidal neuron bursting compared to control (n = 7). Comparisons between groups made via ANOVA plus Holm-Sidak post hoc testing.
SD threshold was determined by progressively increasing the amount of current applied [10 pulses, 10 Hz (100 μs/pulse)], starting with the current needed to produce a half-maximal field potential from a single 100 μs pulse (Fig. 1). If a given current intensity did not trigger SD, the amount of current applied was doubled and re-applied 1 – 2 minutes later until SD occurred. Applied currents ranged from 10 to 10,000 nC. Unless otherwise stated, experimental agents were applied ‘acutely’ (i.e. 15 – 30 minutes before electrophysiological procedures). For OS measurements, SD was triggered 6 times over an hour (i.e., ∼ every 9 minutes).
While the number of SDs that can occur with migraine and HFCM has not been established, others suggest that chronic migraine may occur with only one SD per day. However, this experimental paradigm results in decreased brain excitability in anesthetized animals (Sukhotinsky et al., 2011), suggesting that the number of SDs and recovery period are critical to establishing a migraine model (Kraig et al., 2010). We chose 6 SDs so as to replicate the hyperexcitable phenotype seen in the brain of migraneurs (Palmer et al., 2000; Brennan, 2011; Mulleners et al., 2001; Welch, 2005) and after recurrent SD in hippocampal slice cultures (Mitchell et al., 2010a). Following SD, slice cultures were returned to normal incubation conditions with fresh media until fixation-harvest 24 hours later.
Slice culture excitability change from hydrogen peroxide exposure was assessed by noting the CA3 area field potential response to a single dentate gyrus 100 μs half-maximal current pulse applied 30 min after exposure.
Experimental manipulations
IGF-1 (40 or 100 ng/mL; #4326-RG, R&D Systems, Minneapolis, MN, USA) or insulin (400 μg/mL; #0355, Invitrogen) was added to media either acutely or 3 days before (and during) electrophysiological studies of SD threshold responses. For experiments involving SD and OS, slice culture IGF-1 was refreshed after SD. Hydrogen peroxide (50 or 200 μM; #H1009, Sigma) or ascorbate (2 mM) were added to serum-free media acutely before (and during) electrophysiological recordings. This hydrogen peroxide concentration was chosen to mimic concentrations produced by SD in vivo (Viggiano et al., 2011) and the ascorbate concentration is four-fold that of the serum-free media, consistent with physiological levels (Rice, 1999). Sham control cultures only experienced normal media.
Measurement of oxidative stress
CA3 area OS was determined using CellROX™ Deep Red Reagent (#C10422; Invitrogen), a fixable fluorogenic probe that fluoresces (near infrared) when oxidized. After SD, slice cultures were incubated for 24 hours in normal media supplemented with CellROX™ (5 μM) followed by fixation using 10% phosphate buffered formalin (#SF100-4; Fisher Chemicals, Fair Lawn, NJ, USA) for 24 hours. Then, slice cultures were mounted on gelatin-coated glass slides and coverslipped using Prolong® Gold antifade reagent (#P36930; Invitrogen).
To indirectly assess antioxidant content, slice cultures were exposed to a standard load of OS, namely 50 μmol/L menadione (#47775; Sigma-Aldrich) for 1 hour. Menadione participates in redox cycling reactions at the mitochondrial electron transport chain, leading to production of superoxide anions (Thor et al., 1982). Slices were then incubated in 5 μM CellROX™ for 30 minutes, followed by fixation in 10% phosphate buffered formalin for 24 hours. Hydrogen peroxide could not be used as a mimetic of SD-induced OS here, since we found that it interferes with the CellROX™ reporter molecule (data not shown). Cell death, as measured by Sytox Green (Hulse et al., 2008; Mitchell et al., 2010b), was not observed following hydrogen peroxide or menadione treatment at harvest times described above. There was also no pyramidal neuron death following SD induction, IGF-1 exposure, or any combination thereof (data not shown).
Fluorescence intensity of CellROX™ was measured using a Cool Snap fx CCD camera (Photometrics, Tucson, AZ, USA) on an inverted Leica DM-IRBE microscope (Leica Mikroskopie und Systeme, Wetzlar, Germany) and MetaMorph (v. 7.0.4) software (Molecular Devices, Sunnyvale, CA, USA). Fluorescence intensity (i.e., average fluorescence intensity/area) was registered for a uniform CA3 area of interest (used throughout experiments) at 10× magnification (i.e., 1.70 mm2). Before acquisitions, the imaging system was calibrated to register uniform full image intensity (1500/4096) to a standard (480 nm excitation; 527 nm emission) 125 μmol/L acridine orange (100 mg/L; #A6014; Sigma; St. Louis) solution imaged through a hemacytometer.
Statistical Methods and Figure Preparation
Data was analyzed using SigmaStat (V. 3.5) software (Systat Software, Chicago, IL, USA). Control data for each experiment was set to 1.0 with related group data scaled proportionally to allow for inter-experimental comparisons. All experimental group measurements were compared to same-day sham and/or control cultures and reported as mean ± standard error of mean. Specific statistical tests used are indicated in figure legends. CorelDraw (v. X3; Corel, Ottawa, ON, Canada) and Photoshop (v. CS2; Adobe, San Jose, CA, USA) were used to produce figures.
Results
IGF-1 (and insulin) significantly increased SD threshold
Hippocampal slices were exposed to IGF-1 either acutely (i.e., 15 - 30 minutes), for 3 days, or for 7 days prior to assessing SD threshold. The 7-day IGF-1 exposure was performed phasically to better mimic anticipated effects of EE [i.e., exercise – rest intervals (Will et al., 2004; Kraig et al., 2010)], where slices were exposed to IGF-1-supplemented media in the day and returned to regular media at night. Acute, 3-day, and 7-day exposure to IGF-1 all significantly increased SD threshold compared to control by 24, 75, and 22-fold (Fig. 1). Furthermore, 3-day exposure to insulin [(400 μg/mL); but not lower insulin doses, i.e., 6, 12, and 100 μg/mL (n = 3-9/group)] resulted in a significantly (p = 0.03) higher SD threshold versus control [i.e., 22.60 ± 9.60 (n = 8) and 1.00 ± 0.20 (n = 9), respectively]. However, the insulin dose needed for this protective effect was 15,500-fold higher than IGF-1 (i.e., 70 μM versus 4.5 or 10 nM), suggesting that IGF-1 has greater therapeutic utility against SD. Accordingly, we focused our subsequent work to IGF-1.
IGF-1 significantly reduced OS from SD
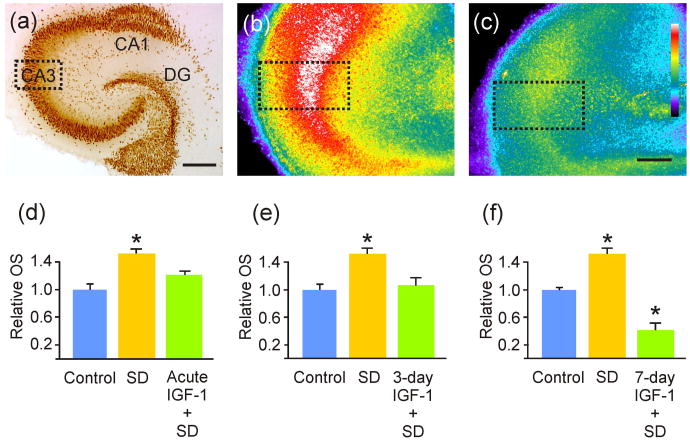
Since SD may increase OS (Viggiano et al., 2011), OS can enhance brain excitability (Gulati et al., 2005; Waldbaum & Patel, 2010; Muller et al., 1993), and IGF-1 is involved in antioxidant signaling (see Discussion), we next tested whether IGF-1 treatment altered SD-induced OS. Results show that acute, 3-day and 7-day treatment with IGF-1 significantly reduced OS from SD (Fig. 2). 7-day exposure was again phasic, as described for SD threshold studies above. While acute treatment with IGF-1 led to a 20% decrease in OS from SD, 3-day exposure to IGF-1 afforded an even greater level of protection, with a 30% decrease in OS from SD, and 7-days offered a 73% decrease in OS from SD.
Figure 2. IGF-1 decreased oxidative stress from spreading depression.
(a) NeuN immunohistochemical labeling of a hippocampal slice culture, for cytoarchitectural reference and to show the CA3 area of interest (dotted line box) used for quantification of oxidative stress (OS) via CellROX™ fluorescence intensity. (b-c) Representative CellROX™-labeled hippocampal slices exposed to SD (b) and to 3-day IGF-1 incubation followed by spreading depression (SD) (c). Dotted line boxes illustrate CA3 areas of interest used for relative OS quantifications. (d) OS was significantly (*p = 0.008) increased from controls after hippocampal slice cultures were exposed to SD, and this effect was abrogated when exposed to IGF-1 acutely (n = 21, 12 and 9 for control, ‘SD’ and ‘SD + IGF-1’ slices, respectively). (e) Similarly, the significantly (*p = 0.007) increased OS induced by SD was abrogated when slices were exposed to IGF-1 for 3 days prior to SD (n = 21, 8 and 6 for ‘SD’ and ‘SD + IGF-1’ slices, respectively). (f) The significant increase in OS from SD (*p < 0.001), when compared to controls, was significantly reduced (*p < 0.001) in slices exposed to IGF-1 for 7 days prior to SD induction (n = 21, 12 and 3 for ‘SD’ and ‘SD + IGF-1’ slices, respectively). Note: IGF-1 exposure was continued for the additional 24 hour CellROX™ incubation. Scale bars = 400 μm (a) and 200 μm (b and c). Comparisons between groups were made via ANOVA plus Holm-Sidak post hoc testing.
SD susceptibility is modulated by OS
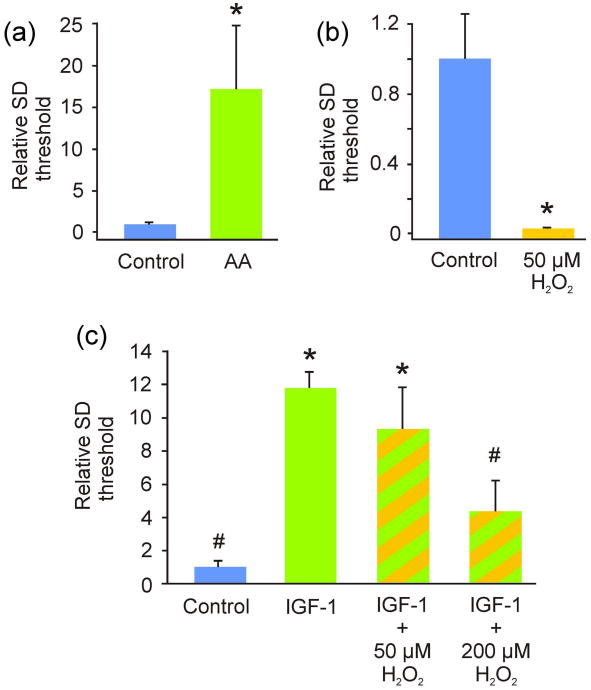
Slices were exposed to either ascorbic acid or hydrogen peroxide and SD threshold was assessed. Ascorbate (2 mM) significantly increased SD threshold, while hydrogen peroxide (50 μM) significantly decreased SD threshold (Fig. 3). Co-exposure to IGF-1 and a higher dose of hydrogen peroxide (200 μM) led to a significant decrease in SD threshold when compared to IGF-1 alone. However, 50 μM hydrogen peroxide co-exposed with IGF-1 was an insufficient oxidant stress to overwhelm the protective effect of IGF-1 on SD susceptibility (Fig. 3). Finally co-incubation of slice cultures with ascorbate and IGF-1 (n = 8) did not significantly raise the threshold for SD versus IGF-1 alone (n = 7) [p = 0.28 with relative SD threshold levels of 7.39 ± 6.16 and 1.00 ± 0.31, respectively].
Figure 3. The exemplary antioxidant ascorbate reduced, whereas the oxidizer hydrogen peroxide increased, spreading depression susceptibility, with the latter effect abrogated by IGF-1.
(a) Average current necessary to induce spreading depression (SD; i.e., SD threshold) was significantly (*p = 0.018) higher when slice cultures were acutely exposed to ascorbate (AA; n = 8) when compared to controls (n = 12). (b) In contrast, average current necessary to induce SD was significantly lower (*p < 0.001) when slice cultures were exposed to 50 μM hydrogen peroxide (H2O2; n = 8 and 11 for control and experimental slices, respectively). (c) While IGF-1 triggered a significant protection from SD susceptibility (*p < 0.0001) and this effect continued when co-administered with 50 μM H2O2, the higher dose of 200 μM H2O2 abrogated this effect to a non-significant difference from control. (n = 14, 16, 5, 9 for control, IGF-1, IGF-1 + 50 μM H2O2, and IGF-1 + 200 μM H2O2, respectively). When compared to IGF-1, SD thresholds of controls and IGF-1 + 200 μM H2O2 were significantly decreased (#p ≤ 0.00001). Comparisons between groups were made via Student's t-test (a, b) or ANOVA plus Holm-Sidak post hoc testing (c).
IGF-1 eliminated effects of SD-mimetics on excitability and OS
We further assessed the ability of IGF-1 to reduce slice culture excitability by decreasing OS. First, we mimicked OS from SD by application of hydrogen peroxide. This exogenously induced OS significantly increased evoked slice hyperexcitability (Fig. 4), like that seen from SD (Mitchell et al. 2010a). Both 3-day and acute exposure to IGF-1 abrogated this hydrogen peroxide-induced hyperexcitability. Second, we additionally mimicked OS from SD by slice exposure to menadione (Fig. 4). As expected, this treatment triggered a significant increase in slice OS, an effect that was abrogated by acute and 3-day exposure to IGF-1. In fact, 3-day exposure to IGF-1 alone could significantly reduce baseline OS from control levels. Furthermore, 7-day exposure to IGF-1 also significantly reduced baseline OS levels by 26% when compared to controls (p = 0.001; n = 11 and 9 for controls and 7-day IGF-1, respectively). The latter is important because exposure to IGF-1 alone, which led to the significant reductions in baseline OS (Fig. 4), triggered a significant increase in spontaneous CA3 bursting (Fig. 5).
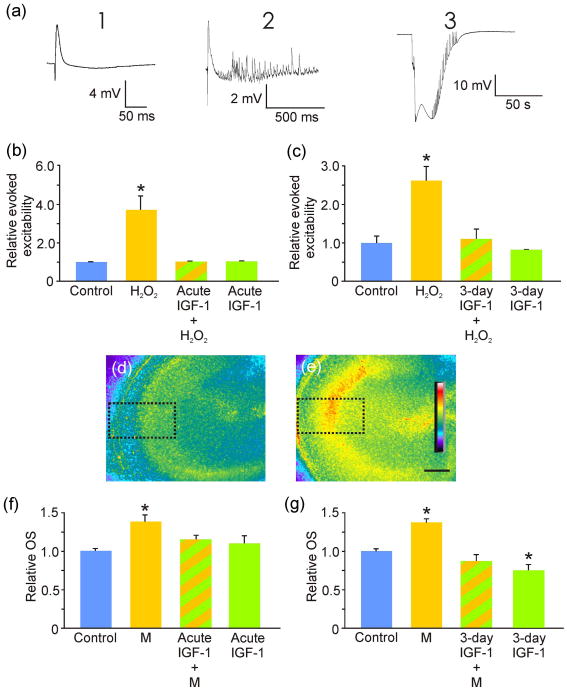
Figure 4. IGF-1 decreased CA3 oxidative stress and its related hyperexcitability.
(a) Slice culture excitability in response to oxidative stress (OS) was further characterized by classifying evoked potential changes to a single current pulse that normally triggered a half-maximal field potential response where a normal field potential (FP; left) was rated “1”; a FP that included stimulus-related bursting activity (center) was rated “2”; and a stimulus that resulted in spreading depression (right) was rated is a “3”. Relative evoked excitability was determined as a sum of responses seen (e.g., responses of “2” and a “3” yielded an overall excitability score of five). Responses were measured 30 minutes after exposure to hydrogen peroxide (H2O2). (b) Exposure to H2O2 (n = 7) triggered a significant (*p < 0.001) increase in evoked excitability compared to control (n = 8) and this increase was abrogated by acute application of IGF-1 (n = 4) to a non-significant difference from control. IGF-1 exposure alone (n = 4) had no significant impact on slice CA3 area evoked excitability (i.e., showed a response of “1”). (c) 3-day exposure to IGF-1 had a similar impact on slice culture OS-increased excitability mimicked by application of H2O2. H2O2 significantly (*p < 0.001) increased slice excitability (n = 15) compared to control (n = 18). Pretreatment with IGF-1 for 3 days (n = 9) reduced the H2O2-induced increased excitability to a non-significant difference from control. (d-e) Exemplary images of control (d) slice culture OS compared to increased slice OS induced by exposure to menadione (e). Calibration bar, 200 μm. Dotted boxes indicate CA3 areas of interest used for relative OS quantifications (f and g). (f) Exposure to menadione (M; n = 12) triggered a significant (*p < 0.001) increase in OS compared to control (n = 18). Acute treatment with IGF-1 (n = 15) reduced OS from menadione to a non-significant (p = 0.15) difference from control (n =18). Acute IGF-1 exposure alone (n = 18) did not reduced slice culture OS from control. (g) Pretreatment with IGF-1 for 3 days (n = 15) also reduced menadione-induced significant increase in OS (*p < 0.001; n = 12) to a non-significant (p = 0.148) difference from control (n = 15). In addition, IGF-1 pretreatment alone (n = 18) significantly (*p = 0.008) reduced slice culture OS compared to control.
Discussion
Here we show that IGF-1 mitigated SD susceptibility and decreased its associated OS. Furthermore, we show that OS-induced hyperexcitability and increased SD susceptibility, and that IGF-1 mitigated these effects. Finally, our results revealed that IGF-1 treatment lowered baseline levels of OS and simultaneously increased spontaneous activity of CA3 pyramidal neurons. These are the first results to indicate that a neuroprotective EE mimetic, IGF-1, prevents SD.
Evidence suggests that EE leads to a physiological increase in neuronal excitability that prevents SD. While SD increases aberrant hyperexcitability (Kruger et al., 1996) that makes brain tissue more susceptible to future SD (Mitchell et al., 2010a; Grinberg et al., 2011), EE increases physiological neuronal excitability associated with improved learning and memory (Kumar et al., 2011; Eckert & Abraham, 2010). EE protects from the aberrant hyperexcitability of seizure (Young et al., 1999; Kraig et al., 2010), and has been shown to reduce SD (Guedes et al., 1996). Furthermore, the neuroprotective effects of EE have also recently been shown to include migraine (Darabaneanu et al., 2011). While various molecular mechanisms of how EE produces these neuroprotective effects have been characterized (Gagné et al., 1998; van Praag et al., 2000; Ekstrand et al., 2008; Herring et al., 2010; Kempermann et al., 2010), the role of IGF-1 is particularly noteworthy.
IGF-1 mediates the neuroprotective effects of EE (Carro et al., 2001), and improves learning and memory (Sonntag et al., 2000). With EE, IGF-1 production is increased and its active uptake by the brain increases in an activity-dependent manner (Nishijima et al., 2010). Once in brain, IGF-1 increases spontaneous hippocampal neuronal activity and improves hippocampus-dependent learning and memory test performance (Lupien et al., 2003; Miltiadous et al., 2011; Xing et al., 2007). Mechanisms by which IGF-1 affects neuronal activity may include increasing ionic conductances (Blair & Marshall, 1997; Kanzaki et al., 1999), modulating neurotransmitter receptor activity (Gonzalez de la Vega et al., 2001; Ramsey et al., 2005), and decreasing generation of reactive oxygen species (Csiszar et al., 2008; Pérez et al., 2008). Important to our work here, increased neuronal activity enhances neural antioxidant production (Papadia et al., 2008). Furthermore, IGF-1 has been shown to similarly increase antioxidant production in multiple peripheral tissues (Jallali et al., 2007; Csiszar et al., 2008). Here we show that IGF-1 reduced OS in control hippocampal slices, a preparation which shows spontaneous, physiological neuronal activity. In fact, this spontaneous activity increased after IGF-1 exposure, a phenomenon that should elevate metabolic activity and therefore the generation of reactive oxygen species. Despite this, we found that net OS significantly declined. We speculate that this results from neural activity-dependent signaling involving increased antioxidant production, as first shown by Papadia and coworkers (2008). While beyond the scope of our current report, future studies are designed to directly confirm that IGF-1 can lead to increased antioxidant production in brain.
We show that SD induces increased tissue OS, as other work has suggested (Viggiano et al., 2011). OS increases hyperexcitability (Gulati et al., 2005; Waldbaum & Patel, 2010; Muller et al., 1993). We confirm and extend these findings to show that OS-induced CA3 hyperexcitability can lead to SD. Furthermore, we show that IGF-1 mitigated the amount of OS generated by SD, SD susceptibility, OS-induced SD susceptibility, as well as the hyperexcitability of OS. Finally, the impact of IGF-1 protection from SD-induced OS increased with time. Together, these results suggest that IGF-1's effects on OS (and, therefore, SD susceptibility) may involve an adaptive response, consistent with physiological-conditioning hormesis (Radak et al., 2008). These effects may help to entrain brain tissue away from the unfettered hyperexcitability needed for SD and toward physiological excitability and decreased OS.
IGF-1 is highly protective in stroke (Liu et al., 2004; Rizk et al., 2007; Fletcher et al., 2009). SDs occur spontaneously in the penumbra of stroke. The number and cumulative duration of the SDs occurring there are proportional to the growth in infarct volume (Dijkhuizen et al., 1999; Mies et al., 1993; Nakamura et al., 2010). Our results may indicate that the mechanism by which IGF-1 decreases stroke size involves decreasing the spontaneous SDs occurring in the penumbra of stroke.
Although we demonstrate the proof of principle that EE-mimetic IGF-1 decreases OS and SD susceptibility, a complete analysis of the optimal dose, duration, and frequency of treatment requires further study. As a first step, we chose to administer 7-day IGF-1 phasically to better mimic the inherently phasic effects of EE, as well as to avoid potentially harmful effects of prolonged tonic application of agents, such as those seen with corticosterone (de Kloet et al., 1999; Zoladz & Diamond, 2009).
We suspect that the 24-fold reduction in SD susceptibility seen with acute IGF-1 exposure that further increased to 75-fold at three days before settling to 22-fold at seven days reflects an adaptive, damped oscillatory response, commonly seen in biological systems (Stark et al., 2007; Paszek et al., 2010; Wang et al., 2012). In contrast, the progressive reduction of OS from IGF-1 by 20, 30, and 73% at these time points suggests a maximal steady-state has not yet been reached. Thus, whether OS too would show a damped oscillatory or a more simple sigmoid response pattern remains unclear. However, collectively these results suggest that with optimal dosing, the ability of IGF-1 to protect against SD via reduced OS can be expected to be at least 20-fold.
While insulin is already recognized as an agent that increases learning and memory (Stockhorst et al., 2004; Craft et al., 2011), its ability to influence SD has not been previously examined. We show that insulin protects against SD and hypothesize that it does so, like IGF-1, via its actions as an EE mimetic (i.e., by increasing processes associated with improved learning and memory, such as CA3 bursting). However, while insulin mitigated SD susceptibility, we show that IGF-1 has this effect at a 15,500-fold smaller dose, suggesting insulin's effects may occur via cross-reactivity with the IGF-1 receptor. We thus conclude that neuroprotective EE mimetics are promising targets against SD, and by extension migraine and HFCM, with IGF-1 shown here to be a novel and potentially effective therapeutic.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (NS-19108), the National Institute of Child Health and Human Disorders (5 PO1 HD 09402), ARRA supplement NS-19108-23S1, the Migraine Research Foundation, the White Foundation, and the Dr. Ralph and Marian Falk Medical Research Trust. We thank Heidi M. Mitchell for assistance in the preparation and maintenance of culture systems and comments referable to the early drafts. We thank Aya D. Pusic for reviewing the manuscript and providing advice throughout this work.
Abbreviations
- EE
environmental enrichment
- HFCM
high frequency and chronic migraine
- IGF-1
insulin-like growth factor-1
- OS
oxidative stress
- SD
spreading depression
Footnotes
The authors have no conflicts or financial interest to disclose.
References
- Blair LA, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- Brennan KC. Turn down the lights!: an irritable occipital cortex in migraine without aura. Neurology. 2011;76:206–207. doi: 10.1212/WNL.0b013e3182074bfb. [DOI] [PubMed] [Google Scholar]
- Bureš J, Burešova O, Křivánek J. The mechanism and applications of Leão's spreading depression of electroencephalographic activity. Prague: Academia; 1974. [Google Scholar]
- Burstein R, Levy D, Jakubowski M. Effects of sensitization of trigeminovascular neurons to triptan therapy during migraine. Rev Neurol (Paris) 2005;161:658–660. doi: 10.1016/s0035-3787(05)85109-4. [DOI] [PubMed] [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnesic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2011;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol. 2008;295:H1882–1894. doi: 10.1152/ajpheart.412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabaneanu S, Overath CH, Rubin D, Luthje S, Sye W, Niederberger U, Gerber WD, Weisser B. Aerobic exercise as a therapy option for migraine: a pilot study. Int J Sports Med. 2011;32:455–460. doi: 10.1055/s-0030-1269928. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? TINS. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, van der Worp HB, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- Eckert MJ, Abraham WC. Physiological effects of enriched environment exposure and LTP induction in the hippocampus in vivo do not transfer faithfully to in vitro slices. Learn Mem. 2010;17:480–484. doi: 10.1101/lm.1822610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus IGF1 for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Gagné J, Gélinas S, Martinoli MG, Foster TC, Ohayon M, Thompson RF, Baudry M, Massicotte G. AMPA receptor properties in adult rat hippocampus following environmental enrichment. Brain Res. 1998;799:16–25. doi: 10.1016/s0006-8993(98)00451-x. [DOI] [PubMed] [Google Scholar]
- Goldberg LD. The cost of migraine and its treatment. Am J Manag Care. 2005;11:S62–67. [PubMed] [Google Scholar]
- Gonzalez de la Vega A, Buno W, Pons S, Garcia-Calderat MS, Garcia-Galloway E, Torres-Aleman I. Insulin-like growth factor I potentiates kainate receptors through a phosphatidylinositol 3-kinase dependent pathway. Neuroreport. 2001;12:1293–1296. doi: 10.1097/00001756-200105080-00047. [DOI] [PubMed] [Google Scholar]
- Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on Lévy flights. PLoS One. 2011;6:e19294. doi: 10.1371/journal.pone.0019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg YY, Kraig RP. Insulin-like growth factor-1 (and insulin) mitigates spreading depression susceptibility: Implications for migraine therapy. Soc Neurosci. 2011;37:Prog #875.07. [Google Scholar]
- Guedes RC, Monteiro JS, Teodosio NR. Malnutrition and brain function: experimental studies using the phenomenon of cortical spreading depression. Rev Bras Biol. 1996;56 Su 1 Pt2:293–301. [PubMed] [Google Scholar]
- Gulati K, Ray A, Pal G, Vijayan VK. Possible role of free radicals in theophylline-induced seizures in mice. Pharmacol Biochem Behav. 2005;82:241–245. doi: 10.1016/j.pbb.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5:148–157. doi: 10.1016/S1474-4422(06)70348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A, Blome M, Ambree O, Sachser N, Paulus W, Keyvani K. Reduction of cerebral oxidative stress following environmental enrichment in mice with Alzheimer-like pathology. Brain Pathol. 2010;20:166–175. doi: 10.1111/j.1750-3639.2008.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Arch Intern Med. 1999;159:813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- Hulse RE, Swenson WG, Kunkler PE, White DM, Kraig RP. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci. 2008;28:12199–12211. doi: 10.1523/JNEUROSCI.3856-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallali N, Ridha H, Thrasivoulou C, Butler P, Cowen T. Modulation of intracellular reactive oxygen species level in chondrocytes by IGF-1, FGF, and TGF-beta1. Connect Tissue Res. 2007;48:149–158. doi: 10.1080/03008200701331516. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Fabel K, Ehninger D, Babu H, Leal-Galicia P, Garthe A, Wolf SA. Why and how physical activity promotes experience-induced brain plasticity. Front Neurosci. 2010;4:189. doi: 10.3389/fnins.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig RP, Mitchell HM, Christie-Pope B, Kunkler PE, White DM, Tang YP, Langan G. TNF-alpha and microglial hormetic involvement in neurological health & migraine. Dose-Response. 2010;8:389–413. doi: 10.2203/dose-response.09-056.Kraig. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger H, Luhmann HJ, Heinemann U. Repetitive spreading depression causes selective suppression of GABAergic function. Neuroreport. 1996;7:2733–2736. doi: 10.1097/00001756-199611040-00065. [DOI] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2011;33:828.e1–828.e17. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Kraig RP. Spreading Depression and Migraine. In: Olesen J, Goadsby P, Ramadan N, Tfelt-Hansen A, Welch KMA, editors. The Headaches. 3. Lippincott-Raven; Philadelphia: 2005. pp. 269–276. [Google Scholar]
- Liu XF, Fawcett JF, Hanson LR, Frey WH., 2nd The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13:16–23. doi: 10.1016/j.jstrokecerebrovasdis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74:512–523. doi: 10.1002/jnr.10791. [DOI] [PubMed] [Google Scholar]
- Mack KJ. Can we help patients with chronic migraine? Neurology. 2011;76:682–683. doi: 10.1212/WNL.0b013e31820d6315. [DOI] [PubMed] [Google Scholar]
- Mies G, Iijima T, Hossmann KA. Correlation between peri-infarct DC shifts and ischaemic neuronal damage in rat. Neuroreport. 1993;4:709–711. doi: 10.1097/00001756-199306000-00027. [DOI] [PubMed] [Google Scholar]
- Miltiadous P, Stamatakis A, Koutsoudaki PN, Tiniakos DG, Stylianopoulou F. IGF-I ameliorates hippocampal neurodegeneration and protects against cognitive deficits in an animal model of temporal lobe epilepsy. Exp Neurol. 2011;231:223–235. doi: 10.1016/j.expneurol.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Mitchell HM, Levasseur V, Kraig RP. TNF-α increases spreading depression susceptibility via reduced GABAergic inhibition - Implications for the transformation of episodic to chronic migraine. Soc Neurosci. 2010a;36:Prog #346.3. [Google Scholar]
- Mitchell HM, White DM, Kraig RP. Strategies for study of neuroprotection from cold-preconditioning. J Vis Exp. 2010b doi: 10.3791/2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13:1167–1177. doi: 10.1523/JNEUROSCI.13-03-01167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulleners WM, Chronicle EP, Palmer JE, Koehler PJ, Vredeveld JW. Visual cortex excitability in migraine with and without aura. Headache. 2001;41:565–572. doi: 10.1046/j.1526-4610.2001.041006565.x. [DOI] [PubMed] [Google Scholar]
- Muller M, Fontana A, Zbinden G, Gahwiler BH. Effects of interferons and hydrogen peroxide on CA3 pyramidal cells in rat hippocampal slice cultures. Brain Res. 1993;619:157–162. doi: 10.1016/0006-8993(93)91607-t. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Strong AJ, Dohmen C, Sakowitz OW, Vollmar S, Sué M, Kracht L, Hashemi P, Bhatia R, Yoshimine T, Dreier JP, Dunn AK, Graf R. Spreading depolarizations cycle around and enlarge focal ischaemic brain lesions. Brain. 2010;133:1994–2006. doi: 10.1093/brain/awq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Garcia Verdugo JM, Leroy F, Soya H, Nuñez A, Torres-Aleman I. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834–846. doi: 10.1016/j.neuron.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Palmer JE, Chronicle EP, Rolan P, Mulleners WM. Cortical hyperexcitability is cortical underinhibition: evidence from a novel functional test of migraine patients. Cephalalgia. 2000;20:525–532. doi: 10.1046/j.1468-2982.2000.00075.x. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Léveillé F, Martel MA, Dakin KA, Hansen H, H, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek P, Jackson DA, White MRH. Oscillatory control of signaling molecules. Curr Opin Gen Develop. 2010;20:670–676. doi: 10.1016/j.gde.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Pérez R, García-Fernández M, Díaz-Sánchez M, Puche JE, Delgado G, Conchillo M, Muntané J, Castilla-Cortázar I. Mitochondrial protection by low doses of insulin-like growth factor- I in experimental cirrhosis. World J Gastroenterol. 2008;14:2731–2739. doi: 10.3748/wjg.14.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Grinberg YY, Mitchell HM, Kraig RP. Modeling neural immune signaling of episodic and chronic migraine using spreading depression in vitro. J Vis Exp. 2011 doi: 10.3791/2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Adams MM, Ariwodola OJ, Sonntag WE, Weiner JL. Functional characterization of des-IGF-1 action at excitatory synapses in the CA1 region of rat hippocampus. J Neurophysiol. 2005;94:247–254. doi: 10.1152/jn.00768.2004. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate compartmentalization in the CNS. Neurotox Res. 1999;1:81–90. doi: 10.1007/BF03033272. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Myatt-Jones J, Rafols J, Dunbar JC. Insulin like growth factor-1 (IGF-1) decreases ischemia-reperfusion induced apoptosis and necrosis in diabetic rats. Endocrine. 2007;31:66–71. doi: 10.1007/s12020-007-0012-0. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark J, Chan C, George AJT. Oscillations in the immune system. Immunol Rev. 2007;216:213–231. doi: 10.1111/j.1600-065X.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav. 2004;83:47–54. doi: 10.1016/j.physbeh.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Sukhotinsky I, Dilekoz E, Wang Y, Qin T, Eikermann-Haerter K, Waeber C, Ayata C. Chronic daily cortical spreading depressions suppress spreading depression susceptibility. Cephalalgia. 2011;31:1601–1608. doi: 10.1177/0333102411425865. [DOI] [PubMed] [Google Scholar]
- Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius, S The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Viggiano A, Viggiano E, Valentino I, Monda M, De Luca B. Cortical spreading depression affects reactive oxygen species production. Brain Res. 2011;1368:11–18. doi: 10.1016/j.brainres.2010.10.062. [DOI] [PubMed] [Google Scholar]
- Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Paszek P, Horton CA, Yue H, White MR, Kell DB, Muldoon MR, Broomhead DS. A systematic survey of the response of a model NF-κB signaling pathway to TNFα stimulation. J Theoret Biol. 2012;297:127–147. doi: 10.1016/j.jtbi.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch KM. Brain hyperexcitability: the basis for antiepileptic drugs in migraine prevention. Headache. 2005;45(1):S25–32. doi: 10.1111/j.1526-4610.2005.4501008.x. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990-2002) Prog Neurobiol. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Xing C, Yin Y, Chang R, Gong X, He X, Xie Z. Effects of insulin-like growth factor 1 on synaptic excitability in cultured rat hippocampal neurons. Exp Neurol. 2007;205:222–229. doi: 10.1016/j.expneurol.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Brankack J, Haas HL. Differences of CA3 bursting in DBA/1 and DBA/2 inbred mouse strains with divergent shuttle box performance. Neuroscience. 1995;64:319–325. doi: 10.1016/0306-4522(94)00400-y. [DOI] [PubMed] [Google Scholar]
- Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Linear and non-linear dose-response functions reveal a hormetic relationship between stress and learning. Dose-Response. 2009;7:132–148. doi: 10.2203/dose-response.08-015.Zoladz. [DOI] [PMC free article] [PubMed] [Google Scholar]