Abstract
Background
Among members of Cryptococcus neoformans- Cryptococcus gattii species complex, C. neoformans is distributed worldwide whereas C. gattii is considered to be more prevalent in the subtropics and tropics including Taiwan. This nationwide study was undertaken to determine the distribution of genotypes, clinical characteristics and outcomes of 219 patients with proven cryptococcosis at 20 hospitals representative of all geographic areas in Taiwan during 1997–2010.
Methods and Findings
Of 219 isolates analyzed, C. neoformans accounted for 210 isolates (95.9%); nine isolates were C. gattii (4.1%). The predominant genotype was VNI (206 isolates). The other genotypes included VNII (4 isolates), VGI (3 isolates) and VGII (6 isolates). Antifungal minimal inhibition concentrations higher than epidemiologic cutoff values (ECVs) were found in nine VNI isolates (7 for amphotericin B). HIV infection was the most common underlying condition (54/219, 24.6%). Among HIV-negative patients, liver diseases (HBV carrier or cirrhosis) were common (30.2%) and 15.4% did not have any underlying condition. Meningoencephalitis was the most common presentation (58.9%), followed by pulmonary infection (19.6%) and “others” (predominantly cryptococcemia) (18.7%). The independent risk factors for 10-week mortality, by multivariate analysis, were cirrhosis of liver (P = 0.014) and CSF cryptococcal antigen titer ≥512 (P = 0.020). All except one of 54 HIV-infected patients were infected by VNI genotype (98.1%). Of the 13 isolates of genotypes other than VNI, 12 (92.3%) were isolated from HIV-negative patients. HIV-infected patients compared to HIV-negative patients were more likely to have meningoencephalitis and serum cryptococcal antigen ≥1∶512. Patients infected with C. gattii compared to C. neoformans were younger, more likely to have meningoencephalitis (100% vs. 57%), reside in Central Taiwan (56% vs. 31%), and higher 10-week crude mortality (44.4% vs. 22.2%).
Conclusions
Cryptococcus neoformans in Taiwan, more prevalent than C. gatii, has a predominant VNI genotype. Isolates with antifungal MIC higher than ECVs were rare.
Introduction
Among members of the Cryptococcus neoformans-Cryptococcus gattii species complex that cause cryptococcosis in humans, C. neoformans (comprising var. grubii [serotype A] and var. neoformans [serotype D]) occur worldwide. In contrast, C. gattii (serotype B and C) is usually limited to the selected regions, particularly the Asia-Pacific region before the occurrence of a C. gattii outbreak in Vancouver Island, Canada [1]. Based on a large global molecular epidemiologic survey Cryptococcus could be divided into eight major genotypes: VNI (serotype A), VNII (serotype A), VNIII (serotype AD), and VNIV (serotype D) of C. neoformans; and VGI, VGII, VGIII, and VGIV of C. gattii using orotidine monophosphate pyrophosphorylase (URA5) gene restriction fragment length polymorphism (RFLP) analysis and M13 polymerase chain reaction (PCR) fingerprinting [2].
Cryptococcosis is associated with significant morbidity and mortality. It can present as meningoencephalitis, pneumonia and cryptococcemia in both immunocompetent and immunocompromised hosts. Outcome and treatment failure are usually associated with underlying conditions, a delay in diagnosis, and absence of a fungicidal drug [3]–[5]. In addition, the emergence of isolates with resistance or elevated minimum inhibition concentration (MIC) above epidemiologic cutoff values (ECVs) is of concern as well [6], [7].
We conducted this nationwide multicenter retrospective study for patients with proven cryptococcosis to address two questions. First, what are the genotypes and antifungal susceptibility of Cryptococcus clinical isolates collected from representative regions in Taiwan? Second, are demographic qualities, underlying conditions, and microbiological characteristics associated with cryptococcosis patient mortality?
Population and Methods
This research was approved by the Research Ethics Committees of the National Taiwan University Hospital (No. 201209035RIC), Mackay Memorial Hospital (No.12MMHIS120), Kaohsiung Medical University Hospital (No.KMUH-IRB-20120239), China Medical University Hospital (No. DMR101-IRB1-240), and National Health Research Institute (No.EC 09602024) and was conducted according to the Declaration of Helsinki. The project involved the use of existing data, records, and clinical isolates without intervention. Informed consent was waived and the data were analyzed anonymously.
Hospital settings and Cryptococcus clinical isolates
Cryptococcus clinical isolates were obtained from 219 patients with proven cryptococcosis managed at 20 hospitals located in the four geographic regions of Taiwan during 1997–2010. The initial patient isolate, regardless of anatomical site, was selected and sent to National Taiwan University Hospital (NTUH) for microbiological characterization.
Genotypes
High-molecular-weight DNA was isolated and genotypes were determined by URA5 gene RFLP analysis [2]. Molecular types were evaluated and compared using M13 PCR-fingerprinting [2]. The computer program BioNumerics version 6.0 (Applied Maths, Kortrijk, Belgium) was used to determine the cluster analysis by the UPGMA method [8]. DNA bands were defined manually with a band position tolerance of 0.8% and an optimization setting of 0.2%. Reference strains included WM 148 (VNI), WM 626 (VNII), WM 628 (VNIII), WM 629 (VNIV), WM 179 (VGI), WM 178 (VGII), WM 161 (VGIII), WM 779 (VGIV) [2], two Australia clinical strains T184 (VNI) and T185 (VGI), and Vancouver Island outbreak strains R265 (VGIIa) and R272 (VGIIb).
Antifungal susceptibility
Susceptibility, as displayed by MIC (µg/ml) levels, to amphotericin B, flucytosine, fluconazole, and voriconazole was determined following the Clinical Laboratory Standards Institute (CLSI) M27-A3 broth microdilution method and incubated at 35°C [9]. All results were read visually at 72 h. The reference strains C. neoformans ATCC 90112, Candida albicans ATCC 90028, and Candida parapsilosis ATCC 22019 were used as internal controls. The ECVs are the MIC values that captured >95% of the observed population in RPMI medium provided in recent studies [6], [7].
Clinical characteristics and outcomes of patients with cryptococcosis
Data were collected retrospectively after isolates were sent for microbiological characterization and included gender, age, underlying conditions such as human immunodeficiency virus (HIV) status and lowest CD4 count during hospitalization, hepatitis B virus (HBV) carrier defined by positive surface antigen (HBsAg) status, and cirrhosis of liver determined by sonography; clinical characteristics included presentation, initial cryptococcal capsular polysaccharide antigen titer in cerebrospinal fluid (CSF) or serum, baseline intracranial opening pressures, neurosurgical intervention, all-cause mortality at 2- and 10-weeks. One patient could possess more than one underlying condition. We did not collect and record treatment details.
Case definition
Proven cryptococcosis was defined and classified into cryptococcal meningoencephalitis, pulmonary cryptococcosis, and others as described previously [10].
Data analysis
The categorical variables were analyzed by number (No.) (%) and the continuous variables were presented as mean ± standard deviation (SD). The association between categorical variables was analyzed with the Chi-square test or Fisher's exact test if the expected number was less than five. The independent and joint effects of several variables to identify significant predictors of mortality were investigated by univariate and multivariate logistic regression analyses. Two-sided P value <0.05 was considered statistically significant. All statistical analyses were performed using the SAS software, version 9.2 (SAS Institute Inc., Cary, NC, US).
Results
Cryptococcus genotypes
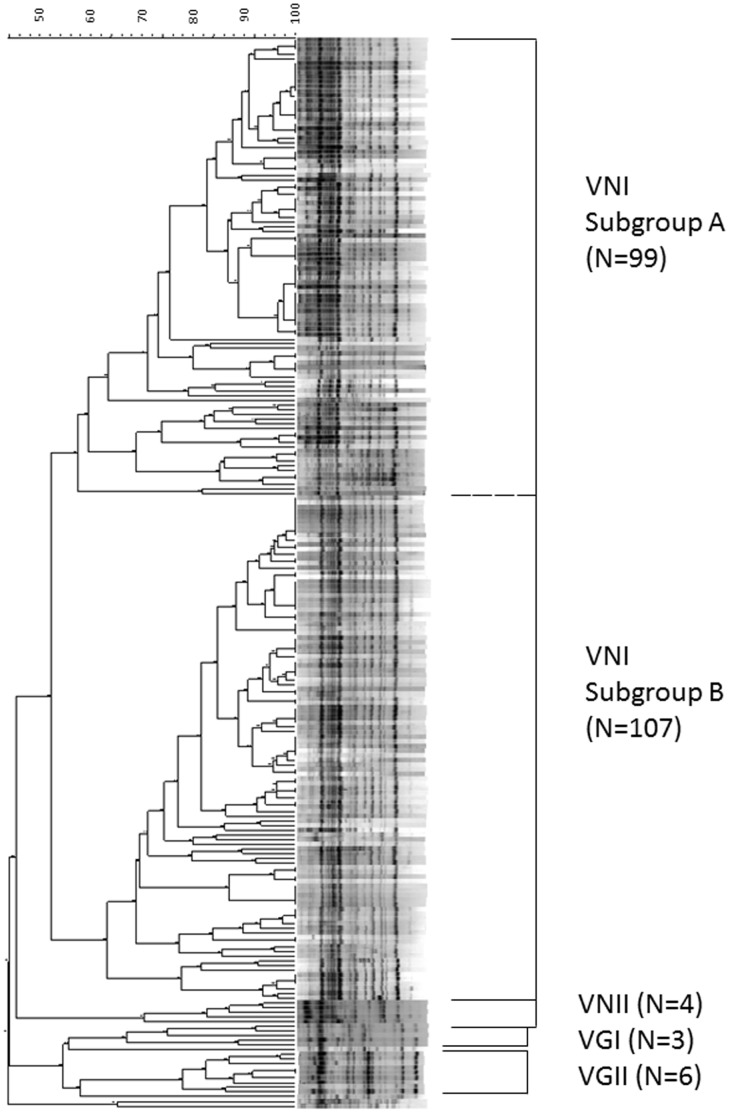
Of 219 Cryptococcus clinical isolates, 210 were C. neoformans (95.9%) and 9 were C. gattii (4.1%). VNI genotype accounted for 206/210 (98.1%) of C. neoformans. Four isolates were VNII. Among the nine isolates of C. gattii, three were VGI and six were VGII. The details of patients with VNII and C. gattii are shown in Table S1 and Table S2, respectively.
Figure 1 shows the M13 PCR-fingerprinting dendrogram of the 219 cryptococcal isolates (details are presented in Figure S1). Genotype VNI can be divided into two subgroups. Subgroup A accounted for 48.1% (99/206) of VNI with 57.4% similarity and subgroup B accounted for 51.9% (107/206) of VNI with 63.2% similarity.
Figure 1. Dendrogram of M13 PCR fingerprint analysis of 219 clinical isolates of Cryptococcus neoformans-Cryptococcus gattii species complex collected in Taiwan during 1997 to 2010 and 12 reference strains.
Antifungal susceptibility
Among the 219 isolates, the susceptibility data of three VNI isolates (T203, T205, and T262) were indeterminate due to very poor growth in RPMI broth at 35°C. The MIC levels of 216 isolates to amphotericin B, flucytosine, fluconazole, and voriconazole are shown in Table 1 . Seven of 203 VNI isolates (3.4%) had amphotericin B MIC levels higher than ECV. One VNI isolate had a flucytosine MIC level higher than ECV. Two of six VGII isolates and one of 203 VNI isolates had fluconazole MIC levels >8 µg/ml, but there were none above this level for 4 VNII isolates and 3 VGI isolates. Fluconazole ECV was 8 µg/ml for VNI and VGI, and was 32 µg/ml for VGII. Therefore, only one VNI isolate of 219 isolates had fluconazole MIC higher than ECV. Detailed information regarding cryptococcosis due to Cryptococcus VNI isolates with antifungal MICs higher than ECVs is shown in Table S3.
Table 1. Susceptibility of 216 cryptococcal clinical isolates to four antifungal agents in Taiwan, 1997–2010.
| Antifungal agent | Genotype | No. of isolates | Minimum inhibitory concentration (µg/mL) | % (No.) above ECV | |||||
| Range | Geometric Mean | MIC50 | MIC90 | ECV | This study | Global studiesa | |||
| Amphotericin B | |||||||||
| VNI | 203 | 0.03–1 | 0.48 | 0.5 | 0.5 | 0.5 | 3.4% (7) | 2.8% | |
| VNII | 4 | 0.13–1 | 0.42 | 0.5 | 1 | NAa | |||
| VGI | 3 | 0.25–0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 0% | 0.8% | |
| VGII | 6 | 0.06–1 | 0.31 | 0.5 | 1 | 1 | 0% | 0.8% | |
| Flucytosine | |||||||||
| VNI | 203 | 0.13–32 | 1.14 | 1 | 2 | 8 | 0.5% (1) | 3.4% | |
| VNII | 4 | 0.13–2 | 0.30 | 0.19 | 2 | NAa | |||
| VGI | 3 | 0.5–1 | 0.63 | 0.5 | 1 | 4 | 0% | 4.3% | |
| VGII | 6 | 1–2 | 1.59 | 2 | 2 | 16 | 0% | 2.9% | |
| Fluconazole | |||||||||
| VNI | 203 | 0.03–16 | 2.35 | 4 | 8 | 8 | 0.5% (1) | 2.9% | |
| VNII | 4 | 0.13–8 | 0.84 | 0.75 | 8 | NAa | |||
| VGI | 3 | 1–4 | 2 | 2 | 4 | 8 | 0% | 1.2% | |
| VGII | 6 | 0.13–16 | 5.04 | 8 | 16 | 32 | 0% | 6.9% | |
| Voriconazole | |||||||||
| VNI | 203 | 0.03–0.25 | 0.06 | 0.06 | 0.13 | 0.25 | 0% | 2.4% | |
| VNII | 4 | 0.03–0.13 | 0.05 | 0.05 | 0.13 | NAa | |||
| VGI | 3 | 0.03–0.06 | 0.04 | 0.03 | 0.06 | 0.5 | 0% | 0% | |
| VGII | 6 | 0.13–0.25 | 0.20 | 0.25 | 0.25 | 0.25 | 0% | 4.1% | |
Epidemiological and clinical characteristics
Table 2 shows the epidemiological and clinical characteristics of the 219 patients with proven cryptococcosis. More than half of the patients were in Northern Taiwan. However, 5 of 9 isolates of C. gattii (55.6%) were from Central Taiwan. The most common five underlying conditions were HIV infection (54 patients, 24.6%), HBV carrier (46 patients, 21.0%), malignancies (44 patients, 20.1%), diabetes mellitus (40 patients, 18.2%), and cirrhosis of liver (31 patients, 14.1%). No underlying condition was identified in 23 patients (10.5%). Meningoencephalitis was the most common presentation (58.9%), followed by pulmonary infection (19.6%) and “others” (predominantly cryptococcemia) (18.7%). The nine patients with C. gattii infection, compared to 210 patients with C. neoformans, were younger (mean 38.6 years vs. 53.1 years) and more likely to have no underlying conditions (44.4% vs. 9.0%), to have meningoencephalitis (100.0% vs. 57.1%) and to undergo neurosurgical intervention (33.3% vs. 9.0%). They also had a higher 10-week mortality (44.4% vs. 22.2%), as seen in Table 2 .
Table 2. Epidemiological and clinical characteristics of 219 patients with proven cryptococcosis hospitalized at 20 hospitals in Taiwan, 1997–2010.
| Characteristics | Cryptococcus neoformans (N = 210) | Cryptococcus gattii (N = 9) | ||
| No. | (%) | No. | (%) | |
| Geographic distribution | ||||
| Northern | 119 | (56.7) | 1 | (11.1) |
| Central | 65 | (30.9) | 5 | (55.6) |
| Southern | 21 | (10.0) | 1 | (11.1) |
| Eastern | 5 | (2.4) | 2 | (22.2) |
| Demographic data | ||||
| Age, range, years | 12 to 94 | 22 to 68 | ||
| Age, mean ± SD, years | 53.1±18.4 | 38.6±13.0 | ||
| Age ≥60 years | 75 | (35.7) | 1 | (11.1) |
| Male | 143 | (72.2) | 5 | (55.6) |
| Underlying conditions | ||||
| HIV infection | 53 | (27.3) | 1 | (11.1) |
| Liver diseases | ||||
| Hepatitis B virus carrier | 46 | (21.9) | 0 | (0.0) |
| Cirrhosis of liver | 31 | (14.8) | 0 | (0.0) |
| Malignancy | ||||
| Hematological malignancy | 13 | (6.2) | 0 | (0.0) |
| Other malignancy | 31 | (14.8) | 0 | (0.0) |
| Diabetes mellitus | 39 | (18.6) | 1 | (11.1) |
| Kidney diseases | 21 | (9.6) | 0 | (0.0) |
| Systemic lupus erythematosus and other rheumatologic diseases | 11 | (5.2) | 0 | (0.0) |
| Cerebrovascular accident | 8 | (3.8) | 1 | (11.1) |
| Tuberculosis | 6 | (2.9) | 0 | (0.0) |
| Solid organ transplantationa | 3 | (1.4) | 1 | (11.1) |
| Idiopathic CD4 lymphocytopenia | 3 | (1.4) | 0 | (0.0) |
| Other diseases | 3 | (1.4) | 0 | (0.0) |
| No underlying conditions | 19 | (9.0) | 4 | (44.4) |
| Classification of cryptococcosis | ||||
| Meningoencephalitis | 120 | (57.1) | 9 | (100.0) |
| Pulmonary cryptococcosis | 43 | (20.5) | 0 | (0.0) |
| Othersb | 47 | (22.4) | 0 | (0.0) |
| Serum cryptococcal capsular antigen | ||||
| Antigen titer ≥512 | 73 | (34.8) | 4 | (44.4) |
| Antigen titer <512 | 57 | (27.1) | 3 | (33.3) |
| Not done | 80 | (38.1) | 2 | (22.2) |
| CSF cryptococcal capsular antigen | ||||
| Antigen titer ≥1∶512 | 76 | (36.2) | 7 | (77.8) |
| Antigen titer <1∶512 | 40 | (19.0) | 2 | (22.2) |
| Not done | 94 | (44.8) | 0 | (0.0) |
| Intracranial pressure | ||||
| Opening pressure≥250 mmH2O | 48 | (22.9) | 6 | (66.7) |
| Opening pressure<250 mmH2O | 42 | (20.0) | 2 | (22.2) |
| Not done or not available | 120 | (57.1) | 1 | (11.1) |
| Neurosurgical intervention | 19 | (9.0) | 3 | (33.3) |
| All-cause mortality | ||||
| 2-week mortality | 22 | (10.5) | 2 | (22.2) |
| 10-week mortality | 60 | (28.6) | 4 | (44.4) |
Abbreviations: SD: standard deviation; CSF: cerebrospinal fluid; HIV: human immunodeficiency virus.
Solid organ transplantation included two liver transplantations and one heart transplantation in C. neoformans infected patients; and one kidney transplantation in C. gattii infected patient.
“Others” included 36 patients with cryptococcemia.
Of 54 HIV-infected patients, 53 were infected by the VNI genotype (98.1%) and one was infected by the VGI genotype, as seen in Table 3 . Excluding five patients without recorded CD4 data, the mean CD4 of 49 HIV-infected patients was 50.0±68.3/mL (ranging from 2 to 318/mL). Of 13 isolates of genotypes other than VNI, twelve (92.3%) were isolated from HIV-negative patients ( Table 3 , Table S1, and Table S2). The 54 HIV-infected patients, as compared to the 149 HIV-negative patients, were younger, predominantly male, and more likely to have meningoencephalitis and serum cryptococcal antigen ≥512. Compared to HIV infected patients, HIV-negative patients were more likely to have pulmonary infection and liver diseases (either HBV carrier or cirrhosis of liver) as the most common underlying conditions (45 patients, 30.2%).
Table 3. Comparisons of genotype distribution and clinical characteristics of cryptococcosis by HIV status, Taiwan, 1997–2010.
| Characteristics | HIV-negative patients (N = 149)a | HIV-infected patients (N = 54)a | P value | ||
| No. | (%) | No. | (%) | ||
| Genotype distribution | |||||
| VNI | 137 | 53 | |||
| VNII | 4 | 0 | |||
| VGI | 2 | 1 | |||
| VGII | 6 | 0 | |||
| Geographic distribution | |||||
| Northern | 84 | (56.4) | 34 | (63.0) | |
| Central | 43 | (28.9) | 14 | (25.9) | |
| Southern | 16 | (10.7) | 5 | (9.3) | |
| Eastern | 6 | (4.0) | 0 | (0.0) | |
| Demographic data | |||||
| Age ≥60 years | 75 | (50.3) | 1 | (1.9) | <0.001 |
| Male | 94 | (63.1) | 51 | (94.4) | <0.001 |
| Underlying conditions | |||||
| Liver diseases | |||||
| Hepatitis B virus carrier | 33 | (22.1) | 13 | (24.1) | 0.845 |
| Cirrhosis of liverb | 30 | (20.1) | 1 | (1.9) | 0.001 |
| Diabetes mellitus | 40 | (26.8) | 0 | (0.0) | <0.001 |
| Malignancy | |||||
| Hematological malignancy | 5 | (3.4) | 3 | (5.6) | 0.686 |
| Other malignancy | 33 | (22.1) | 3 | (5.6) | 0.005 |
| Kidney diseases | 20 | (13.4) | 1 | (1.9) | 0.014 |
| Solid organ transplantation | 4 | (2.7) | 0 | (0.0) | 0.576 |
| No underlying conditions | 23 | (15.4) | 0 | (0.0) | 0.002 |
| Classification of cryptococcosis | 0.002 | ||||
| Meningoencephalitis | 80 | (53.7) | 44 | (81.5) | |
| Pulmonary cryptococcosis | 35 | (23.5) | 3 | (5.6) | |
| Othersc | 34 | (22.8) | 7 | (13.0) | |
| Serum cryptococcal capsular antigen | 0.001 | ||||
| Antigen titer ≥512 | 43 | (28.9) | 34 | (63.0) | |
| Antigen titer <512 | 49 | (32.9) | 11 | (20.4) | |
| Not doned | 57 | (38.3) | 9 | (16.7) | |
| CSF c cryptococcal capsular antigen | 0.661 | ||||
| Antigen titer ≥1∶512 | 50 | (33.6) | 33 | (61.1) | |
| Antigen titer <1∶512 | 27 | (18.1) | 15 | (27.8) | |
| Not doned | 72 | (48.3) | 6 | (11.1) | |
| Intracranial pressure | 0.101 | ||||
| Opening pressure≥250 mmH2O | 32 | (21.5) | 22 | (40.7) | |
| Opening pressure<250 mmH2O | 33 | (22.1) | 11 | (20.4) | |
| Not done or not availabled | 84 | (56.4) | 21 | (38.9) | |
| Neurosurgical intervention | 15 | (10.1) | 7 | (13.0) | 0.592 |
| All-cause mortality | |||||
| 2-week mortality | 19 | (12.8) | 5 | (9.3) | 0.468 |
| 10-week mortality | 52 | (34.9) | 12 | (22.2) | 0.100 |
Abbreviation: HIV: human immunodeficiency virus.
Of 219 patients with cryptococcosis, the HIV status of 16 patients was not available. Therefore, 203 cases were included for analysis.
One patient could possess more than one underlying condition; 18 HIV-negative patients had both cirrhosis of liver and HBV infection.
“Others” included 25 patients with cryptococcemia in HIV-negative group and seven cryptococcemia in HIV-infected group.
Data which were not done or not available were excluded from statistical analysis.
Of nine patients infected by the VNI genotype and with antifungal MICs above ECVs, five patients had HIV infections, six had meningoencephalitis, and three had cryptococcemia. The all-cause mortality at 10 weeks was 33.3% (3/9), as shown in Table S3. We did not collect data, such as prior use of antifungal agent or drug interaction, to explain the reason for elevated MICs.
Risk factors for mortality at 2 weeks and 10 weeks
The outcomes of 19 patients at 2-weeks and 24 patients at 10-weeks were not available as patients transferred to other hospitals. All-cause mortality at 2-weeks and 10-weeks were shown in Table 1 . The significant risk factors for 2-week mortality of cryptococcosis, according to univariate analysis, were geographic distribution in Eastern Taiwan (P = 0.041), and classification of “others” (predominantly cryptococcemia) (P = 0.011). Under multivariate analysis the risk factors for 2-week mortality were geographic distribution in Eastern Taiwan (P = 0.043; odds ratio (OR), 10.7; 95% confidence interval (CI), 1.1–106.1) and classification of “others” (P = 0.018; OR, 13.3; 95% CI, 1.6–112.4).
Risk factors associated with 10-week mortality for 195 patients with cryptococcosis are shown in Table 4 . The significant factors under univariate analysis were age ≥60 years (P = 0.016), cirrhosis of liver (P = 0.001), kidney diseases (P = 0.035), meningoencephalitis (P = 0.038), other cryptococcosis (P<0.001) and CSF cryptococcal antigen titer ≥1∶512 (P = 0.019). Multivariate analysis showed cirrhosis of liver (P = 0.014; OR, 3.8; 95% CI, 1.3–11.16) and CSF antigen titer ≥1∶512 (P = 0.020; OR, 3.3; 95% CI, 1.2–9.0) as independent predictors for mortality.
Table 4. Risk factors associated with 10-week mortality for 195 patients with cryptococcosis in Taiwan.
| Characteristics | Died (N = 64) | Lived (N = 131) | Odds ratio | 95% confidence interval | P value | ||
| No. | (%) | No. | (%) | ||||
| Demographic data | |||||||
| Age ≥60 years | 32 | (50.0) | 42 | (32.1) | 2.2 | 1.1–3.9 | 0.016 |
| Male | 41 | (64.1) | 98 | (74.8) | 0.6 | 0.3–1.1 | 0.12 |
| Underlying conditions | |||||||
| HIV infection | 12 | (18.8) | 39 | (29.8) | 0.5 | 0.3–1.1 | 0.10 |
| Hepatitis B virus carrier | 15 | (23.4) | 28 | (21.4) | 1.1 | 0.5–2.3 | 0.76 |
| Cirrhosis of liver | 18 | (28.1) | 12 | (9.2) | 3.9 | 1.7–8.7 | 0.001 |
| Kidney diseases | 11 | (17.2) | 9 | (6.9) | 2.7 | 1.1–7.0 | 0.03 |
| Classification of cryptococcosis | |||||||
| Pulmonary | 5 | (7.8) | 33 | (25.2) | 1.0 | ||
| Meningoencephalitis | 37 | (57.8) | 83 | (63.4) | 2.9 | 1.1–8.1 | 0.04 |
| Othersa | 22 | (34.4) | 15 | (11.4) | 10.4 | 3.3–32.9 | <0.001 |
| Serum cryptococcal capsular antigen | |||||||
| Antigen titer ≥1∶512 | 26 | (40.6) | 47 | (35.9) | 1.4 | 0.7–2.9 | 0.41 |
| Antigen titer <1∶512 | 17 | (26.6) | 42 | (32.1) | 1.0 | ||
| Not doneb | 21 | (32.8) | 42 | (32.1) | |||
| CSF cryptococcal capsular antigen | |||||||
| Antigen titer ≥1∶512 | 29 | (45.3) | 51 | (38.9) | 3.2 | 1.2–8.6 | 0.02 |
| Antigen titer <1∶512 | 6 | (9.4) | 34 | (26.0) | 1.0 | ||
| Not doneb | 29 | (45.3) | 46 | (35.1) | |||
| Intracranial pressure | |||||||
| Opening pressure ≥250 mmH2O | 16 | (25.0) | 37 | (28.2) | 1.0 | 0.4–2.6 | 0.92 |
| Opening pressure <250 mmH2O | 12 | (18.8) | 29 | (22.1) | 1.0 | ||
| Not done or not availableb | 36 | (56.3) | 65 | (49.6) | |||
| Neurosurgical intervention | 9 | (14.1) | 13 | (9.9) | 1.5 | 0.6–3.7 | 0.43 |
Abbreviation: CSF: cerebrospinal fluid.
“Others” included 19 patients with cryptococcemia died and 12 patients with cryptococcemia lived.
Data which were not done or not available were excluded from statistical analysis.
Discussion
The current study provides the first nationwide description of the microbiological and clinical epidemiology of cryptococcosis in Taiwan. The majority of isolates in Taiwan were C. neoformans genotype VNI (96%). This is in agreement with the worldwide distribution of Cryptococcus which is VNI in Ibero-America (68%) [2], Vietnam (71%) [11], India (89%) [12], Malaysia (89%) [13], China (93%) [14] and Korea (96%) [15].
Cryptococcosis in HIV-negative patients was common (73%) in Taiwan (this study) as well as in China (84% to 96%) [14], [16], [17]. However, HIV-negative patients accounted for 60% in an Indian study [12], 57% in Australia and New Zealand [18], 23% of a French cohort [19] and 18% in Mexican [20]. Only 15% patients were no underlying condition in Taiwan (this study). This was very different from reports in China (68%) [16] and Vietnam (81%) [11]; and yet was close to a study in Korea (19%) [15], USA (22%) [10] and results of another review from China (16%) [17]. Regarding the distribution of underlying conditions and their impact on 10-week mortality, this study showed that HIV infection was the most common underlying condition (25%), but not a risk factor associated with mortality of cryptococcosis ( Table 4 ). Liver diseases (either HBV carrier or cirrhosis) were the most common underlying conditions among HIV-negative patients in Taiwan (30%, Table 3 ) and in China (12%) [17]. Furthermore, cirrhosis of liver was an independent predictor of mortality in this study ( Table 4 ) and our previous single center study of cryptococcemia [21]. High CSF antigen titers have been associated with death at 10 weeks in a cohort of Italian HIV-positive patients [22] and HIV uninfected patients in Vietnam [11] and our previous study [23]. Our current study confirmed this finding as well. Thus, a threshold of 1∶512 or higher should help monitor patients with cryptococcosis, regardless of their HIV status.
In this study, we found clinical presentation of patients with C. gattii infection were more likely than those with C. neoformans infection to have meningoencephalitis, were younger, and were less likely to have underlying conditions ( Table 2 ), which was concordant with an Australian study [18]. The past studies from a center in northern Taiwan (i.e. NTUH) revealed that clinical cases of C. gattii decreased from 59% (17/29) during 1982–1994 to 13% (4/30) during 1995–1997 [24], and 1% (1/100) during 1999–2004 [25]. Another report from a center in southern Taiwan showed 15% (5/34) clinical cases during 1998–2002 were C. gattii [26]. Although the ecological niches of C. gattii are poorly defined in Taiwan [27], Chaturvedi V. et al. suggested a hypothetical lifecycle of C. gattii whereby it cycles through plants, soil, air, and water [28]. Loss of tree coverage in mountainous areas following numerous landslides washed into the estuaries in recent years might explain part of the reason why there has been a decrease in C. gattii in Taiwan. We speculate that the global distribution of C. gattii, as shown in Table 5 , might be related to ocean circulation to allow distribution and thriving of C. gattii propagules into new ecological niches.
Table 5. The global distribution of clinical isolates of Cryptococcus gattii by genotype in the literature reviewed.
| Report year | Collection year | Region | No. of isolates | Reference | ||||
| Total | VGI | VGII | VGIII | VGIV | ||||
| 1996 | 1965–1994 | Australia | 48 | 44 | 3 | 1 | 0 | [33] |
| 2003 | 1961–2001 | South American | 33 | 3 | 13 | 16 | 1 | [2] |
| 2004 | 1999–2002 | Canada, BC | 21 | 1 | 20 | 0 | 0 | [8] |
| 2005 | NA | Papua New Guinea | 37 | 31 | 2 | 4 | 0 | [34] |
| 2005 | NA | Australia, NT | 21 | 9 | 12 | 0 | 0 | [34] |
| 2005 | NA | India | 5 | 0 | 5 | 0 | 0 | [12] |
| 2006 | 1987–2004 | Colombia | 16 | 1a | 14b | 1 | 0 | [35] |
| 2006 | 1998–2003 | Hong Kong | 3 | 1 | 2 | 0 | 0 | [36] |
| 2007 | 2004–2005 | USA, Northwest | 5 | 1 | 4 | 0 | 0 | [37] |
| 2008 | 1994–2006 | China, 16 provinces | 9 | 9 | 0 | 0 | 0 | [16] |
| 2008 | 1981–2005 | China, Southeastern | 9 | 8 | 1 | 0 | 0 | [14] |
| 2009 | 2006–2008 | USA, Northwest | 14 | 0 | 14 | 0 | 0 | [38] |
| 2009 | 1994–2004 | Mexico | 8 | 2 | 2 | 2 | 2 | [20] |
| 2009 | 2007 | USA, Southeastern | 1 | 1 | 0 | 0 | 0 | [39] |
| 2010 | 2003–2004 | Malaysia | 11c | 4 | 4 | 0 | 0 | [13] |
| 2010 | 1998–2007 | Vietnam | 10 | 9 | 1 | 0 | 0 | [11] |
| 2010 | 1990–2008 | Korea | 2 | 0 | 1 | 1 | 0 | [15] |
| 2010 | 2007 | Japan | 1 | 0 | 1 | 0 | 0 | [40] |
| 2012 | 2005–2007 | India | 4 | 0 | 0 | 0 | 4 | [41] |
| 2012 | 2011 | USA, Southeastern | 1 | 1 | 0 | 0 | 0 | [42] |
| 2012 | 1997–2010 | Taiwan | 9 | 3 | 6 | 0 | 0 | Current Study |
Abbreviations: NT: Northern Territory; BC: British Columbia; NA: not available.
Mating type a.
11 strains with mating type a were included.
Three untyped C. gattii were included.
Recently, Espinel-Ingroff A. et al. suggested the epidemiologic cutoff values (ECVs) (highest wild type susceptibility endpoint) of antifungal susceptibility for reference [6], [7] as the Clinical and Laboratory Standards Institute (CLSI) does not provide clinical breakpoints (CBPs) for Cryptococcus species [9]. While CBPs predict the clinical outcome of therapy, the ECVs could monitor the emergence of strains with reduced susceptibility (due to mutation) to the agent being evaluated. In the current study, only nine of 219 isolates had MICs higher than ECVs ( Table 1 ). Of them, seven isolates (3.4%) of the VNI genotype had amphotericin B MIC levels higher than ECV, while the global study showed 2.8% [6]. Regarding fluconazole MIC, the values of MIC50 and MIC90 in this study ( Table 1 ) and ECVs in global studies [7] were higher for VGII than for VGI, VNI, and VNII. This indicates antifungal susceptibility for Cryptococcus should be species-specific and molecular type-specific [6], [7]. It seems likely that the differences seen among the C. neoformans- C. gattii species complex are due to intrinsic heteroresistance to fluconazole [29], chromosome duplication during prolonged azole therapy [30], and possible involvement of phosphoinositide-dependent kinase (PDK1), protein kinase C (PKC), and target of rapamycin (TOR) signaling pathways in basal fluconazole tolerance [31].
The strengths of this study are the large number of cryptococcal clinical isolates collected from hospitals representative of all regions of Taiwan during a 13 year period, the use of molecular methods for genotyping, assessment of antifungal susceptibility, and characterization of the risk factors for 10-week mortality. The weaknesses inherent in a study of this kind were the inability to collect sufficient isolates of rare genotypes or those with MICs higher than ECV to determine the impact on outcome. Generally only one isolate per infection is tested, although it has been revealed that 20% of patients with cryptococcosis can be infected by multiple strains or molecular types [32].The geographic distribution according to hospital location might not represent the places where exposure to Cryptococcus occurred. Besides, we could not evaluate treatment responses of an individual drug because antifungal regimens and dosages were modified in many of the patients and confounded by the underlying conditions.
In conclusion, the major genotype of Cryptococcus clinical isolates in Taiwan was VNI. Only nine of 219 patients were infected by C. gattii. Isolates with antifungal MICs higher than ECVs were rare. HIV infection was the most common underlying condition and all except one such patient was infected by the VNI genotype. Liver diseases were the most common underlying conditions in HIV-negative patients. Cirrhosis of liver and high CSF cryptococcal antigen levels were independent predictors of 10-week mortality.
Supporting Information
Details of dendrogram of M13 PCR fingerprint analysis of 219 clinical isolates of Cryptococcus neoformans- Cryptococcus gattii species complex collected in Taiwan during 1997 to 2010 and 12 reference strains.
(TIF)
Microbiological, epidemiological, and clinical characteristics and outcomes of cryptococcosis due to VNII genotype in Taiwan, 1997 to 2010.
(DOC)
Microbiological, epidemiological, and clinical characteristics and outcomes of Cryptococcus gattii in Taiwan, 1997 to 2010.
(DOC)
Microbiological, epidemiological, and clinical characteristics and outcomes of cryptococcosis due to Cryptococcus VNI isolates with antifungal minimum inhibition concentration above epidemiologic cutoff values in Taiwan, 1997 to 2010.
(DOC)
Acknowledgments
Additional members of the Taiwan Infectious Diseases Study Network (TIDSnet) for cryptococcosis include Chung-Ming Lee, Mackay Memorial Hospital, Taipei; Bor-Shen Hu, Taipei City Hospital, Taipei; Tsrang-Neng Jang, Shin Kong Wu Ho-Su Memorial Hospital, Taipei; Chia-Ying Liu, Far Eastern Memorial Hospital, Taipei; Shey-Chiang Su, Mackay Memorial Hospital, Hsinchu; Wen-Chien Ko, National Cheng Kung University Hospital, Tainan; Yao-Shen Chen, Kaohsiung Veterans General Hospital, Kaohsiung; Jen-Chih Tsai, Tzu Chi General Hospital, Hualien; Cheng-Chih Lin, Mackay Memorial Hospital, Taitung. The authors have declared that no competing interests exist.
The authors wish to thank Dr. Anastasia P. Litvintseva and Dr. John R. Perfect at Duke University Medical Center, USA, and Dr. David Ellis at the Adelaide Women's and Children's Hospital, Australia, for providing the genotyping reference strains, the Vancouver Island outbreak strains, and Australian clinical isolates, respectively. Valuable assistance during the course of experiments provided by Li-Fan Chen at National Taiwan University Hospital, Shu-Ling Weng and Chun-Kuei Liu at Mackay Memorial Hospital. The authors wish to thank Fang-Ju Sun at Mackay Memorial Hospital for assistance in statistical analysis. The authors wish to thank Calvin M. Kunin at University of Arizona, USA for his critical review of this manuscript.
Funding Statement
This work was supported by grants NSC98-2314- B-002-066-MY3 (YCC), NSC100-314- B-195-006 (HKT), NSC100-2320- B-010 -011 (WLC) from the National Science Council of Taiwan, and DOH100-TD- B-111-001 (YCC) from the Department of Health, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Harris J, Lockhart S, Chiller T (2012) Cryptococcus gattii: where do we go from here? Med Mycol 50: 113–129 doi:10.3109/13693786.2011.607854. [DOI] [PubMed] [Google Scholar]
- 2. Meyer W, Castaneda A, Jackson S, Huynh M, Castaneda E, et al. (2003) Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis 9: 189–195 doi:10.3201/eid0902.020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nucci M, Perfect JR (2008) When primary antifungal therapy fails. Clin Infect Dis 46: 1426–1433 doi:10.1086/587101. [DOI] [PubMed] [Google Scholar]
- 4. Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, et al. (2007) Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 45: 76–80 doi:10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 5. Dromer F, Bernede-Bauduin C, Guillemot D, Lortholary O, French Cryptococcosis Study G (2008) Major role for amphotericin B-flucytosine combination in severe cryptococcosis. PLoS ONE 3: e2870 doi:10.1371/journal.pone.0002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Espinel-Ingroff A, Chowdhary A, Cuenca-Estrella M, Fothergill A, Fuller J, et al. (2012) Cryptococcus neoformans - Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for amphotericin B and flucytosine. Antimicrob Agents Chemother 56: 3107–3113 doi:10.1128/AAC.06252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinel-Ingroff A, Aller AI, Canton E, Castanon-Olivares LR, Chowdhary A, et al. (2012) Cryptococcus neoformans - Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 56: 5898–5906 doi:10.1128/AAC.01115-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, et al. (2004) A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A 101: 17258–17263 doi:10.1073/pnas.0402981101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. Wayne, PA: CLSI. [Google Scholar]
- 10. Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, et al. (2001) Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 33: 690–699 doi:10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 11. Chau TT, Mai NH, Phu NH, Nghia HD, Chuong LV, et al. (2010) A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam - high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis 10: 199 doi:10.1186/1471-2334-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jain N, Wickes BL, Keller SM, Fu J, Casadevall A, et al. (2005) Molecular epidemiology of clinical Cryptococcus neoformans strains from India. J Clin Microbiol 43: 5733–5742 doi:10.1128/JCM.43.11.5733-5742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tay ST, Rohani MY, Hoo TS, Hamimah H (2010) Epidemiology of cryptococcosis in Malaysia. Mycoses 53: 509–514 doi:10.1111/j.1439-0507.2009.01750.x. [DOI] [PubMed] [Google Scholar]
- 14. Feng X, Yao Z, Ren D, Liao W, Wu J (2008) Genotype and mating type analysis of Cryptococcus neoformans and Cryptococcus gattii isolates from China that mainly originated from non-HIV-infected patients. FEMS Yeast Res 8: 930–938 doi:10.1111/j.1567-1364.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 15. Choi YH, Ngamskulrungroj P, Varma A, Sionov E, Hwang SM, et al. (2010) Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res 10: 769–778 doi:10.1111/j.1567-1364.2010.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen J, Varma A, Diaz MR, Litvintseva AP, Wollenberg KK, et al. (2008) Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg Infect Dis 14: 755–762 doi:10.3201/eid1405.071312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuchong C, Fubin C, Jianghan C, Fenglian W, Nan X, et al. (2012) Cryptococcosis in China (1985–2010): review of cases from Chinese database. Mycopathologia 173: 329–335 doi:10.1007/s11046-011-9471-1. [DOI] [PubMed] [Google Scholar]
- 18. Chen S, Sorrell T, Nimmo G, Speed B, Currie B, et al. (2000) Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 31: 499–508 doi:10.1086/313992. [DOI] [PubMed] [Google Scholar]
- 19. Dromer F, Mathoulin-Pelissier S, Launay O, Lortholary O, French Cryptococcosis Study G (2007) Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med 4: e21 doi:10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olivares LR, Martinez KM, Cruz RM, Rivera MA, Meyer W, et al. (2009) Genotyping of Mexican Cryptococcus neoformans and C. gattii isolates by PCR-fingerprinting. Med Mycol 47: 713–721 doi:10.3109/13693780802559031. [DOI] [PubMed] [Google Scholar]
- 21. Jean SS, Fang CT, Shau WY, Chen YC, Chang SC, et al. (2002) Cryptococcaemia: clinical features and prognostic factors. QJM 95: 511–518. [DOI] [PubMed] [Google Scholar]
- 22. Antinori S, Galimberti L, Magni C, Casella A, Vago L, et al. (2001) Cryptococcus neoformans infection in a cohort of Italian AIDS patients: natural history, early prognostic parameters, and autopsy findings. Eur J Clin Microbiol Infect Dis 20: 711–717. [DOI] [PubMed] [Google Scholar]
- 23. Shih CC, Chen YC, Chang SC, Luh KT, Hsieh WC (2000) Cryptococcal meningitis in non-HIV-infected patients. QJM 93: 245–251. [DOI] [PubMed] [Google Scholar]
- 24. Chen YC, Chang SC, Shih CC, Hung CC, Luhbd KT, et al. (2000) Clinical features and in vitro susceptibilities of two varieties of Cryptococcus neoformans in Taiwan. Diagn Microbiol Infect Dis 36: 175–183 doi:S0732-8893(99)00137-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 25. Liaw SJ, Wu HC, Hsueh PR (2010) Microbiological characteristics of clinical isolates of Cryptococcus neoformans in Taiwan: serotypes, mating types, molecular types, virulence factors, and antifungal susceptibility. Clin Microbiol Infect 16: 696–703 doi:10.1111/j.1469-0691.2009.02930.x. [DOI] [PubMed] [Google Scholar]
- 26. Chang WN, Huang CR, Lei CB, Lee PY, Chien CC, et al. (2004) Serotypes of clinical cerebrospinal fluid Cryptococcus neoformans isolates from southern Taiwan and their in vitro susceptibilities to amphotericin B, fluconazole, and voriconazole. Jpn J Infect Dis 57: 113–115. [PubMed] [Google Scholar]
- 27.Lee CK (2011) [Isolation of Cryptococcus species and Tricosporon asahii from environment in southern Taiwan] [master thesis]. Kaohsiung City, Taiwan (R.O.C.): National Sun Yat-Sen University. 52 p. [Google Scholar]
- 28. Chaturvedi V, Chaturvedi S (2011) Cryptococcus gattii: a resurgent fungal pathogen. Trends Microbiol 19: 564–571 doi:10.1016/j.tim.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varma A, Kwon-Chung KJ (2010) Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 54: 2303–2311 doi:10.1128/AAC.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sionov E, Lee H, Chang YC, Kwon-Chung KJ (2010) Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6: e1000848 doi:10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee H, Khanal Lamichhane A, Garraffo HM, Kwon-Chung KJ, Chang YC (2012) Involvement of PDK1, PKC and TOR signalling pathways in basal fluconazole tolerance in Cryptococcus neoformans . Mol Microbiol 84: 130–146 doi:10.1111/j.1365-2958.2012.08016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desnos-Ollivier M, Patel S, Spaulding AR, Charlier C, Garcia-Hermoso D, et al. (2010) Mixed infections and In Vivo evolution in the human fungal pathogen Cryptococcus neoformans. MBio 1. doi:10.1128/mBio.00091-10. [DOI] [PMC free article] [PubMed]
- 33. Sorrell TC, Chen SC, Ruma P, Meyer W, Pfeiffer TJ, et al. (1996) Concordance of clinical and environmental isolates of Cryptococcus neoformans var. gattii by random amplification of polymorphic DNA analysis and PCR fingerprinting. J Clin Microbiol 34: 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campbell LT, Currie BJ, Krockenberger M, Malik R, Meyer W, et al. (2005) Clonality and recombination in genetically differentiated subgroups of Cryptococcus gattii. Eukaryot Cell 4: 1403–1409 doi:10.1128/EC.4.8.1403-1409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Escandon P, Sanchez A, Martinez M, Meyer W, Castaneda E (2006) Molecular epidemiology of clinical and environmental isolates of the Cryptococcus neoformans species complex reveals a high genetic diversity and the presence of the molecular type VGII mating type a in Colombia. FEMS Yeast Res 6: 625–635 doi:10.1111/j.1567-1364.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 36. Lui G, Lee N, Ip M, Choi KW, Tso YK, et al. (2006) Cryptococcosis in apparently immunocompetent patients. QJM 99: 143–151 doi:10.1093/qjmed/hcl014. [DOI] [PubMed] [Google Scholar]
- 37. MacDougall L, Kidd SE, Galanis E, Mak S, Leslie MJ, et al. (2007) Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis 13: 42–50 doi:10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Byrnes EJ 3rd, Bildfell RJ, Frank SA, Mitchell TG, Marr KA, et al. (2009) Molecular evidence that the range of the Vancouver Island outbreak of Cryptococcus gattii infection has expanded into the Pacific Northwest in the United States. J Infect Dis 199: 1081–1086 doi:10.1086/597306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Byrnes EJ 3rd, Li W, Lewit Y, Perfect JR, Carter DA, et al. (2009) First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PLoS ONE 4: e5851 doi:10.1371/journal.pone.0005851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okamoto K, Hatakeyama S, Itoyama S, Nukui Y, Yoshino Y, et al. (2010) Cryptococcus gattii genotype VGIIa infection in man, Japan, 2007. Emerg Infect Dis 16: 1155–1157 doi:10.3201/eid1607.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cogliati M, Chandrashekar N, Esposto MC, Chandramuki A, Petrini B, et al. (2012) Cryptococcus gattii serotype-C strains isolated in Bangalore, Karnataka, India. Mycoses 55: 262–268 doi:10.1111/j.1439-0507.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 42. Sellers B, Hall P, Cine-Gowdie S, Hays AL, Patel K, et al. (2012) Cryptococcus gattii: an emerging fungal pathogen in the southeastern United States. Am J Med Sci 343: 510–511 doi:10.1097/MAJ.0b013e3182464bc7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of dendrogram of M13 PCR fingerprint analysis of 219 clinical isolates of Cryptococcus neoformans- Cryptococcus gattii species complex collected in Taiwan during 1997 to 2010 and 12 reference strains.
(TIF)
Microbiological, epidemiological, and clinical characteristics and outcomes of cryptococcosis due to VNII genotype in Taiwan, 1997 to 2010.
(DOC)
Microbiological, epidemiological, and clinical characteristics and outcomes of Cryptococcus gattii in Taiwan, 1997 to 2010.
(DOC)
Microbiological, epidemiological, and clinical characteristics and outcomes of cryptococcosis due to Cryptococcus VNI isolates with antifungal minimum inhibition concentration above epidemiologic cutoff values in Taiwan, 1997 to 2010.
(DOC)