Abstract
Several Western guidelines recommend the routine use of pharmacologic thromboprophylaxis for cancer surgery patients to prevent venous thromboembolism (VTE). However, the necessity of routine pharmacologic perioperative thromboprophylaxis in Asian gastric cancer (GC) patients has not been clearly determined. To determine the necessity of routine perioperative pharmacologic thromboprophylaxis in Korean gastric cancer patients, the incidence of postoperative VTE was prospectively evaluated in gastric cancer patients receiving surgery. Among 610 GC patients who had received surgery, 375 patents underwent routine duplex Doppler ultrasonography (DUS) on days 5–12 following surgery to detect VTE and then VTE-related symptoms and signs were checked at 4 weeks after surgery (cohort A). The 235 patients that declined DUS were registered to cohort B and the occurrence of postoperative VTE was retrospectively analyzed. In cohort A, symptomatic or asymptomatic VTE until 4 weeks after surgery was detected in 9 patients [2.4%; 95% confidence interval (CI); 0.9–3.9]. Tumor stage was a significant factor related to VTE development [stage I, 1.4%; stage II/III, 2.4%; stage IV, 9.7% (P = 0.008)]. In multivariate analysis, patients with stage IV had a higher postoperative VTE development [odds ratio, 8.18 (95% CI, 1.54–43.42)] than those with stage I. In cohort B, a low incidence of postoperative VTE was reaffirmed; only one postoperative VTE case (0.4%) was observed. In conclusion, the incidence of postoperative VTE in Korean GC patients was only 2.4%. Risk-stratified applications of perioperative pharmacologic thromboprophylaxis are thought to be more appropriate than the routine pharmacologic thromboprophylaxis in Korean GC patients receiving surgery.
Introduction
Venous thromboembolism (VTE), including extremity deep vein thrombosis (DVT) and pulmonary embolism (PE), is attributed to several risk factors including old age, immobilization, surgery and others [1]. Especially in cancer surgery, the risk for VTE increases during the perioperative period [2], [3]. Therefore, several Western guidelines recommend the routine use of pharmacologic thromboprophylaxis for cancer surgery patients to prevent VTE [3], [4], [5], [6], [7].
Gastric cancer (GC) is particularly prevalent in eastern Asia. According to Western guidelines, all GC patients should receive pharmacologic prophylaxis such as low molecular weight heparin (LMWH) [3], [4], [5], [6]. Although there is no firm evidence from prospective studies, many Asian cancer surgeons believe that the incidence of postoperative VTE is not so high as they must follow Western guidelines. In our previous retrospective study, postoperative VTE was observed in only 0.2% of GC patients receiving surgery [8]. Our data strongly suggested that the incidence of postoperative VTE in Korean GC patients is much lower than that of Western patients [8], in whom the incidence has been reported to be a lot higher [9], [10]. Since most previous studies insisting on a low incidence of VTE in Asian patients have been retrospectively conducted [8], [11], the necessity of routine pharmacologic perioperative thromboprophylaxis in Asian cancer patients has not been clearly determined.
Due to ethnic differences in incidences of VTE between Asian and Western cancer patients, studies focusing on VTE in Asian patients are clearly required. Moreover, as GC is prevalent in Asia, large prospective studies on the incidence of postoperative VTE in Asian GC patients are required to justify risk-stratified application of perioperative pharmacologic thromboprophylaxis.
Materials and Methods
Patient population; a prospective cohort (cohort A)
This prospective study, conducted at Seoul National University Bundang Hospital (SNUBH), was carried out to investigate the postoperative incidence of VTE in GC patients. The pharmacologic prophylaxis for VTE in GC patients receiving surgery was not routine clinical practice at SNUBH. Patients who were admitted for GC surgery and met the eligibility criteria were consecutively enrolled between May 2010 and July 2011.
Patients with ≥ 20 years-of-age and who had pathologically confirmed adenocarcinoma of the stomach or gastroesophogeal junction were included. All patients received major abdominal cancer surgery for curative or palliative intent. Major surgery was defined as a surgical procedure lasting > 30 minutes [3]. All patients did not receive prophylactic pharmacologic anticoagulation. However, mechanical prophylaxis (elastic bandage or stockings) were allowed. The exclusion criteria were as follows: history of VTE, a known hypercoagulable state or congenital thrombophilia; concurrent VTE at the time of admission for GC surgery; a prior or concomitant malignancy, except for patients who were disease-free for 5 years after curative therapy; a history of taking antiplatelet or anticoagulant agents less than 2 days prior to the operation; comorbidities that required pharmacologic anticoagulation during the perioperative period (i.e., atrial fibrillation or cerebrovascular infarct); and pregnancy.
In the prospective cohort (cohort A; N = 375), all patients underwent duplex and color Doppler ultrasonography (DUS) on lower extremities to screen for DVT regardless of postoperative symptom development. All patient demographics and laboratory data were collected before surgery. A variation of the Elixhauser Comorbidity index was used for comorbidities [12], [13], [14]. Comorbidities that indicated the presence of another cancer or transient conditions (i.e., electrolyte disturbance or transient arrhythmia) were excluded; however, hyperlipidemia was included as one comorbidity entity [11]. The tumor stage was based on the final pathology reports.
The detection of VTE in the cohort A
All cohort A patients underwent a DUS between 5–12 days following GC surgery. The DUS was performed by two experienced radiologists (S.I.C., 12 years and E.J.C., 10 years for vascular DUS imaging). All imaging was performed using a HDI 5000 ultrasound (Philips Medical Systems, Bothell, WA) equipped with a high-resolution 5–9 MHz linear-array transducer, from the distal 3–4 cm of the external iliac vein to the distal calf veins. DUS included imaging in the transverse and longitudinal planes using both gray-scale and color DUS.
DVT was defined when the following conditions were seen: (a) echogenic material within lumen, (b) non-compressibility of the affected vein, or (c) nonvisualized flow in color Doppler imaging [15], [16]. To check for non-compressibility, the deep veins were evaluated at 1-cm intervals from the common femoral vein to the calf veins. At times, blood flow echogenicity resulting from blood stasis and erythrocyte aggregation contributed to false-positive results. In these conditions, dynamic tests such as flow augmentation produced by passive limb raising or upstream muscle compression were performed to exclude false positive results [17].
A routine postoperative follow-up visit was performed at 4 weeks (window period, ± 1 week) following surgery and then every 3–6 months. Symptoms and signs related to VTE were checked during the surgical admission and outpatient clinic follow-up periods. Whenever VTE-related symptoms were clinically suspected, the study protocol guided that a DUS or computed tomography angiography (CT angiography) for low extremities or pulmonary vasculature should be conducted.
Patient population; a retrospective cohort (cohort B)
During the study period, 235 patients who did not want to undergo DUS following surgery were registered to cohort B (Figure 1). These patients met the same eligibility criteria as patients in cohort A. Data collection in cohort B patients was done to reaffirm the result observed in cohort A. In cohort B, DUS or CT angiography was only performed for patients with suspected symptoms related to VTE. Most of the clinical data for cohort B were retrieved from the prospectively maintained database in the department of surgery at SNUBH [8]. However, data on VTE development were retrospectively collected from an electronic medical chart review.
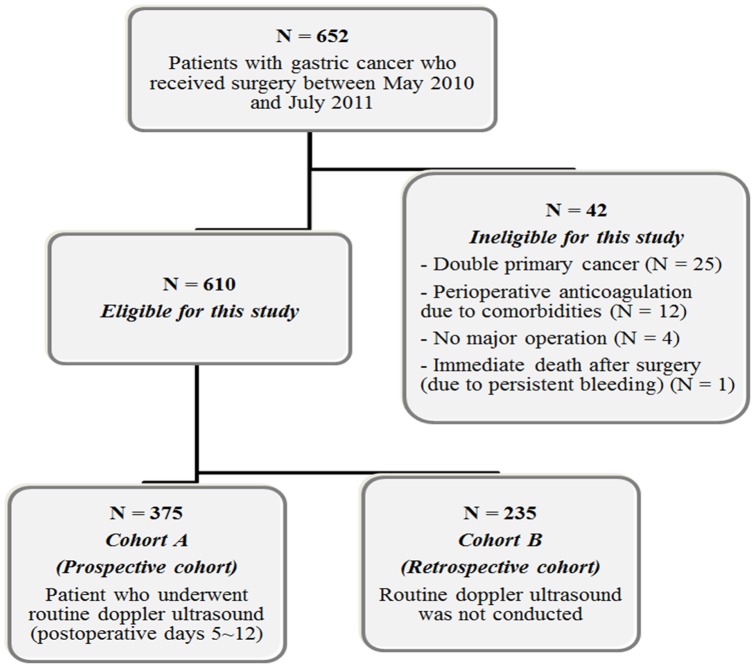
Figure 1. Flow of patients in this study.
Statistical and ethical considerations
The primary objective was to find the incidence of symptomatic or asymptomatic VTE following surgery in cohort A patients. The incidence of postoperative VTE was defined as the cases detected by routine DUS (on days 5–12 after surgery) plus any additional VTE cases detected by DUS or other studies (until 4 weeks after surgery). The secondary objective was to identify risk factors for the development of VTE in this population.
We assumed that the actual incidence of postoperative VTE would be approximately 6% and this incidence would be lower than 10%. The calculated sample size was 375 with 80% power and one-sided significance level of 0.025. Although the enrollment of 420 patients was initially planned in consideration for a 10% drop-out rate, this study was completed when 375 cohort A patients were enrolled because there had been no drop-out cases at 4 weeks (window period, ± 1 week) after surgery.
Chi-square or linear-by-linear association tests were conducted to compare percentages in cross-tabulations and the t-test was used to compare means. In a multivariate analysis to investigate risk factors for VTE, a logistic regression model was applied. Two-sided P-values ≤ 0.05 were considered significant and SPSS software was used (SPSS, Inc., Chicago, IL). The declaration of Helsinki was followed for this study. The patients in cohort A provided written informed consent prior to this study. The exemption of acquiring written consents from cohort B patients was permitted by the Institutional Review Board (IRB) at SNUBH (IRB study number: B-1002/094-007). This study was approved by IRB and was registered to ClinicalTrials.gov (NCT01345773).
Results
Patient characteristics and incidence of VTE in the cohort A
Among the 610 patients who met eligibility criteria, 375 were enrolled in cohort A (Figure 1). Patient characteristics are presented in Table 1. In the cohort A, stage distribution was as follows: stage I (58.4%); stage II (15.5%); stage III (17.9%); stage IV (8.3%). Laparoscopic surgery was performed in 74.4%. A partial gastrectomy (subtotal or proximal gastrectomy) was conducted in 75.2%. The mean operation time was 179 minutes. There was no postoperative mortality. All patients (N = 375) were followed up at 4 weeks (window period, ± 1 week) and 366 patients (97.6%) were followed up at 12 weeks after surgery. Of the 9 patients whose follow-up was lost at 3 months, 6 patients were transferred to hospitals near the patients' residence and 3 patients died from cancer progression.
Table 1. Patient characteristics.
| Variable | Cohort A (N = 375) | Cohort B (N = 235) | P-value |
| Gender | 0.874* | ||
| Male | 253 (67.5%) | 160 (68.1%) | |
| Female | 122 (32.5%) | 75 (31.9%) | |
| Age | 0.995* | ||
| Median (range) | 61 (23–88) | 62 (22–91) | |
| < 70 years | 268 (71.5%) | 168 (71.5%) | |
| ≥ 70 years | 107 (28.5%) | 67 (28.5%) | |
| No. of comorbidites | 0.779* | ||
| 0 | 117 (31.2%) | 66 (28.1%) | |
| 1 | 121 (32.3%) | 79 (33.6%) | |
| 2 | 84 (22.4%) | 59 (25.1%) | |
| ≥ 3 | 53 (14.1%) | 31 (13.2%) | |
| Preoperative laboratory [mean ± (SD)] | |||
| White blood cell (/μL) | 6832 (± 2227) | 6782 (± 2129) | 0.781† |
| Hemoglobin (g/dL) | 13.5 (± 2.1) | 13.4 (± 2.3) | 0.514† |
| Platelet (× 103/μL) | 240 (± 82) | 250 (± 77) | 0.145† |
| Stage | 0.324* | ||
| Stage I | 219 (58.4%) | 150 (63.8%) | |
| Stage II | 58 (15.5%) | 26 (11.1%) | |
| Stage III | 67 (17.9%) | 44 (18.7%) | |
| Stage IV | 31 (8.3%) | 15 (6.4%) | |
| Tumor location in stomach | 0.257* | ||
| Low third | 211 (56.3%) | 139 (59.1%) | |
| Middle third | 84 (22.4%) | 58 (24.7%) | |
| Upper third | 72 (19.2%) | 31 (13.2%) | |
| Whole | 8 (2.1%) | 7 (3.0%) | |
| Gross morphology | 0.094* | ||
| Early gastric cancer | 196 (52.3%) | 139 (59.1%) | |
| Borrmann type 1 | 12 (3.2%) | 2 (0.9%) | |
| Borrmann type 2 | 23 (6.1%) | 16 (6.8%) | |
| Borrmann type 3 | 121 (32.3%) | 71 (30.2%) | |
| Borrmann type 4 | 23 (6.1%) | 7 (3.0%) | |
| Lauren classification | 0.946* | ||
| Intestinal | 181 (48.3%) | 113 (48.1%) | |
| Diffuse | 176 (46.9%) | 112 (47.7%) | |
| Mixed | 18 (4.8%) | 10 (4.3%) | |
| Tumor differentiation | 0.054* | ||
| Well differentiated | 41 (10.9%) | 33 (14.0%) | |
| Moderately differentiated | 138 (36.8%) | 81 (34.5%) | |
| Poorly differentiated | 131 (34.9%) | 74 (31.5%) | |
| Signet ring cell | 51 (13.6%) | 45 (19.1%) | |
| Mucinous or other | 14 (3.7%) | 2 (0.9%) | |
| Surgical extent | 0.021* | ||
| Partial gastrectomy | 282 (75.2%) | 197 (83.8%) | |
| Total gastrectomy | 87 (23.2%) | 33 (14.0%) | |
| Other palliative surgery (no gastrectomy) | 6 (1.6%) | 5 (2.1%) | |
| Surgical procedure | 0.026* | ||
| Laparoscopic surgery | 279 (74.4%) | 193 (82.1%) | |
| Open surgery | 96 (25.6%) | 42 (17.9%) | |
| Operation duration | |||
| Mean (± SD, minutes) | 179 ± 60 | 175 ± 58 | 0.495† |
| ≤ 2 hours | 80 (21.3%) | 48 (20.4%) | 0.180* |
| > 2 and ≤ 3 hours | 142 (37.9%) | 106 (45.1%) | |
| > 3 hours | 153 (40.8%) | 81 (34.5%) | |
| Surgical outcome | 0.636* | ||
| R0 | 349 (93.1%) | 221 (94%) | |
| R1/R2 | 26 (6.9%) | 14 (6.0%) |
Abbreviations: SD, standard deviation.
chi-square test, †t-test.
In multivariate analysis using a logistic regression model, the clinical parameters with P<0.20 in univariate analyses (age, number of comorbidities, WBC counts, hemoglobin level, surgical procedure [laparoscopic vs. open surgery] and stage) were included. A backward stepwise conditional logistic regression was used with P = 0.10 as the entry and P = 0.10 as the removal criteria.
Abbreviations: OR, odds ratio; CI, confidence interval; WBC, white blood cell.
In cohort A, VTE was detected in 9 patients [2.4%; 95% confidence interval (CI): 0.9–3.9] within the 4 weeks after surgery. The median time to VTE detection was 7 days (range, 6–25). VTE was detected in 8 patients by routine DUS; all 8 DVT events were asymptomatic distal calf vein thrombosis, but one patient had subtle dyspnea and a CT revealed a PE simultaneously. In the remaining one patient, on the 25th day after surgery, asymptomatic PE was incidentally detected in a chest CT performed to evaluate the tumor status before initiating palliative chemotherapy and an asymptomatic proximal DVT was also simultaneously detected by additional DUS. No other VTE case was detected between 4 and 12 weeks after surgery. The further detailed characteristics of VTE events are shown in Table 2.
Table 2. Characteristics of patients who developed VTE during postoperative periods.
| Group | Sex/Age | Comorbidity | Stage | Surgery | Surgical outcome | Operation time | Time to ambulation | VTE event | Time to VTE events after surgery | Treatment |
| Cohort A | F/48 | Hypertension | pT1aN0M0, Stage IA | Laparoscopic subtotal gastrectomy | R0 | 120 min | Within 1 day | Asymptomatic DVT, - Left calf vein | 7 days | Observation, spontaneously disappeared |
| Cohort A | F/73 | Parkinson's disease | pT1aN0M0, Stage IA | Laparoscopic subtotal gastrectomy | R0 | 155 min | Within 1 day | Asymptomatic DVT, - Left calf vein | 7 days | Observation, spontaneously disappeared |
| Cohort A | M/43 | None | pT2N0M0, Stage IB | Laparoscopic subtotal gastrectomy | R0 | 160 min | Within 1 day | Asymptomatic DVT, - Right calf vein | 7 days | Observation, spontaneously disappeared |
| Cohort A | M/73 | Diabetes, Hypertension, Anemia | pT3N2M0, Stage IIIA | Laparoscopic total gastrectomy | R0 | 360 min | Within 1 day | Asymptomatic DVT, - Right calf vein | 6 days | LMWH, disappeared |
| Cohort A | F/76 | Anemia | pT4aN3bM0, Stage IIIC | Open total gastrectomy | R0 | 170 min | Within 1 day | Asymptomatic DVT, - Both calf veins | 7 days | LMWH, disappeared |
| Cohort A | M/66 | Hypertension, Anemia, Peripheral arterial disease | pT4aN3bM0, Stage IIIC | Open total gastrectomy | R0 | 325 min | Within 2 days | Asymptomatic DVT, - Right calf vein | 7 days | LMWH, disappeared |
| Cohort A | M/53 | Obesity, Anemia | pT4aN3bM1, Stage IV | Open total gastrectomy | R2 | 165 min | Within 2 days | Asymptomatic DVT, - Left calf vein | 7 days | LMWH, disappeared |
| Cohort A | F/76 | Hypertension, Congestive heart failure, Atrial fibrillation Anemia | pT4aN2M1, Stage IV | Open total gastrectomy | R2 | 165 min | Within 1 day | Asymptomatic DVT,- Right trifurcation level and calf veins Symptomatic PE | 6 days | LMWH, disappeared |
| Cohort A | M/78 | Hypertension, Anemia | cT4N+M1, Stage IV | Laparoscopic gastrojejunostomy | R2 | 55 min | Within 1 day | Asymptomatic DVT,- Left superficial femoral vein to tibioperoneal trunk and peroneal vein Asymptomatic PE | 25 days | LMWH, disappeared |
| Cohort B | M/68 | None | pT4bN2M0, Stage IIIB | Open total gastrectomy | R0 | 340 min | Within 3 days | Asymptomatic PE | 4 days | Observation, spontaneously disappeared |
Abbreviations: VTE, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; LMWH, low molecular weight heparin.
Risk factors for VTE development
Risk factors for postoperative VTE were analyzed in cohort A patients. The results of univariate analyses are presented in Table 3. The tumor stage was a significant factor related to VTE development (P = 0.008). Compared with the low VTE incidences in patients with stage I (1.4%) and II/III (2.4%), stage IV patients had 9.7% of VTE incidence. The number of comorbidities showed a borderline significance for developing VTE (P = 0.086). Patients aged ≥ 70 years had higher incidences of postoperative VTE than those aged < 70 years (4.7% vs. 1.5%); however, this was not statistically significant (P = 0.126). Laparoscopic surgery showed a numerically lower VTE incidence than open surgery, but this was not also significant (P = 0.190). Although the surgical outcomes (R0 vs. R1/R2) and extent (partial gastrectomy, total gastrectomy or palliative surgery without a gastrectomy) were associated with different postoperative VTE incidences (P<0.05), those parameters were clearly correlated with tumor stages. All patients who received palliative surgery without a gastrectomy (N = 6) and the 22 of 26 patients who had postoperative residual tumors (R1/R2 surgery) had stage IV. When 344 patients with stage I to III were separately analyzed, the extent of the surgery did not influence the postoperative VTE incidence [total gastrectomy (3/70, 4.3%) vs. partial gastrectomy (3/274, 1.1%); P = 0.101].
Table 3. The incidence of VTE according to clinical parameters.
| Variable | VTE (-) (N = 366) | VTE (+) (N = 9) | P-value | |
| Gender | 0.480* | |||
| Male | 248 (98.0%) | 5 (2.0%) | ||
| Female | 118 (96.7%) | 4 (3.3%) | ||
| Age | 0.126* | |||
| < 70 years | 264 (98.5%) | 4 (1.5%) | ||
| ≥ 70 years | 102 (95.3%) | 5 (4.7%) | ||
| BMI | 0.236† | |||
| BMI < 21 | 85 (96.6%) | 3 (3.4%) | ||
| 21≤ BMI < 25 | 171 (97.2%) | 5 (2.8%) | ||
| BMI ≥ 25 | 110 (99.1%) | 1 (0.9%) | ||
| Smoking | 0.893† | |||
| Never smoker | 228 (97.4%) | 6 (2.6%) | ||
| Ex-smoker | 59 (98.3%) | 1 (1.7%) | ||
| Current smoker | 79 (97.5%) | 2 (2.5%) | ||
| No. of comorbidities | 0.086† | |||
| 0 | 116 (99.1%) | 1 (0.9%) | ||
| 1 | 118 (97.5%) | 3 (2.5%) | ||
| 2 | 82 (97.6%) | 2 (2.4%) | ||
| ≥ 3 | 50 (94.3%) | 3 (5.7%) | ||
| WBC counts (/μL) | 0.188† | |||
| 4000 ≤ WBC < 10000 | 329 (97.9%) | 7 (2.1%) | ||
| WBC < 4000 | 13 (92.9%) | 1 (7.1%) | ||
| WBC ≥ 10000 | 24 (96.0%) | 1 (4.0%) | ||
| Hemoglobin (g/dL) | 0.140* | |||
| Hemoglobin ≥ 10.0 | 340 (98.0%) | 7 (2.0%) | ||
| Hemoglobin < 10.0 | 26 (92.9%) | 2 (7.1%) | ||
| Platelet counts (× 103/μL) | 0.957† | |||
| 130 ≤ Platelet < 400 | 341 (97.7%) | 8 (2.3%) | ||
| Platelet < 130 | 13 (100.0%) | 0 (0.0%) | ||
| Platelet ≥ 400 | 12 (92.3%) | 1 (7.7%) | ||
| Stage | 0.008† | |||
| Stage I | 216 (98.6%) | 3 (1.4%) | ||
| Stage II/III | 122 (97.6%) | 3 (2.4%) | ||
| Stage IV | 28 (90.3%) | 3 (9.7%) | ||
| Tumor location in stomach | 0.278† | |||
| Low third | 217 (98.6%) | 3 (1.4%) | ||
| Middle third | 61 (95.3%) | 3 (4.7%) | ||
| Upper third | 79 (96.3%) | 3 (3.7%) | ||
| Whole | 9 (100.0%) | 0 (0.0%) | ||
| Gross morphology | 0.271† | |||
| Early gastric cancer | 193 (98.5%) | 3 (1.5%) | ||
| Borrmann type 1 | 11 (91.7%) | 1 (8.3%) | ||
| Borrmann type 2 | 23 (100.0%) | 0 (0.0%) | ||
| Borrmann type 3 | 117 (96.7%) | 4 (3.3%) | ||
| Borrmann type 4 | 22 (95.7%) | 1 (4.3%) | ||
| Lauren classification | 0.531† | |||
| Intestinal | 176 (97.2%) | 5 (2.8%) | ||
| Diffuse | 172 (97.7%) | 4 (2.3%) | ||
| Mixed | 18 (100%) | 0 (0%) | ||
| Tumor differentiation | 0.632† | |||
| Well differentiated | 41 (100%) | 0 (0%) | ||
| Moderately differentiated | 133 (96.4%) | 5 (3.6%) | ||
| Poorly differentiated | 129 (98.5%) | 2 (1.5%) | ||
| Signet ring cell | 50 (98.0%) | 1 (2.0%) | ||
| Mucinous or other | 13 (92.9%) | 1 (7.1%) | ||
| Surgical extent | 0.001† | |||
| Partial gastrectomy‡ | 279 (98.9%) | 3 (1.1%) | ||
| Total gastrectomy | 82 (94.3%) | 5 (5.7%) | ||
| Other palliative surgery (no gastrectomy) | 5 (83.3%) | 1 (16.7%) | ||
| Surgical procedure | 0.190* | |||
| Laparoscopic surgery | 274 (98.2%) | 5 (1.8%) | ||
| Open surgery | 92 (95.8%) | 4 (4.2%) | ||
| Operation duration | 0.440† | |||
| ≤ 2 hours | 78 (97.5%) | 2 (2.5%) | ||
| > 2 and ≤ 3 hours | 137 (96.5%) | 5 (3.5%) | ||
| > 3 hours | 151 (98.7%) | 2 (1.3%) | ||
| Surgical outcome | 0.019* | |||
| R0 | 343 (98.3%) | 6 (1.7%) | ||
| R1/R2 | 23 (88.5%) | 3 (11.5%) | ||
| Time to ambulation after surgery | 0.682† | |||
| ≤ 24 hours | 271 (97.5%) | 7 (2.5%) | ||
| > 24 and ≤ 48 hours | 82 (97.6%) | 2 (2.4%) | ||
| > 72 hours | 13 (100%) | 0 (0%) | ||
Fisher's exact test, †linear-by-linear association.
Subtotal gastrectomy was conducted in 271 patients and proximal gastrectomy in 11 patients.
Abbreviations: VTE, venous thromboembolism; BMI, body mass index; WBC, white bleed cell.
In multivariate analysis, the clinical parameters with P<0.20 in univariate analyses were included (Table 4). Only the disease stage was predictive of postoperative VTE development: patients with stage IV had a higher incidence of postoperative VTE with an odds ratio (OR) of 8.18 (95% CI, 1.54–43.42) compared with those with stage I. However, the risk of VTE in patients with stage II/III was not different from those with stage I. Although there was no statistical significance, elderly patients (age ≥ 70 years) had a trend of developing higher VTE than patients aged < 70 years (OR 3.42; 95% CI, 0.88–13.33; P = 0.076).
Table 4. Multivariate analysis (logistic regression analysis) for the postoperative development of venous thromboembolism.
| OR | 95% CI | P-value | |
| Age | |||
| < 70 years | 1.00 | – | – |
| ≥ 70 years | 3.42 | 0.88–13.33 | 0.076 |
| Stage | |||
| stage I | 1.00 | – | – |
| stage II/III | 1.68 | 0.33–8.53 | 0.529 |
| stage IV | 8.18 | 1.54–43.42 | 0.014 |
| Constant | 0.008 | – | < 0.001 |
The development of VTE in the cohort B
Compared with patients in the cohort A, the extent of gastrectomy and surgical procedure (laparoscopic vs. open surgery) showed a different distribution in cohort B patients (N = 235, Table 1). At 4 weeks, the dropout rate during follow-up was 1.3% (3/235). Three patients dropped out before the 21st day after surgery without suspected VTE symptoms or postoperative complications; these 3 patients were referred to nearby hospitals for the further follow-up.
In the cohort B, symptomatic postoperative VTE did not develop. Only one case of asymptomatic PE in a segmental branch of the right lower lobe pulmonary artery was incidentally found in an abdominal CT performed to evaluate postoperative complications. The PE in this case was spontaneously resolved without treatment (Table 2).
Discussion
This is the largest prospective study on the incidence of postoperative VTE in GC patients. It demonstrated that postoperative VTE is very rare (2.4%; 95% CI, 0.9–3.9) in Korean GC patients. As routine pharmacologic prophylaxis is generally considered when the incidence of postoperative VTE is ≥ 10% [10], our study shows that risk-stratified applications of perioperative pharmacologic thromboprophylaxis is more appropriate than the routine pharmacologic thromboprophylaxis in Asian GC patients receiving surgery.
It has been demonstrated that Asians have a lower incidence of VTE [3], [8], [11], [12], [13], [18], [19], [20], [21], [22], [23], [24]. In a Korean prospective study which included 107 patients with various gastrointestinal cancers, the postoperative VTE incidence detected by DUS was 7.5% [21]. Our previous retrospective studies on patients with stomach or colorectal cancer reported the incidence of postoperative VTE much lower than Western patients [8], [11]. Another Korean study by Jeong et al. reported no cases of symptomatic VTE among 182 GC patients following a gastrectomy that had not received LMWH prophylaxis [24]. In the present study, the incidence of postoperative VTE detected by DUS was only 2.4% in prospective cohort A (N = 375). To reaffirm the low incidence of VTE observed in cohort A, a separate analysis on retrospective cohort B (N = 235) was done and only one patient (0.4%) was found to have postoperative VTE. Our study clearly shows that the incidence of postoperative VTE in Korean GC patients is much lower than that of Western patients.
In our study, only the disease stage was predictive of postoperative VTE and the incidence of VTE tended to increase in elderly patients in the multivariate analysis (Table 4). An advanced stage has been consistently reported to be predictive of VTE in previous studies [3], [5], [8], [11], [12], [13], [22] and an older age is also a well-known risk factor [3], [5], [8], [12], [13], [19], [25], [26]. Considering the low incidence of overall postoperative VTE in our patient cohorts, the risk-stratified application of perioperative pharmacologic thromboprophylaxis for selected GC patients such as those with stage IV are thought to be more appropriate in Korea than routine pharmacologic thromboprophylaxis.
Although GC has been reported to have high risk of VTE development in Western studies [27], [28], the reasons why the incidence of postoperative VTE in our Korean GC patients is too low need to be further discussed. In a Japanese study conducted on abdominal surgery patients that consisted of general, gynecologic and urologic surgery (N = 173), the VTE incidence detected by venography was 24.3%, which was almost comparable to ranges reported in the West [29]. Use of a venography, having a higher sensitivity for VTE detection than DUS, may be one of the reasons for an increased VTE detection as compared to our study. However, DUS is the current standard method for VTE detection as a venography is a cumbersome procedure. Including many intra-pelvic surgery cases (53%), which was related to more frequent postoperative VTE development than upper abdominal surgery, may be another reason of increased detection of VTE. Moreover, the number of GC patients recruited in that study was too small (N = 33) [29]. Therefore, results of the Japanese study cannot be generalized to Asian GC patients. The lower incidence of postoperative VTE in our study might be attributed to the application of mechanical thromboprophylaxis (elastic bandage or stockings), tumor characteristics and a frequent use of laparoscopic surgery. The Japanese study had mechanical thromboprophylaxis performed in about half of the patients [29], whereas mechanical prophylaxis was routinely used for our study. Although there are conflicting results, these mechanical methods might have played an important role in reducing postoperative VTE in our study [24], [30], [31]. Mechanical thromboprophylaxis has been preferred to pharmacologic thromboprophylaxis by most Asian surgeons because of concerns about increased postoperative bleeding related to LMWH [20], [24], [31]. Another reason for the low incidence of postoperative VTE in Korean GC patients may be due to the increased number of cases with early GC (EGC). Since endoscopic surveillance is commonly conducted for early diagnosis of GC in Asian countries including Korea and Japan [32], EGC becomes more common. In the present study, stage I disease was about 60% and this proportion of EGC is in a similar range to those reported from other Korean institutions [33], [34], [35], [36]. Japan is known to have a higher proportion of EGC cases as compared to Korea [37], [38]. As advanced stage is most predictive of VTE development in GC [8], [22], the high prevalence of EGC in eastern Asian countries may be one of reasons for decreased postoperative VTE. In addition, a frequent use of laparoscopic gastrectomy in the patient population might be another explanation. In retrospective studies mostly composed of patients with benign diseases, a lower incidence of VTE after laparoscopic surgery compared with open surgery was reported [39], [40]. In Asian countries including Korea and Japan, despite the lack of long term survival data from well-designed randomized trials [41], [42], [43], a laparoscopy-assisted gastrectomy for EGC is rapidly gaining popularity based on the benefits of a shorter hospital stay, earlier mobilization and functional recovery. As a large-scaled phase 3 study comparing laparoscopic and open surgery in EGC patients has completed patient enrollment and is awaiting survival outcomes [43], laparoscopic gastrectomy is expected to be more popular if long term survival outcomes are shown to be similar between laparoscopic and open gastrectomy. In the present study, 74.4% of patients received laparoscopic surgery and 74.1% of patients were able to ambulate within 24 hours after surgery (Table 3).
Although our study was conducted at a single institution, the situations at other Korean institutions are similar to our institution as most Korean GC patients receive surgery at experienced tertiary high-volume centers [44]. Therefore, our results are thought to be generalized in Korea [21], [24]. However, the generalization of our results for all Asian cancer patients needs to be very cautious. In large prospective studies conducted for Asian patients receiving major orthopedic surgery, postoperative VTE incidence was in a range similar to that of Western patients; the aggressiveness of orthopedic surgery and prolonged immobilization are thought to overwhelm the ethnic advantage of Asian patients [45], [46]. As mentioned above, intra-pelvic surgery was reported to be related to a higher postoperative VTE than upper abdominal surgery [29]. In addition, the propensity of developing VTE may be different according to different ethnic groups even within Asian countries [46]. Therefore, prospective studies on the necessity of routine perioperative pharmacologic thromboprophylaxis in Asian cancer patients need to be conducted, probably in each Asian country separately.
In summary, the incidence of postoperative VTE was 2.4% in Korean GC patients and only advanced stage was related to the frequent development of postoperative VTE. Risk-stratified applications of perioperative pharmacologic thromboprophylaxis are thought to be appropriate in Korean GC patients. More prospective studies on the postoperative incidence of VTE in Asian cancer patients are also warranted.
Acknowledgments
We are grateful to the Medical Research Collaborating Center (MRCC) at Seoul National University Bundang Hospital for statistical assistance.
Funding Statement
This study was partially supported by research grants from the Seoul National University Bundang Hospital Research Fund (02-2010-037) and Sanofi-Aventis. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, et al. (2000) Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 160: 809–815. [DOI] [PubMed] [Google Scholar]
- 2. White RH, Zhou H, Romano PS (2003) Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 90: 446–455. [DOI] [PubMed] [Google Scholar]
- 3. Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, et al. (2007) American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 25: 5490–5505. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Streiff MB, Farge D, Mandala M, Debourdeau P, et al. (2009) Venous thromboembolism prophylaxis and treatment in cancer: a consensus statement of major guidelines panels and call to action. J Clin Oncol 27: 4919–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mandala M, Falanga A, Roila F (2010) Venous thromboembolism in cancer patients: ESMO Clinical Practice Guidelines for the management. Ann Oncol 21 Suppl 5v274–276. [DOI] [PubMed] [Google Scholar]
- 6. Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, et al. (2012) Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141: e227S–277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khorana AA (2007) The NCCN Clinical Practice Guidelines on Venous Thromboembolic Disease: strategies for improving VTE prophylaxis in hospitalized cancer patients. Oncologist 12: 1361–1370. [DOI] [PubMed] [Google Scholar]
- 8. Lee KW, Bang SM, Kim S, Lee HJ, Shin DY, et al. (2010) The incidence, risk factors and prognostic implications of venous thromboembolism in patients with gastric cancer. J Thromb Haemost 8: 540–547. [DOI] [PubMed] [Google Scholar]
- 9. Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H (2001) Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 88: 913–930. [DOI] [PubMed] [Google Scholar]
- 10. Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, et al. (2008) Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 381S–453S. [DOI] [PubMed] [Google Scholar]
- 11. Choi S, Lee KW, Bang SM, Kim S, Lee JO, et al. (2011) Different characteristics and prognostic impact of deep-vein thrombosis/pulmonary embolism and intraabdominal venous thrombosis in colorectal cancer patients. Thromb Haemost 106: 1084–1094. [DOI] [PubMed] [Google Scholar]
- 12. Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, et al. (2006) Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol 24: 1112–1118. [DOI] [PubMed] [Google Scholar]
- 13. Chew HK, Wun T, Harvey DJ, Zhou H, White RH (2007) Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol 25: 70–76. [DOI] [PubMed] [Google Scholar]
- 14. Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 15. Appelman PT, De Jong TE, Lampmann LE (1987) Deep venous thrombosis of the leg: US findings. Radiology 163: 743–746. [DOI] [PubMed] [Google Scholar]
- 16. Cronan JJ, Dorfman GS, Grusmark J (1988) Lower-extremity deep venous thrombosis: further experience with and refinements of US assessment. Radiology 168: 101–107. [DOI] [PubMed] [Google Scholar]
- 17. Gaitini D (2006) Current approaches and controversial issues in the diagnosis of deep vein thrombosis via duplex Doppler ultrasound. J Clin Ultrasound 34: 289–297. [DOI] [PubMed] [Google Scholar]
- 18. Oh SY, Kim JH, Lee KW, Bang SM, Hwang JH, et al. (2008) Venous thromboembolism in patients with pancreatic adenocarcinoma: lower incidence in Asian ethnicity. Thromb Res 122: 485–490. [DOI] [PubMed] [Google Scholar]
- 19. Jang MJ, Bang SM, Oh D (2011) Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost 9: 85–91. [DOI] [PubMed] [Google Scholar]
- 20. Liew NC, Moissinac K, Gul Y (2003) Postoperative venous thromboembolism in Asia: a critical appraisal of its incidence. Asian J Surg 26: 154–158. [DOI] [PubMed] [Google Scholar]
- 21. Kim IG, Kim KH, Seo HJ, Kim JI, Ahn CH, et al. (2004) Deep vein thrombosis after surgery for gastrointestinal cancer; incidence and correlation with risk factors. J Korean Soc Vasc Surg 20: 237–241. [Google Scholar]
- 22. Chew HK, Wun T, Harvey D, Zhou H, White RH (2006) Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 166: 458–464. [DOI] [PubMed] [Google Scholar]
- 23. Mukherjee D, Lidor AO, Chu KM, Gearhart SL, Haut ER, et al. (2008) Postoperative venous thromboembolism rates vary significantly after different types of major abdominal operations. J Gastrointest Surg 12: 2015–2022. [DOI] [PubMed] [Google Scholar]
- 24. Jeong O, Ryu SY, Park YK, Kim YJ (2010) The effect of low molecular weight heparin thromboprophylaxis on bleeding complications after gastric cancer surgery. Ann Surg Oncol 17: 2363–2369. [DOI] [PubMed] [Google Scholar]
- 25. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, et al. (1998) Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 158: 585–593. [DOI] [PubMed] [Google Scholar]
- 26. Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, et al. (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151: 933–938. [PubMed] [Google Scholar]
- 27. Levitan N, Dowlati A, Remick SC, Tahsildar HI, Sivinski LD, et al. (1999) Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 78: 285–291. [DOI] [PubMed] [Google Scholar]
- 28. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111: 4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakon M, Maehara Y, Yoshikawa H, Akaza H (2006) Incidence of venous thromboembolism following major abdominal surgery: a multi-center, prospective epidemiological study in Japan. J Thromb Haemost 4: 581–586. [DOI] [PubMed] [Google Scholar]
- 30. Clarke-Pearson DL, Dodge RK, Synan I, McClelland RC, Maxwell GL (2003) Venous thromboembolism prophylaxis: patients at high risk to fail intermittent pneumatic compression. Obstet Gynecol 101: 157–163. [DOI] [PubMed] [Google Scholar]
- 31. Inada K, Shirai N, Hayashi M, Matsumoto K, Hirose M (1983) Postoperative deep venous thrombosis in Japan. Incidence and prophylaxis. Am J Surg 145: 775–779. [DOI] [PubMed] [Google Scholar]
- 32. Lee EH, Lee HY, Choi KS, Jun JK, Park EC, et al. (2011) Trends in Cancer Screening Rates among Korean Men and Women: Results from the Korean National Cancer Screening Survey (KNCSS), 2004–2010. Cancer Res Treat 43: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, et al. (2012) Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg 214: 88–96. [DOI] [PubMed] [Google Scholar]
- 34. Kim DH, Oh CA, Oh SJ, Choi MG, Noh JH, et al. (2012) Validation of seventh edition AJCC gastric cancer staging modifications. J Surg Oncol 105: 26–30. [DOI] [PubMed] [Google Scholar]
- 35. Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, et al. (2011) Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg 98: 255–260. [DOI] [PubMed] [Google Scholar]
- 36. Jeong O, Park YK (2011) Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer 11: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jung HC (2003) Gastric cancer screening. Korean J Gastroenterol 41: 72–78. [Google Scholar]
- 38. Hisamichi S, Sugawara N, Fukao A (1988) Effectiveness of gastric mass screening in Japan. Cancer Detect Prev 11: 323–329. [PubMed] [Google Scholar]
- 39. Buchberg B, Masoomi H, Lusby K, Choi J, Barleben A, et al. (2011) Incidence and risk factors of venous thromboembolism in colorectal surgery: does laparoscopy impart an advantage? Arch Surg 146: 739–743. [DOI] [PubMed] [Google Scholar]
- 40. Nguyen NT, Hinojosa MW, Fayad C, Varela E, Konyalian V, et al. (2007) Laparoscopic surgery is associated with a lower incidence of venous thromboembolism compared with open surgery. Ann Surg 246: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 41. Lee JH, Han HS, Lee JH (2005) A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 19: 168–173. [DOI] [PubMed] [Google Scholar]
- 42. Hosono S, Arimoto Y, Ohtani H, Kanamiya Y (2006) Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol 12: 7676–7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, et al. (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251: 417–420. [DOI] [PubMed] [Google Scholar]
- 44. Ahn HS, Yook JH, Park CH, Park YK, Yu W, et al. (2011) General perioperative management of gastric cancer patients at high-volume centers. Gastric Cancer 14: 178–182. [DOI] [PubMed] [Google Scholar]
- 45. Leizorovicz A, Turpie AG, Cohen AT, Wong L, Yoo MC, et al. (2005) Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost 3: 28–34. [DOI] [PubMed] [Google Scholar]
- 46. Piovella F, Wang CJ, Lu H, Lee K, Lee LH, et al. (2005) Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost 3: 2664–2670. [DOI] [PubMed] [Google Scholar]