Abstract
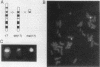
A familial, constitutionally rearranged human chromosome 17 is deleted for much of the DNA in its centromeric region but retains full mitotic centromere activity. Fluorescence in situ hybridization, pulsed-field gel electrophoresis, and Southern blot analysis of the residual centromeric region revealed a approximately 700-kb centromeric array of tandemly repeated alpha satellite DNA that was only approximately 20 to 30% as large as a normal array. This deletion was associated with a reduction in the amount of the centromere-specific antigen CENP-B detected by indirect immunofluorescence. The coincidence of the primary constriction, the small residual array of alpha satellite DNA, and the reduced amount of detectable CENP-B support the hypothesis that CENP-B is associated with alpha satellite DNA. Furthermore, the finding that both the deleted chromosome 17 and its derivative supernumerary fragment retained mitotic function and possess centromeric protein antigens suggests that human centromeres are structurally and functionally repetitive.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo T., Willard H. F., de la Chapelle A. Determination of the breakpoints of 1;7 translocations in myelodysplastic syndrome by in situ hybridization using chromosome-specific alpha satellite DNA from human chromosomes 1 and 7. Cytogenet Cell Genet. 1989;50(1):49–53. doi: 10.1159/000132718. [DOI] [PubMed] [Google Scholar]
- Balczon R. D., Brinkley B. R. Tubulin interaction with kinetochore proteins: analysis by in vitro assembly and chemical cross-linking. J Cell Biol. 1987 Aug;105(2):855–862. doi: 10.1083/jcb.105.2.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M. W., Tan E., Brinkley B. R. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981 Oct;91(1):95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Valdivia M. M., Tousson A., Brenner S. L. Compound kinetochores of the Indian muntjac. Evolution by linear fusion of unit kinetochores. Chromosoma. 1984;91(1):1–11. doi: 10.1007/BF00286479. [DOI] [PubMed] [Google Scholar]
- Chikashige Y., Kinoshita N., Nakaseko Y., Matsumoto T., Murakami S., Niwa O., Yanagida M. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell. 1989 Jun 2;57(5):739–751. doi: 10.1016/0092-8674(89)90789-7. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Clarke L., Baum M. P. Functional analysis of a centromere from fission yeast: a role for centromere-specific repeated DNA sequences. Mol Cell Biol. 1990 May;10(5):1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L. Centromeres of budding and fission yeasts. Trends Genet. 1990 May;6(5):150–154. doi: 10.1016/0168-9525(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Cooke C. A., Bernat R. L., Earnshaw W. C. CENP-B: a major human centromere protein located beneath the kinetochore. J Cell Biol. 1990 May;110(5):1475–1488. doi: 10.1083/jcb.110.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallapiccola B., Mastroiacovo P., Gandini E. Centric fission of chromosome no. 4 in the mother of two patients with trisomy 4p. Hum Genet. 1976 Jan 28;31(1):121–125. doi: 10.1007/BF00270409. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Cooke C. A. Proteins of the inner and outer centromere of mitotic chromosomes. Genome. 1989;31(2):541–552. doi: 10.1139/g89-103. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Migeon B. R. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92(4):290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Ratrie H., 3rd, Stetten G. Visualization of centromere proteins CENP-B and CENP-C on a stable dicentric chromosome in cytological spreads. Chromosoma. 1989 Jun;98(1):1–12. doi: 10.1007/BF00293329. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Rattner J. B. A map of the centromere (primary constriction) in vertebrate chromosomes at metaphase. Prog Clin Biol Res. 1989;318:33–42. [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes J. A., Green D. K., Malloy P., Sumner A. T. Flow cytometry measurements of human chromosome kinetochore labeling. Cytometry. 1989 Mar;10(2):134–142. doi: 10.1002/cyto.990100204. [DOI] [PubMed] [Google Scholar]
- Fishel B., Amstutz H., Baum M., Carbon J., Clarke L. Structural organization and functional analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol. 1988 Feb;8(2):754–763. doi: 10.1128/mcb.8.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryns J. P., Bulcke J., Hens L., Van den Berghe H. Balanced transmission of centromeric fission products in man. Hum Genet. 1980;54(1):127–128. doi: 10.1007/BF00279063. [DOI] [PubMed] [Google Scholar]
- Hahnenberger K. M., Baum M. P., Polizzi C. M., Carbon J., Clarke L. Construction of functional artificial minichromosomes in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1989 Jan;86(2):577–581. doi: 10.1073/pnas.86.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. A case of centric fission in man. Humangenetik. 1975;26(3):257–259. doi: 10.1007/BF00281462. [DOI] [PubMed] [Google Scholar]
- Hill A., Bloom K. Genetic manipulation of centromere function. Mol Cell Biol. 1987 Jul;7(7):2397–2405. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist G. P., Dancis B. Telomere replication, kinetochore organizers, and satellite DNA evolution. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4566–4570. doi: 10.1073/pnas.76.9.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T. C. A possible function of constitutive heterochromatin: the bodyguard hypothesis. Genetics. 1975 Jun;79 (Suppl):137–150. [PubMed] [Google Scholar]
- Hsu T. C., Pathak S., Chen T. R. The possibility of latent centromeres and a proposed nomenclature system for total chromosome and whole arm translocations. Cytogenet Cell Genet. 1975;15(1):41–49. doi: 10.1159/000130497. [DOI] [PubMed] [Google Scholar]
- Jørgensen A. L., Bostock C. J., Bak A. L. Chromosome-specific subfamilies within human alphoid repetitive DNA. J Mol Biol. 1986 Jan 20;187(2):185–196. doi: 10.1016/0022-2836(86)90227-5. [DOI] [PubMed] [Google Scholar]
- Mahtani M. M., Willard H. F. Pulsed-field gel analysis of alpha-satellite DNA at the human X chromosome centromere: high-frequency polymorphisms and array size estimate. Genomics. 1990 Aug;7(4):607–613. doi: 10.1016/0888-7543(90)90206-a. [DOI] [PubMed] [Google Scholar]
- Manuelidis L. Chromosomal localization of complex and simple repeated human DNAs. Chromosoma. 1978 Mar 22;66(1):23–32. doi: 10.1007/BF00285813. [DOI] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Sugimoto K., Okazaki T. Alphoid satellite DNA is tightly associated with centromere antigens in human chromosomes throughout the cell cycle. Exp Cell Res. 1989 Mar;181(1):181–196. doi: 10.1016/0014-4827(89)90192-4. [DOI] [PubMed] [Google Scholar]
- Merry D. E., Pathak S., Hsu T. C., Brinkley B. R. Anti-kinetochore antibodies: use as probes for inactive centromeres. Am J Hum Genet. 1985 Mar;37(2):425–430. [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. R., Gosden J. R., Miller D. A. A cloned sequence, p82H, of the alphoid repeated DNA family found at the centromeres of all human chromosomes. Chromosoma. 1985;92(5):369–377. doi: 10.1007/BF00327469. [DOI] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Schultes N. P., Szostak J. W. Chromosome length controls mitotic chromosome segregation in yeast. Cell. 1986 May 23;45(4):529–536. doi: 10.1016/0092-8674(86)90284-9. [DOI] [PubMed] [Google Scholar]
- Nakaseko Y., Adachi Y., Funahashi S., Niwa O., Yanagida M. Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J. 1986 May;5(5):1011–1021. doi: 10.1002/j.1460-2075.1986.tb04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R. B. Mitosis. Adv Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Oakey R., Tyler-Smith C. Y chromosome DNA haplotyping suggests that most European and Asian men are descended from one of two males. Genomics. 1990 Jul;7(3):325–330. doi: 10.1016/0888-7543(90)90165-q. [DOI] [PubMed] [Google Scholar]
- Peretti D., Maraschio P., Lambiase S., Lo Curto F., Zuffardi O. Indirect immunofluorescence of inactive centromeres as indicator of centromeric function. Hum Genet. 1986 May;73(1):12–16. doi: 10.1007/BF00292655. [DOI] [PubMed] [Google Scholar]
- Peterson J. B., Ris H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J Cell Sci. 1976 Nov;22(2):219–242. doi: 10.1242/jcs.22.2.219. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta A. F., Cooke C. A., Earnshaw W. C. Structure of the human centromere at metaphase. Trends Biochem Sci. 1990 May;15(5):181–185. doi: 10.1016/0968-0004(90)90158-8. [DOI] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Saunders M., Fitzgerald-Hayes M., Bloom K. Chromatin structure of altered yeast centromeres. Proc Natl Acad Sci U S A. 1988 Jan;85(1):175–179. doi: 10.1073/pnas.85.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surosky R. T., Newlon C. S., Tye B. K. The mitotic stability of deletion derivatives of chromosome III in yeast. Proc Natl Acad Sci U S A. 1986 Jan;83(2):414–418. doi: 10.1073/pnas.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer B. R., Moses M. J., Rosenbaum J. L. Assembly of microtubules onto kinetochores of isolated mitotic chromosomes of HeLa cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4023–4027. doi: 10.1073/pnas.72.10.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-Smith C., Brown W. R. Structure of the major block of alphoid satellite DNA on the human Y chromosome. J Mol Biol. 1987 Jun 5;195(3):457–470. doi: 10.1016/0022-2836(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waye J. S., Mitchell A. R., Willard H. F. Organization and genomic distribution of "82H" alpha satellite DNA. Evidence for a low-copy or single-copy alphoid domain located on human chromosome 14. Hum Genet. 1988 Jan;78(1):27–32. doi: 10.1007/BF00291229. [DOI] [PubMed] [Google Scholar]
- Waye J. S., Willard H. F. Structure, organization, and sequence of alpha satellite DNA from human chromosome 17: evidence for evolution by unequal crossing-over and an ancestral pentamer repeat shared with the human X chromosome. Mol Cell Biol. 1986 Sep;6(9):3156–3165. doi: 10.1128/mcb.6.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevrick R., Willard H. F. Long-range organization of tandem arrays of alpha satellite DNA at the centromeres of human chromosomes: high-frequency array-length polymorphism and meiotic stability. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9394–9398. doi: 10.1073/pnas.86.23.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet. 1985 May;37(3):524–532. [PMC free article] [PubMed] [Google Scholar]
- Willard H. F., Holmes M. T. A sensitive and dependable assay for distinguishing hamster and human X-linked steroid sulfatase activity in somatic cell hybrids. Hum Genet. 1984;66(2-3):272–275. doi: 10.1007/BF00286615. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Wevrick R., Warburton P. E. Human centromere structure: organization and potential role of alpha satellite DNA. Prog Clin Biol Res. 1989;318:9–18. [PubMed] [Google Scholar]