Abstract
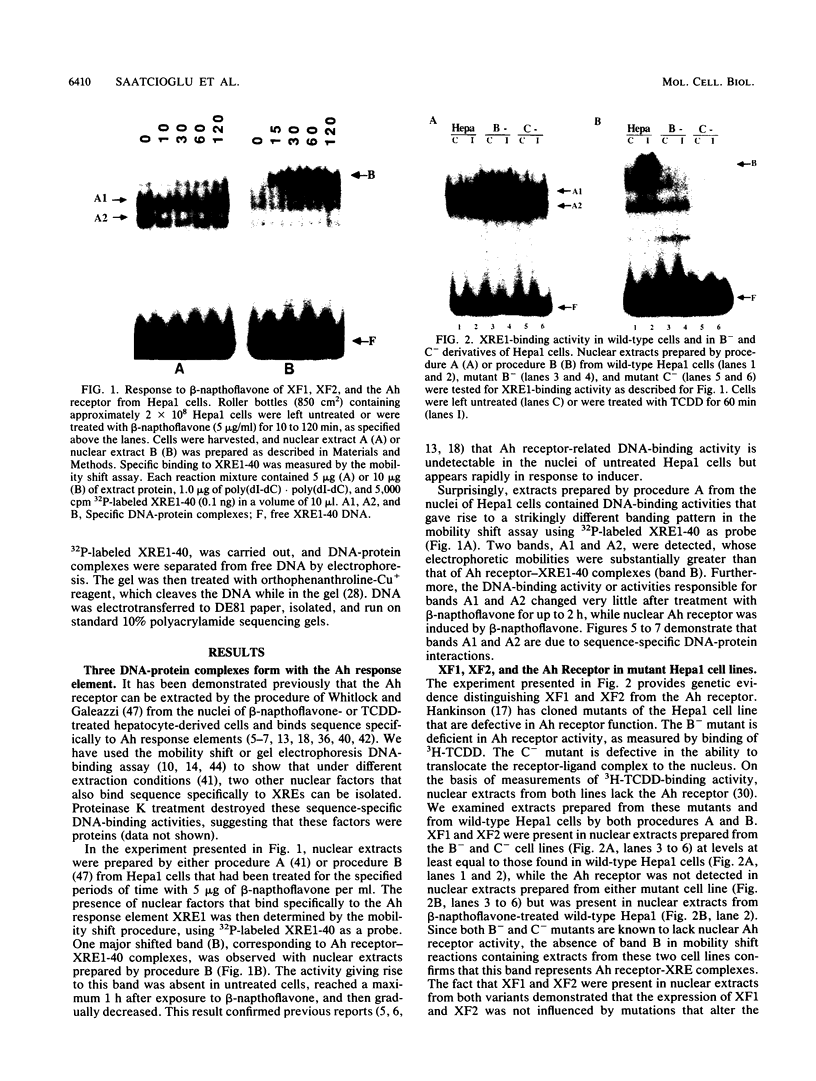
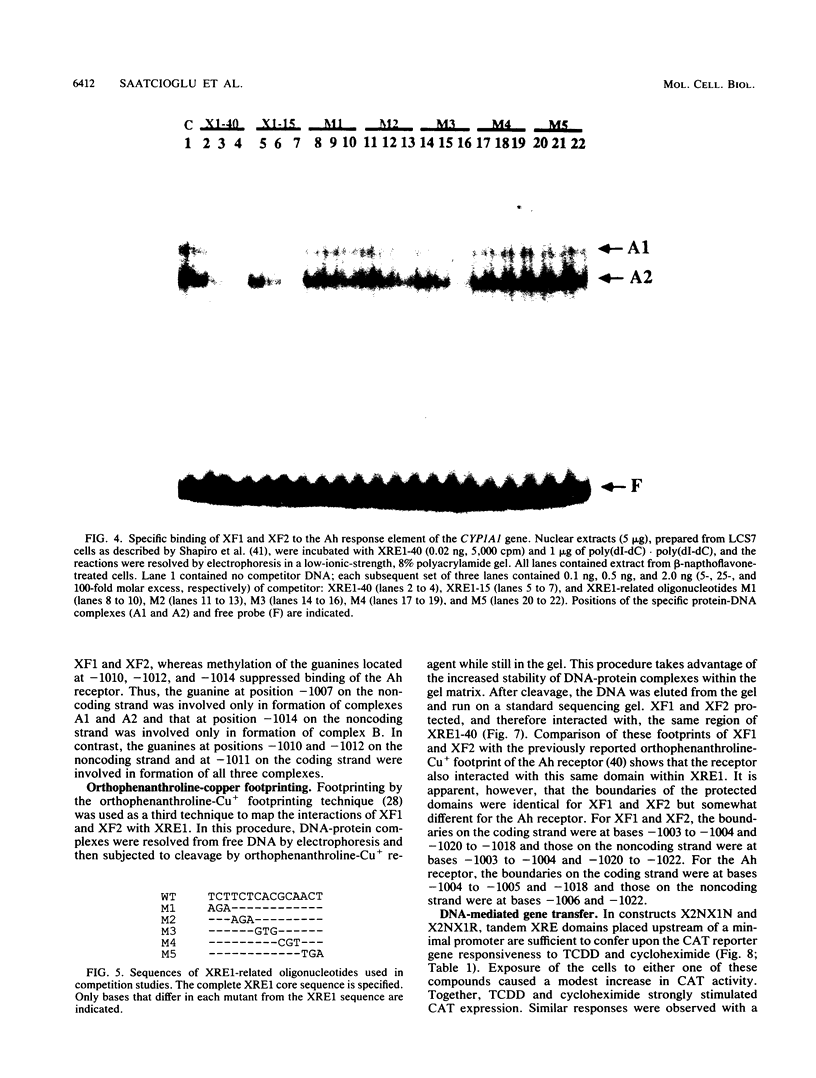
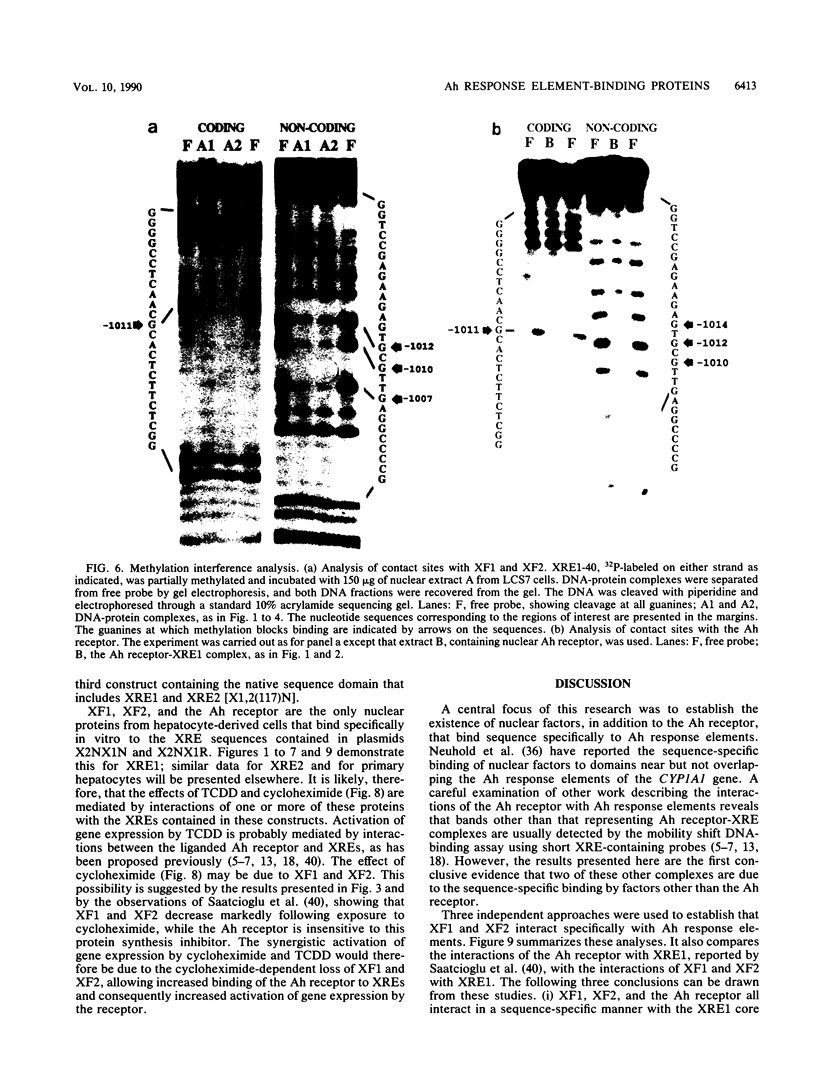
Three nuclear factors, the Ah receptor, XF1, and XF2, bind sequence specifically to the Ah response elements or xenobiotic response elements (XREs) of the cytochrome P450IA1 (P450c) gene. The interactions of these factors with the Ah response element XRE1 were compared by three independent methods, methylation interference footprinting, orthophenanthroline-Cu+ footprinting, and mobility shift competition experiments, using a series of synthetic oligonucleotides with systematic alterations in the XRE core sequence. These studies established the following (i) all three factors interact sequence specifically with the core sequence of XRE1; (ii) the pattern of contacts made with this sequence by the Ah receptor are different from those made by XF1 and XF2; and (iii) although XF1 and XF2 can be distinguished by the mobility shift assay, the sequence specificities of their interactions with XRE1 are indistinguishable. Further characterization revealed the following additional differences among these three factors: (i) XF1 and XF2 could be extracted from nuclei under conditions quite different from those required for extraction of the Ah receptor; (ii) XF1 and XF2 were present in the nuclei of untreated cells and did not respond to polycyclic compounds, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and beta-napthoflavone, while nuclear Ah receptor was undetectable in untreated cells and rapidly increased in response to TCDD; (iii) inhibition of protein synthesis did not affect the TCDD-induced appearance of the Ah receptor but substantially decreased the constitutive activities of XF1 and XF2, suggesting that the Ah receptor must be present in untreated cells in an inactive form that can be rapidly activated by polycyclic compounds, while the constitutive expression of XF1 and XF2 depends on the continued synthesis of a relatively unstable protein; (iv) the receptor-deficient and nuclear translocation-defective mutants of the hepatoma cell line Hepa1, which are known to lack nuclear Ah receptor, expressed normal levels of XF1 and XF2, suggesting that the former factor is genetically distinct from the latter two; and (v) a divalent metal ion, probably Zn2+, is known to be an essential cofactor for the Ah receptor but was not required for the DNA-binding activities of XF1 and XF2. Together, these findings indicate that the Ah receptor is distinct from XF1 and XF2, while the latter two activities may be related. Because the DNA-binding domains of these three factors overlap substantially, their binding to XREs is probably mutually exclusive, which suggests that the interplay of these factors at Ah response elements may be important to the regulation of CYP1A1 gene transcription. The results of preliminary transfection experiments with constructs harboring XREs upstream of the chloramphenicol acetyltransferase gene driven by a minimal simian virus 40 promoter are presented that are consistent with this hypothesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Schlegel-Haueter S. E. Study of liver differentiation in vitro. J Cell Biol. 1981 May;89(2):216–222. doi: 10.1083/jcb.89.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y. Temperature-sensitive adult liver cell line dependent on glucocorticoid for differentiation. Mol Cell Biol. 1983 Jun;3(6):1013–1020. doi: 10.1128/mcb.3.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Xanthopoulos K. G., Darnell J. E., Jr A liver-specific DNA-binding protein recognizes multiple nucleotide sites in regulatory regions of transthyretin, alpha 1-antitrypsin, albumin, and simian virus 40 genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3840–3844. doi: 10.1073/pnas.85.11.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresteil T., Jaiswal A. K., Eisen H. J. Transcriptional control of human cytochrome P1-450 gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin in human tissue culture cell lines. Arch Biochem Biophys. 1987 Feb 15;253(1):233–240. doi: 10.1016/0003-9861(87)90656-4. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Protein-DNA interactions at recognition sites for the dioxin-Ah receptor complex. J Biol Chem. 1989 Oct 5;264(28):16478–16482. [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988 Nov 25;263(33):17221–17224. [PubMed] [Google Scholar]
- Drouin J., Charron J., Gagner J. P., Jeannotte L., Nemer M., Plante R. K., Wrange O. Pro-opiomelanocortin gene: a model for negative regulation of transcription by glucocorticoids. J Cell Biochem. 1987 Dec;35(4):293–304. doi: 10.1002/jcb.240350404. [DOI] [PubMed] [Google Scholar]
- Fagan J. B., Pastewka J. V., Chalberg S. C., Gozukara E., Guengerich F. P., Gelboin H. V. Noncoordinate regulation of the mRNAs encoding cytochromes P-450BNF/MC-B and P-450ISF/BNF-G. Arch Biochem Biophys. 1986 Jan;244(1):261–272. doi: 10.1016/0003-9861(86)90116-5. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Nishi C., Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986 Feb 11;14(3):1465–1477. doi: 10.1093/nar/14.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985 Oct 25;13(20):7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W., Schleif R. A dimer of AraC protein contacts three adjacent major groove regions of the araI DNA site. Proc Natl Acad Sci U S A. 1985 May;82(10):3129–3133. doi: 10.1073/pnas.82.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines R. N., Mathis J. M., Jacob C. S. Identification of multiple regulatory elements on the human cytochrome P450IA1 gene. Carcinogenesis. 1988 Sep;9(9):1599–1605. doi: 10.1093/carcin/9.9.1599. [DOI] [PubMed] [Google Scholar]
- Israel D. I., Estolano M. G., Galeazzi D. R., Whitlock J. P., Jr Superinduction of cytochrome P1-450 gene transcription by inhibition of protein synthesis in wild type and variant mouse hepatoma cells. J Biol Chem. 1985 May 10;260(9):5648–5653. [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Durrin L. K., Fisher J. M., Whitlock J. P., Jr Control of gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Multiple dioxin-responsive domains 5'-ward of the cytochrome P1-450 gene. J Biol Chem. 1986 May 25;261(15):6647–6650. [PubMed] [Google Scholar]
- Jones P. B., Durrin L. K., Galeazzi D. R., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci U S A. 1986 May;83(9):2802–2806. doi: 10.1073/pnas.83.9.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Tagashira Y., Sogawa K., Fujii-Kuriyama Y. Titration of mRNAs for cytochrome P-450c and P-450d under drug-inductive conditions in rat livers by their specific probes of cloned DNAs. J Biol Chem. 1984 Aug 25;259(16):10145–10149. [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. Tissue-specific expression of the mouse dioxin-inducible P(1)450 and P(3)450 genes: differential transcriptional activation and mRNA stability in liver and extrahepatic tissues. Mol Cell Biol. 1986 May;6(5):1471–1477. doi: 10.1128/mcb.6.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M. D., Sigman D. S. Footprinting DNA-protein complexes in situ following gel retardation assays using 1,10-phenanthroline-copper ion: Escherichia coli RNA polymerase-lac promoter complexes. Biochemistry. 1987 Nov 17;26(23):7234–7238. doi: 10.1021/bi00397a006. [DOI] [PubMed] [Google Scholar]
- Laimins L. A., Khoury G., Gorman C., Howard B., Gruss P. Host-specific activation of transcription by tandem repeats from simian virus 40 and Moloney murine sarcoma virus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6453–6457. doi: 10.1073/pnas.79.21.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legraverend C., Hannah R. R., Eisen H. J., Owens I. S., Nebert D. W., Hankinson O. Regulatory gene product of the Ah locus. Characterization of receptor mutants among mouse hepatoma clones. J Biol Chem. 1982 Jun 10;257(11):6402–6407. [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. The role of ribonucleic acid and protein synthesis in microsomal aryl hydrocarbon hydroxylase induction in cell culture. The independence of transcription and translation. J Biol Chem. 1970 Jan 10;245(1):160–168. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Gonzalez F. J., Jaiswal A. K., Nebert D. W. Dioxin-inducible enhancer region upstream from the mouse P(1)450 gene and interaction with a heterologous SV40 promoter. DNA. 1986 Oct;5(5):403–411. doi: 10.1089/dna.1986.5.403. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco D. S., Boyum K. W., Merchant S. N., Chalberg S. C., Fagan J. B. Transcriptional and post-transcriptional regulation of the genes encoding cytochromes P-450c and P-450d in vivo and in primary hepatocyte cultures. J Biol Chem. 1988 Jun 25;263(18):8671–8676. [PubMed] [Google Scholar]
- Pasco D. S., Fagan J. B. Efficient DNA-mediated gene transfer into primary cultures of adult rat hepatocytes. DNA. 1989 Sep;8(7):535–541. doi: 10.1089/dna.1.1989.8.535. [DOI] [PubMed] [Google Scholar]
- Pfeifer K., Arcangioli B., Guarente L. Yeast HAP1 activator competes with the factor RC2 for binding to the upstream activation site UAS1 of the CYC1 gene. Cell. 1987 Apr 10;49(1):9–18. doi: 10.1016/0092-8674(87)90750-1. [DOI] [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Aryl hydrocarbon (Ah) receptor DNA-binding activity. Sequence specificity and Zn2+ requirement. J Biol Chem. 1990 Jun 5;265(16):9251–9258. [PubMed] [Google Scholar]
- Shapiro D. J., Sharp P. A., Wahli W. W., Keller M. J. A high-efficiency HeLa cell nuclear transcription extract. DNA. 1988 Jan-Feb;7(1):47–55. doi: 10.1089/dna.1988.7.47. [DOI] [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr The potential role of DNA methylation in the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1989 Oct 25;264(30):17754–17758. [PubMed] [Google Scholar]
- Sogawa K., Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. Location of regulatory elements responsible for drug induction in the rat cytochrome P-450c gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8044–8048. doi: 10.1073/pnas.83.21.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Teifeld R. M., Fagan J. B., Pasco D. S. Transient superinducibility of cytochrome P450c (CYP1A1) mRNA and transcription. DNA. 1989 Jun;8(5):329–338. doi: 10.1089/dna.1.1989.8.329. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Galeazzi D. R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin receptors in wild type and variant mouse hepatoma cells. Nuclear location and strength of nuclear binding. J Biol Chem. 1984 Jan 25;259(2):980–985. [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Gelboin H. V. Induction of aryl hydrocarbon (benzo(a)pyrene) hydroxylase in liver cell culture by temporary inhibition of protein synthesis. J Biol Chem. 1973 Sep 10;248(17):6114–6121. [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]