Abstract
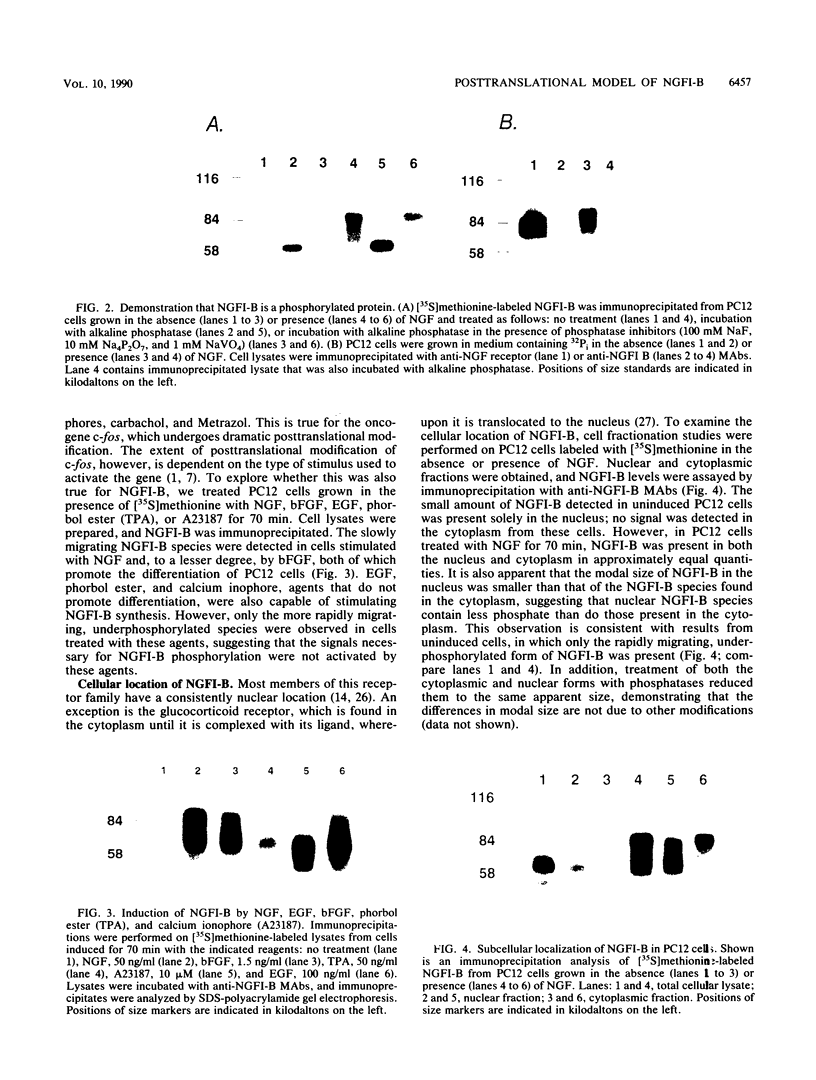
The NGFI-B gene is rapidly activated by a variety of stimuli that induce cells to differentiate or proliferate. It encodes a protein with a predicted molecular mass of congruent to 61 kDa and is a member of the thyroid/steroid hormone receptor gene family. To characterize this protein, monoclonal antibodies were raised against a bacterial TrpE-NGFI-B fusion protein that encompasses a large portion (Glu-410 to Leu-527) of the carboxy-terminal domain of NGFI-B. These antibodies detected a protein that was rapidly synthesized in response to nerve growth factor (NGF) and migrated as a broad band on sodium dodecyl sulfate-polyacrylamide gels with an apparent molecular mass that ranged from 63 to 88 kDa. Pulse-chase analysis demonstrated that NGFI-B was rapidly posttranslationally modified and was a short-lived protein. NGFI-B was found to be a phosphorylated protein, and the multiple NGFI-B species coalesced into a single, more rapidly migrating species when treated with alkaline phosphatase. PC12 cells grown in the absence of NGF contained low levels of NGFI-B that was underphosphorylated. Epidermal growth factor, phorbol ester, and the calcium ionophore A23187 stimulated the synthesis of NGFI-B that was composed largely of underphosphorylated, rapidly migrating species. In contrast, basic fibroblast growth factor, which promotes differentiation of PC12 cells, induced the synthesis of NGFI-B species similar to those synthesized in response to NGF treatment. The underphosphorylated NGFI-B found in uninduced PC12 cells was found only in the nucleus, whereas NGFI-B in NGF-stimulated PC12 cells was present in approximately equal quantities in the cytoplasm and nucleus. Consistent with the cellular distribution observed in nonstimulated PC12 cells, the highly phosphorylated species were predominantly cytoplasmic whereas the more rapidly migrating forms were nuclear.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Zerial M., Lemaire P., Almendral J., Bravo R., Charnay P. A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J. 1988 Jan;7(1):29–35. doi: 10.1002/j.1460-2075.1988.tb02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Barium modulates c-fos expression and post-translational modification. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8521–8524. doi: 10.1073/pnas.83.22.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M. L., Fahrner T. J., Aykent S., Milbrandt J. The zinc finger protein NGFI-A exists in both nuclear and cytoplasmic forms in nerve growth factor-stimulated PC12 cells. J Biol Chem. 1990 Sep 5;265(25):15253–15260. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Schrader W. T., O'Malley B. W. Hormone-dependent regulation of chicken progesterone receptor deoxyribonucleic acid binding and phosphorylation. Endocrinology. 1989 Dec;125(6):3051–3058. doi: 10.1210/endo-125-6-3051. [DOI] [PubMed] [Google Scholar]
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Hazel T. G., Nathans D., Lau L. F. A gene inducible by serum growth factors encodes a member of the steroid and thyroid hormone receptor superfamily. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8444–8448. doi: 10.1073/pnas.85.22.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeck W., Rusconi S., Groner B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem. 1989 Aug 25;264(24):14396–14402. [PubMed] [Google Scholar]
- Joseph L. J., Le Beau M. M., Jamieson G. A., Jr, Acharya S., Shows T. B., Rowley J. D., Sukhatme V. P. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7164–7168. doi: 10.1073/pnas.85.19.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Auricchio F. Hormone binding of estradiol-17 beta receptor: evidence for its regulation by cytoplasmic phosphorylation and nuclear dephosphorylation. Prevention of dephosphorylation by antiestrogens. J Steroid Biochem. 1981 Dec;15:369–373. doi: 10.1016/0022-4731(81)90299-5. [DOI] [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Estradiol receptor: phosphorylation on tyrosine in uterus and interaction with anti-phosphotyrosine antibody. EMBO J. 1986 Nov;5(11):2867–2872. doi: 10.1002/j.1460-2075.1986.tb04581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988 May;1(3):183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor rapidly induces c-fos mRNA in PC12 rat pheochromocytoma cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4789–4793. doi: 10.1073/pnas.83.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nielsen C. J., Sando J. J., Pratt W. B. Evidence that dephosphorylation inactivates glucocorticoid receptors. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1398–1402. doi: 10.1073/pnas.74.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990 May;87(9):3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortí E., Mendel D. B., Smith L. I., Munck A. Agonist-dependent phosphorylation and nuclear dephosphorylation of glucocorticoid receptors in intact cells. J Biol Chem. 1989 Jun 15;264(17):9728–9731. [PubMed] [Google Scholar]
- Perrot-Applanat M., Logeat F., Groyer-Picard M. T., Milgrom E. Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology. 1985 Apr;116(4):1473–1484. doi: 10.1210/endo-116-4-1473. [DOI] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryseck R. P., Macdonald-Bravo H., Mattéi M. G., Ruppert S., Bravo R. Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. EMBO J. 1989 Nov;8(11):3327–3335. doi: 10.1002/j.1460-2075.1989.tb08494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan P. L., Evans R. M., Horwitz K. B. Phosphotryptic peptide analysis of human progesterone receptor. New phosphorylated sites formed in nuclei after hormone treatment. J Biol Chem. 1989 Apr 15;264(11):6520–6528. [PubMed] [Google Scholar]
- Sheridan P. L., Francis M. D., Horwitz K. B. Synthesis of human progesterone receptors in T47D cells. Nascent A- and B-receptors are active without a phosphorylation-dependent post-translational maturation step. J Biol Chem. 1989 Apr 25;264(12):7054–7058. [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Käs E., Carothers A. M., Chasin L. A. Deletion of the diploid dihydrofolate reductase locus from cultured mammalian cells. Cell. 1983 Jun;33(2):405–412. doi: 10.1016/0092-8674(83)90422-1. [DOI] [PubMed] [Google Scholar]
- Watson M. A., Milbrandt J. The NGFI-B gene, a transcriptionally inducible member of the steroid receptor gene superfamily: genomic structure and expression in rat brain after seizure induction. Mol Cell Biol. 1989 Oct;9(10):4213–4219. doi: 10.1128/mcb.9.10.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikström A. C., Bakke O., Okret S., Brönnegård M., Gustafsson J. A. Intracellular localization of the glucocorticoid receptor: evidence for cytoplasmic and nuclear localization. Endocrinology. 1987 Apr;120(4):1232–1242. doi: 10.1210/endo-120-4-1232. [DOI] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






