Abstract
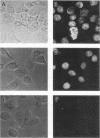
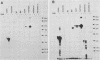
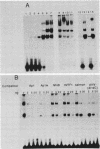
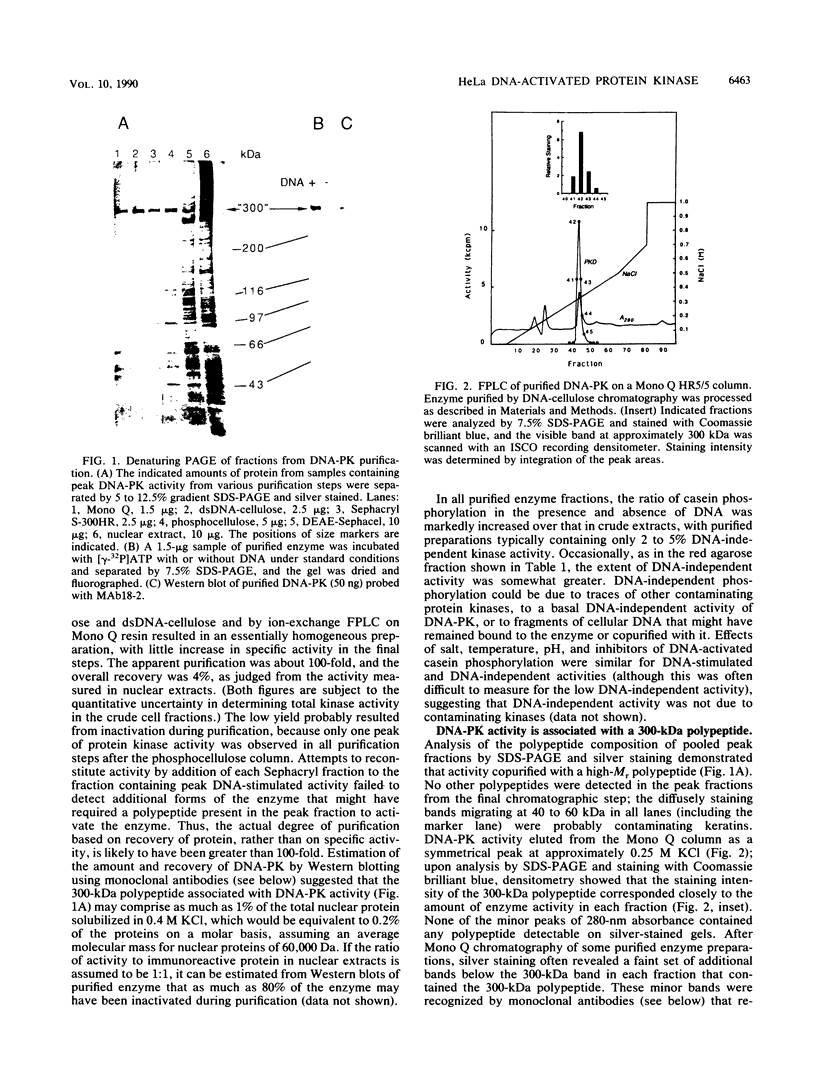
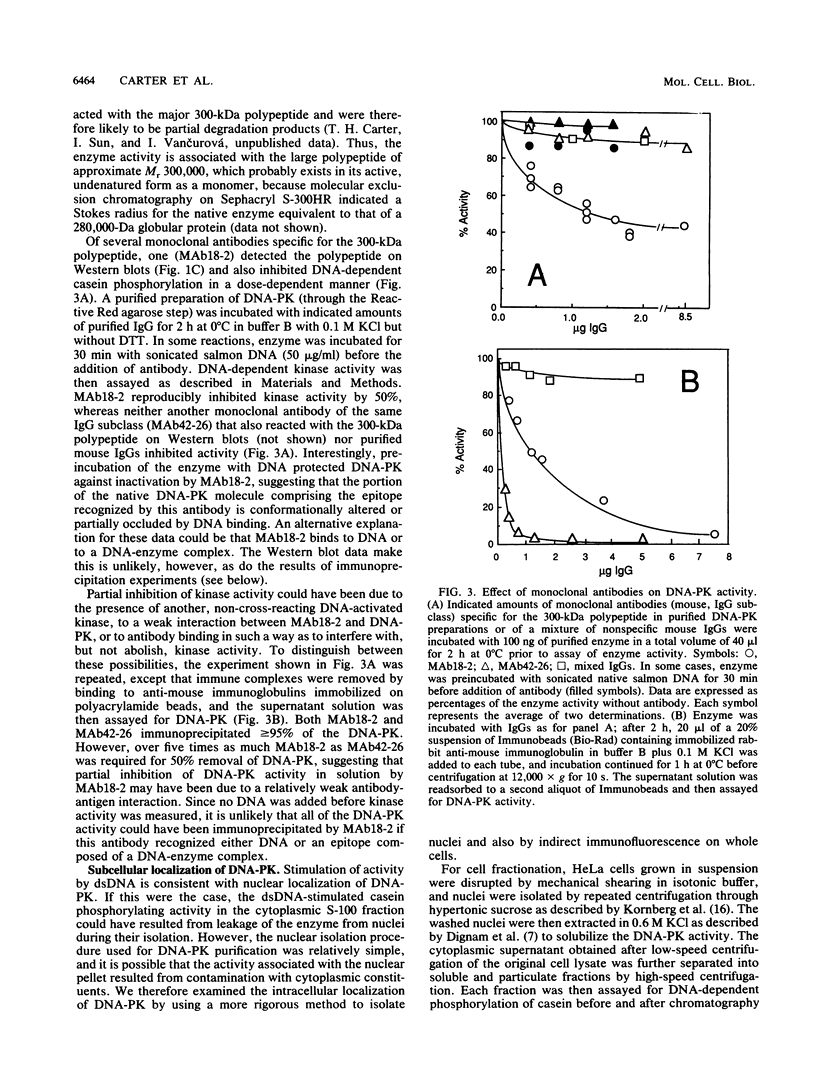
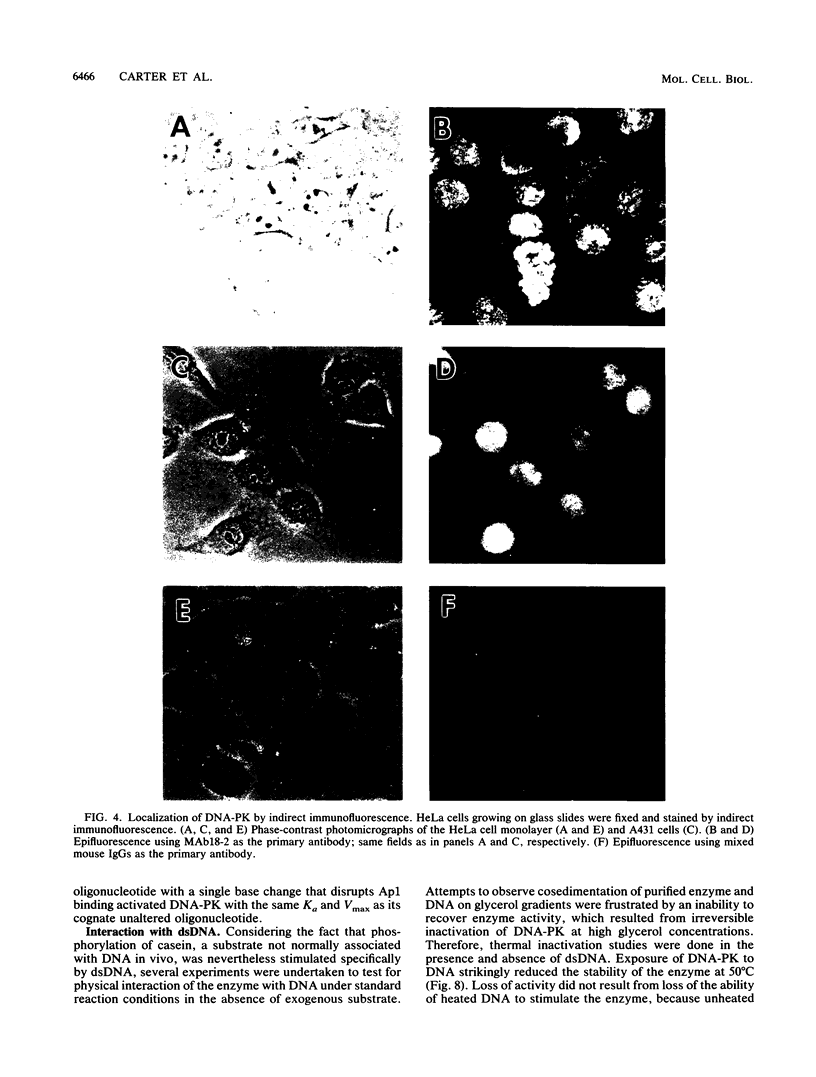
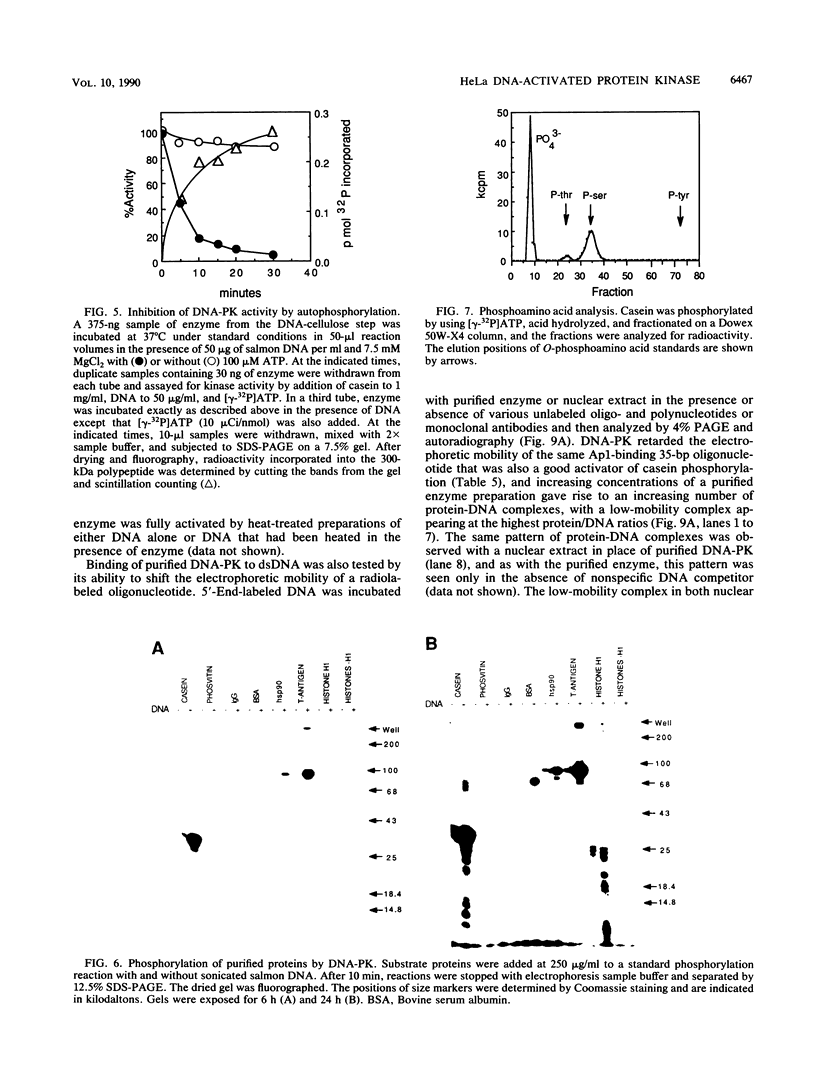
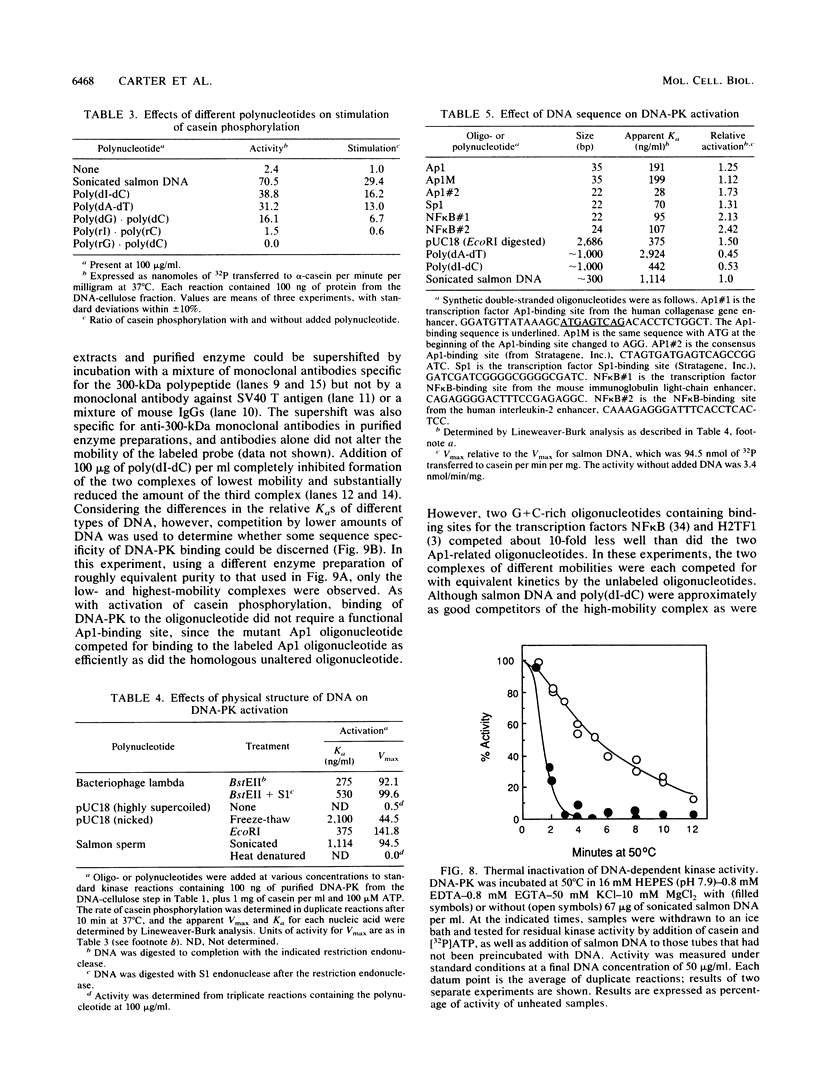
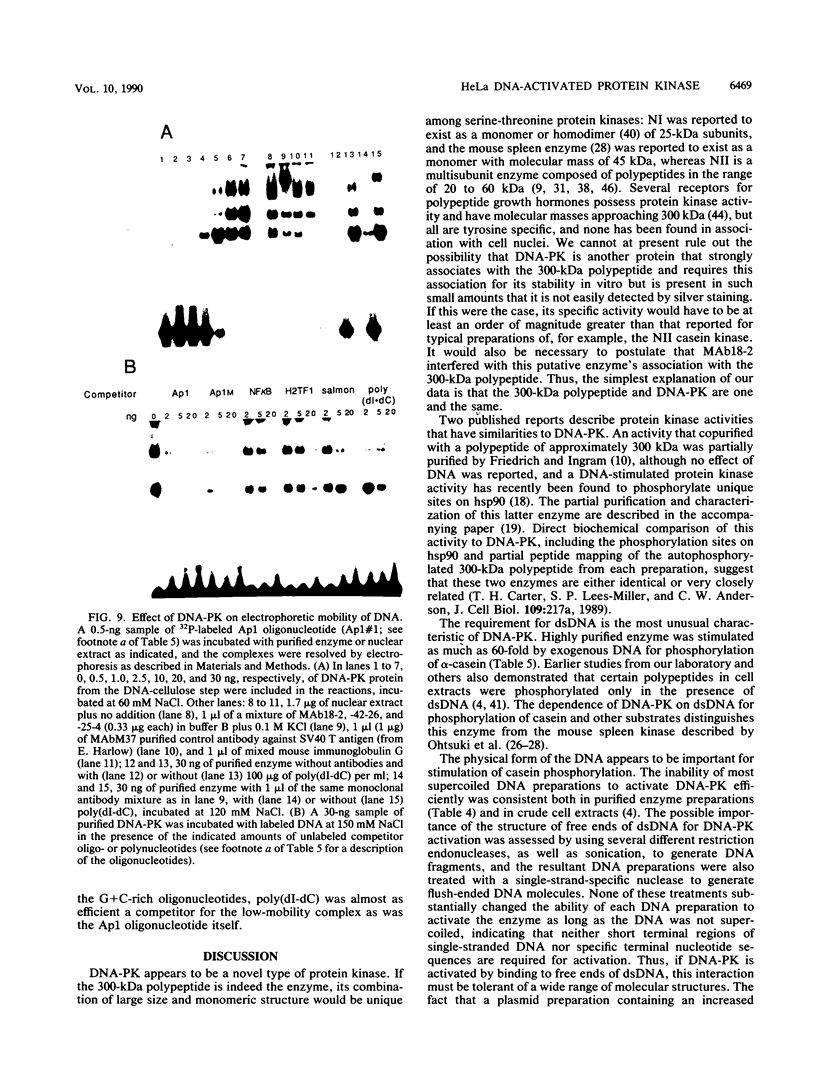
A DNA-activated protein kinase (DNA-PK) was purified from nuclei of HeLa cells. Activity was associated with a single high-molecular-mass (approximately-300,000 Da) polypeptide when analyzed by gel filtration, denaturing polyacrylamide gel electrophoresis, and Western immunoblotting using a monoclonal antibody that also inhibits enzyme activity. Nuclear localization was indicated by subcellular fractionation and confirmed by immunofluorescence on whole cells. Double-stranded DNA stimulated phosphorylation of the 300-kDa polypeptide in purified preparations as well as phosphorylation of the exogenous substrates alpha-casein, simian virus 40 large T antigen, and the human heat shock protein hsp90. Autophosphorylation led to inactivation of the enzyme. The phosphorylation of casein was stimulated over 30-fold by DNA and was specific for serine and threonine residues. Bovine serum albumin and histone H1 were poor substrates for DNA-PK, and no phosphorylation of immunoglobulin G or histones other than H1 was observed. Supercoiled or heat-denatured DNA and synthetic double-stranded RNA or RNA-DNA copolymers did not stimulate casein phosphorylation by DNA-PK. Interaction of the enzyme with DNA in the absence of exogenous substrates was demonstrated by thermal inactivation and gel mobility shifts. These characteristics identify DNA-PK as distinct from other protein kinases described in the literature and suggest that activation by DNA is an important feature of the enzyme's in vivo function.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Sharp P. A. Two transcription factors, NF-kappa B and H2TF1, interact with a single regulatory sequence in the class I major histocompatibility complex promoter. Proc Natl Acad Sci U S A. 1988 Feb;85(3):723–727. doi: 10.1073/pnas.85.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Kopman C. R., James C. B. DNA-stimulated protein phosphorylation in HeLa whole cell and nuclear extracts. Biochem Biophys Res Commun. 1988 Dec 15;157(2):535–540. doi: 10.1016/s0006-291x(88)80282-1. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Milovanovic Z. Z., Babiss L. E., Fisher P. B. Accelerated onset of viral transcription in adenovirus-infected HeLa cells treated with the tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate. Mol Cell Biol. 1984 Mar;4(3):563–566. doi: 10.1128/mcb.4.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P. R., Lue P. F., Liew C. C., Gornall A. G. Purification and properties of rat liver nuclear protein kinases. Can J Biochem. 1972 Dec;50(12):1249–1259. doi: 10.1139/o72-170. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T. D., Ingram V. M. Identification of a novel casein kinase activity in HeLa cell nuclei. Biochim Biophys Acta. 1989 Jul 21;992(1):41–48. doi: 10.1016/0304-4165(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Hara T., Takahashi K., Endo H. Reversal of heparin inhibition of nuclear protein kinase NII by polyamines and histones. FEBS Lett. 1981 Jun 1;128(1):33–36. doi: 10.1016/0014-5793(81)81072-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Kuroda Y., Ku Y., Nishizuka Y. Stimulation by polydeoxyribonucleotide of histone phosphorylation by guanosine 3':5'-monophosphate-dependent protein kinase. Biochem Biophys Res Commun. 1979 Mar 15;87(1):200–206. doi: 10.1016/0006-291x(79)91666-8. [DOI] [PubMed] [Google Scholar]
- Hunt T. Maturation promoting factor, cyclin and the control of M-phase. Curr Opin Cell Biol. 1989 Apr;1(2):268–274. doi: 10.1016/0955-0674(89)90099-9. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kato T., Lowry O. H. Distrbution of enzymes between nucleus and cytoplasm of single nerve cell bodies. J Biol Chem. 1973 Mar 25;248(6):2044–2048. [PubMed] [Google Scholar]
- Kornberg R. D., LaPointe J. W., Lorch Y. Preparation of nucleosomes and chromatin. Methods Enzymol. 1989;170:3–14. doi: 10.1016/0076-6879(89)70039-2. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Powers E. A., Ruff V. A., Jaken S., Kaufmann S. Type 3 protein kinase C localization to the nuclear envelope of phorbol ester-treated NIH 3T3 cells. J Cell Biol. 1989 Aug;109(2):685–695. doi: 10.1083/jcb.109.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees-Miller S. P., Anderson C. W. The human double-stranded DNA-activated protein kinase phosphorylates the 90-kDa heat-shock protein, hsp90 alpha at two NH2-terminal threonine residues. J Biol Chem. 1989 Oct 15;264(29):17275–17280. [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S., Nurse P. Substrates for p34cdc2: in vivo veritas? Cell. 1990 May 18;61(4):549–551. doi: 10.1016/0092-8674(90)90463-o. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Hilz H., Eppenberger H. M., Dutly F. Rapid and reversible translocation of the catalytic subunit of cAMP-dependent protein kinase type II from the Golgi complex to the nucleus. EMBO J. 1985 Nov;4(11):2801–2806. doi: 10.1002/j.1460-2075.1985.tb04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Konno Y., Imajoh S., Suzuki K. A novel phorbol ester receptor/protein kinase, nPKC, distantly related to the protein kinase C family. Cell. 1988 Jun 3;53(5):731–741. doi: 10.1016/0092-8674(88)90091-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Shiraishi H., Sato T., Ishida N. Biochemical characterization of a specific phosphate acceptor of nuclear cyclic AMP-independent protein kinase. Biochim Biophys Acta. 1982 Oct 28;719(1):32–39. doi: 10.1016/0304-4165(82)90303-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuki K., Shiraishi H., Yamada E., Nakamura M., Ishida N. A non-histone chromatin protein that is a specific phosphate acceptor of nuclear cAMP-independent protein kinase from mouse spleen cells. J Biol Chem. 1980 Mar 25;255(6):2391–2395. [PubMed] [Google Scholar]
- Ohtsuki K., Yamada E., Nakamura M., Ishida N. Mouse spleen cell nuclear protein kinases and the stimulating effect of dsDNA on NHP phosphorylation by cyclic AMP-independent protein kinase in vitro. J Biochem. 1980 Jan;87(1):35–45. doi: 10.1093/oxfordjournals.jbchem.a132745. [DOI] [PubMed] [Google Scholar]
- Potuzak H., Wintersberger U. DNA covalently linked to carboxymethyl-cellulose and its application in affinity chromatography. FEBS Lett. 1976 Mar 15;63(1):167–170. doi: 10.1016/0014-5793(76)80218-9. [DOI] [PubMed] [Google Scholar]
- Quarless S. A. Identification of an ionic strength sensitive nuclear protein kinase activity from the cervical carcinoma HeLa. Biochem Biophys Res Commun. 1985 Dec 31;133(3):981–987. doi: 10.1016/0006-291x(85)91232-x. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Bell L. E., Siefken D. A., Jacob S. T. A heparin-sensitive nuclear protein kinase. Purification, properties, and increased activity in rat hepatoma relative to liver. J Biol Chem. 1981 Jul 25;256(14):7468–7477. [PubMed] [Google Scholar]
- Sagata N., Watanabe N., Vande Woude G. F., Ikawa Y. The c-mos proto-oncogene product is a cytostatic factor responsible for meiotic arrest in vertebrate eggs. Nature. 1989 Nov 30;342(6249):512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Taira H., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Characteristics of a double-stranded RNA-activated protein kinase system partially purified from interferon treated Ehrlich ascites tumor cells. J Biol Chem. 1978 Sep 10;253(17):5915–5921. [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sikorska M., Whitfield J. F., Walker P. R. The regulatory and catalytic subunits of cAMP-dependent protein kinases are associated with transcriptionally active chromatin during changes in gene expression. J Biol Chem. 1988 Feb 25;263(6):3005–3011. [PubMed] [Google Scholar]
- Simpson R. T. Protein kinase in HeLA nucleosomes: a reevaluation of the interactions of histomes with the ends of core particle DNA. Nucleic Acids Res. 1978 Apr;5(4):1109–1119. doi: 10.1093/nar/5.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Thornburg W., Lindell T. J. Purification of rat liver nuclear protein kinase NII. J Biol Chem. 1977 Oct 10;252(19):6660–6665. [PubMed] [Google Scholar]
- Thornburg W., O'Malley A. F., Lindell T. J. Purification of rat liver nuclear protein kinase NI. J Biol Chem. 1978 Jul 10;253(13):4638–4641. [PubMed] [Google Scholar]
- Verma R., Chen K. Y. Spermine inhibits the phosphorylation of the 11,000- and 10,000-dalton nuclear proteins catalyzed by nuclear protein kinase NI in NB-15 mouse neuroblastoma cells. J Biol Chem. 1986 Feb 25;261(6):2890–2896. [PubMed] [Google Scholar]
- Walker A. I., Hunt T., Jackson R. J., Anderson C. W. Double-stranded DNA induces the phosphorylation of several proteins including the 90 000 mol. wt. heat-shock protein in animal cell extracts. EMBO J. 1985 Jan;4(1):139–145. doi: 10.1002/j.1460-2075.1985.tb02328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Zandomeni R., Weinmann R. Inhibitory effect of 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole on a protein kinase. J Biol Chem. 1984 Dec 10;259(23):14804–14811. [PubMed] [Google Scholar]
- Zandomeni R., Zandomeni M. C., Shugar D., Weinmann R. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J Biol Chem. 1986 Mar 5;261(7):3414–3419. [PubMed] [Google Scholar]