Abstract
Autoantibodies to topoisomerase I (topo I), RNA polymerase III (RNAPIII), centromere, U3RNP/fibrillarin, Th, PM-Scl, and U1RNP found in scleroderma (SSc) are associated with unique clinical subsets. The effects of race and gender on autoantibody prevalence and clinical manifestations were examined. Autoantibodies in sera from 105 SSc (include 75 Caucasian, 24 African-American, 6 others; 89 females and 16 males) were analyzed by immunofluorescence and immunoprecipitation. Clinical information was from database. SSc-related autoantibodies seldom coexist except for anti-topo I and anti-U1RNP. Anti-topo I (35% vs 15%), anti-U3RNP (30% vs 3%, p=0.0005), and anti-U1RNP (30% vs 13%) were more common in African-Americans vs Caucasians. Anti-centromere (17%) and anti-PM-Scl (only in 8% of female) were found only in Caucasians. In race/gender combination, all three African-American males had anti-topo I (p=0.04). Anti-U3RNP (35% vs 3%, p=0.0005) and anti-U1RNP were common in African-American females. In African-American, all nucleolar dominant staining sera had anti-U3RNP; nuclear pattern was topo I (50%), U1RNP (19%), and RNAPIII (13%). In Caucasian, nucleolar was anti-Th (43%) and PM-Scl (29%); nuclear pattern was RNAPIII (29%), topo I (24%), and U1RNP (18%). Anti-topo I, anti-RNAPIII, and anti-U3RNP were associated with diffuse SSc while anti-centromere, anti-Th, and anti-U1 with limited disease. Proximal scleroderma was less common in African-American with anti-topo I (38% vs 91% in Caucasian, p=0.04). The production of SSc-related autoantibodies is gender and race dependent, and this can be highly relevant in understanding their clinical significance.
Keywords: Autoantibodies, Gender, Race, Scleroderma, Systemic sclerosis
Introduction
Specific autoantibodies in systemic autoimmune rheumatic diseases are useful biomarkers associated with certain diagnoses and/or unique clinical manifestations [1, 2]. Several autoantibodies, including anti-topoisomerase I (topo I), anti-centromere (ACA), anti-RNA polymerase III (RNAPIII), U3RNP/fibrillarin, Th/To, and PM-Scl, have been reported to be associated with scleroderma (systemic sclerosis, SSc); each of these is associated with certain clinical features and defines a unique clinical subset [1, 3, 4]. Anti-topo I, RNAPIII, and U3RNP are associated with diffuse scleroderma whereas anti-centromere and anti-Th are associated with limited scleroderma. Examples of strong association of SSc-related autoantibodies and clinical manifestations include anti-topo I with severe interstitial lung disease (ILD) and RNAPIII with scleroderma renal crisis [3, 4]. Thus, identifying autoantibodies is clinically useful not only in helping diagnosis but also in predicting development of certain clinical manifestations and prognosis [2, 4]. However, significant differences in prevalence and specificity of autoantibodies and clinical features in SSc patients depending on race and ethnicity have been reported [3, 5]. Anti-centromere antibodies can be determined by immunofluorescence antinuclear antibody (ANA) test alone [2]. Anti-topo I ELISA has been used widely in clinical practice although difference in specificity illustrated by high prevalence of anti-topo I in systemic lupus erythematosus patients in some reports [6, 7] is a concern [1, 8]. Anti-RNAPIII ELISA was approved by FDA a few years ago and has been used more and more as indicated by the increasing number of published papers using anti-RNAPIII ELISA kit [2, 9]. However, availability of tests for other autoantibody specificities is limited. Thus, despite reported racial difference and differences even within Caucasians of different ethnicity in prevalence of SSc-related autoantibodies, comprehensive studies that include most of SSc-related autoantibodies are limited to data available from small numbers of institutes [3, 5]. In the present study, the prevalence of SSc-related autoantibodies and associated clinical features were characterized comparing different race and gender.
Materials and methods
Patients
From patients enrolled to the University of Florida Center for Autoimmune Diseases (n=1,542) registry during 2000–2008, 105 patients (Caucasian 75, African-American 23, Latin 5, Asian 1, mixed 1; 89 females and 16 males) who fulfilled the ACR SSc classification criteria [10] were studied. Mixed connective tissue disease (MCTD) or overlap syndrome that fulfills the criteria for more than one systemic autoimmune rheumatic disease was not separated from SSc. Clinical information was from the database and medical record. Interstitial lung disease was defined by chest X-ray or high-resolution computed tomography. The protocol was approved by the Institutional Review Board. This study meets and is in compliance with all ethical standards in medicine, and informed consent was obtained from all patients according to the Declaration of Helsinki.
Autoantibody analysis
Autoantibodies in sera from the initial visit of each patient were analyzed by immunofluorescence and immunoprecipitation. Immunofluorescence antinuclear antibodies (ANA, HEp-2 ANA slides; INOVA Diagnostics, San Diego, CA, USA) were performed using a 1:80-diluted serum followed by FITC goat anti-human IgG (γ-chain specific, Southern Biotechnology, Birmingham, AL, USA). Analysis of protein components of autoantigens were by immunoprecipitation of 35S-methionine-labeled K562 cell extract and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 8% and 12.5% acrylamide SDS-PAGE [11]. RNA components were analyzed by immunoprecipitation of unlabeled K562 cells, urea-PAGE, and silver staining (Silver Stain Plus, Bio-Rad, Hercules, CA, USA) [5]. Specificities of autoantibodies were determined using reference sera.
Statistical analysis
Prevalence of autoantibodies or clinical manifestations was compared by Fisher’s exact test using Prism 4.0c for Macintosh (GraphPad Software, Inc., San Diego, CA, USA). P<0.05 was considered significant.
Results
SSc-related autoantibodies were determined using immunofluorescence and immunoprecipitation. Prevalence of autoantibodies is summarized in Table 1.
Table 1.
Prevalence of autoantibodies in Caucasian, African-American, and Latin American patients with scleroderma
| Totala | Caucasian American | African-American | Latin American | |
|---|---|---|---|---|
| N | 105 | 75 | 23 | 5 |
| Male, % | 15 | 17 | 13 | 0 |
| Age (mean±SD) | 50.6±13.3 | 52.4±12.1 | 47.5±15.9 | 41.8±15.4 |
| Topo I | 19% (20/105) | 15% (11/75)1 | 35% (8/23)1,2 | 20% (1/5) |
| RNAP III | 16% (17/105) | 19% (14/75) | 9% (2/23) | 0% |
| Centromere | 14% (15/105) | 17% (13/75)3 | 03,4 | 40% (2/5) |
| U3RNP | 9% (9/105) | 3% (2/75)5 | 30% (7/23)5 | 0% |
| Th | 8% (8/105) | 9% (7/75) | 4% (1/23) | 0% |
| U1RNP | 18% (19/105) | 13% (10/75)6 | 30% (7/23)6 | 40% (2/5) |
| PM-Scl | 5% (5/105) | 6% (5/75) | 0% | 0% |
| None of the above | 17% (18/105) | 20% (15/75) | 9% (2/23) | 0% |
p=0.07;
p=0.04 vs non-African-American;
p=0.03;
p=0.04 vs non-African-American;
p=0.0005;
p=0.11
Total includes one each of Asian and mixed patient in addition to Caucasian, African-American, and Latin
Prevalence of autoantibodies in different race
Anti-topo I was the most common specificity (35%) in African-Americans, and also this was more prevalent than other race (p=0.07 vs Caucasian, p=0.04 vs non-African-American). Anti-RNAPIII (19%) was the most common in Caucasians followed by ACA (17%) and anti-topo I (15%). Although the numbers of non-Caucasian patients are small, ACA (p=0.03 vs African-American) was not found in African-Americans. Anti-PM-Scl (6%) antibodies were found only in Caucasian, and seven of eight cases of anti-Th were also Caucasian. In contrast, anti-U3RNP (30% vs 3% in Caucasian, p=0.0006) and anti-U1RNP (30% vs 13% in Caucasian) were as common as anti-topo I in African-Americans. As a total, 80% of Caucasian, 91% of African-American, and 100% of Latin SSc patients had one or more SSc-related autoantibodies on the list.
Race, ANA patterns, and autoantibody specificities
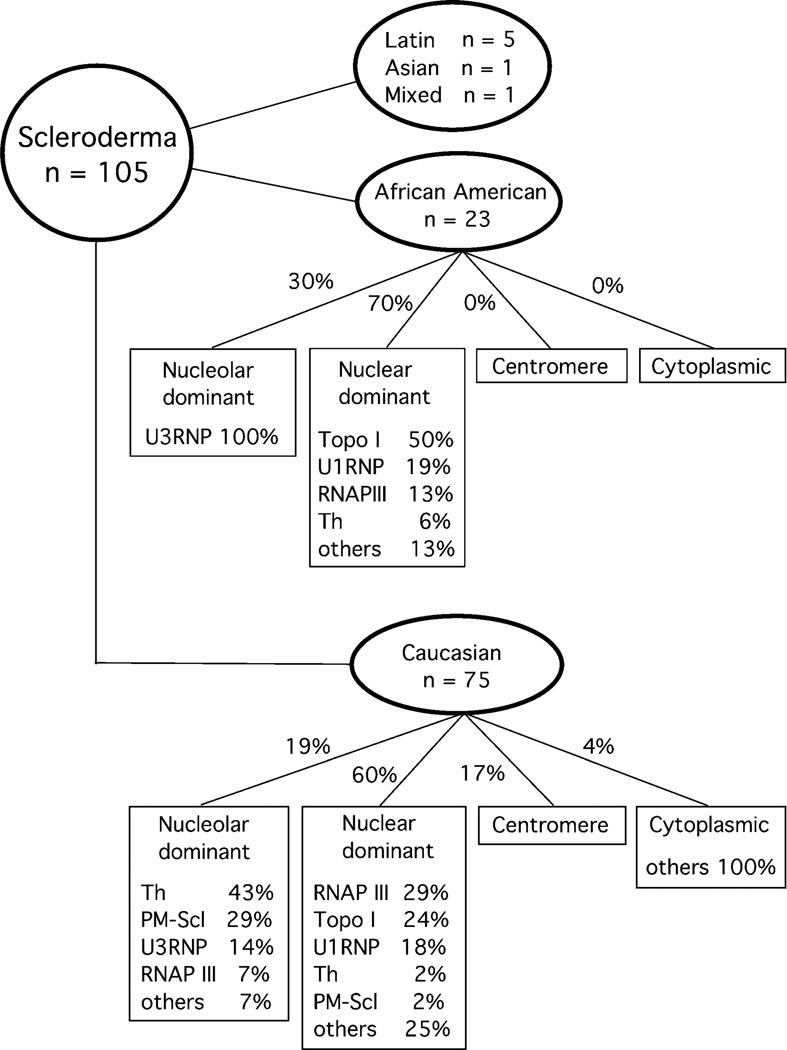
African-American and Caucasian SSc patients were grouped based on the immunofluorescent patterns: nucleolar dominant, nuclear dominant, centromere, and cytoplasmic. Many sera have more than one of the nuclear, nucleolar, or cytoplasmic staining, but sera were classified based on the brightest staining pattern when 1:80 diluted sera were tested. Majority of sera were classified as nuclear or nucleolar and dominant cytoplasmic staining was uncommon. Percentage of specific autoantibodies detected in each group is shown (Fig. 1). In African-Americans, all seven nucleolar dominant staining sera were anti-U3RNP. In striking contrast, anti-Th (43%) and PM-Scl (29%) were the most common for nucleolar staining dominant sera in Caucasians. For nuclear dominant pattern, 50% had anti-topo I, 19% had anti-U1RNP (Note: Patients with both anti-topo I and U1RNP were in anti-topo I group in this analysis), and RNAPIII 13% in African-Americans whereas in Caucasians anti-RNAPIII (29%) was the most common followed by topo I (24%) and U1RNP (18%). In our cohort, ACA was found only in 17% of Caucasian and not in African-American (Table 1). These data indicate that common specificities that can be expected based on the ANA patterns are quite different between African- and Caucasian-Americans and that considering race and ANA patterns will be helpful to predict specific autoantibodies even when specific test results are not available.
Fig. 1.
Prevalence of autoantibodies in scleroderma patients classified based on race and patterns of immunofluorescence antinuclear antibodies. ANA patterns were classified based on the dominant immunofluorescence staining pattern using 1:80 diluted sera. Patients with both anti-topo I and U1RNP were classified in anti-topo I group in this analysis
Coexistence of other scleroderma-related autoantibodies
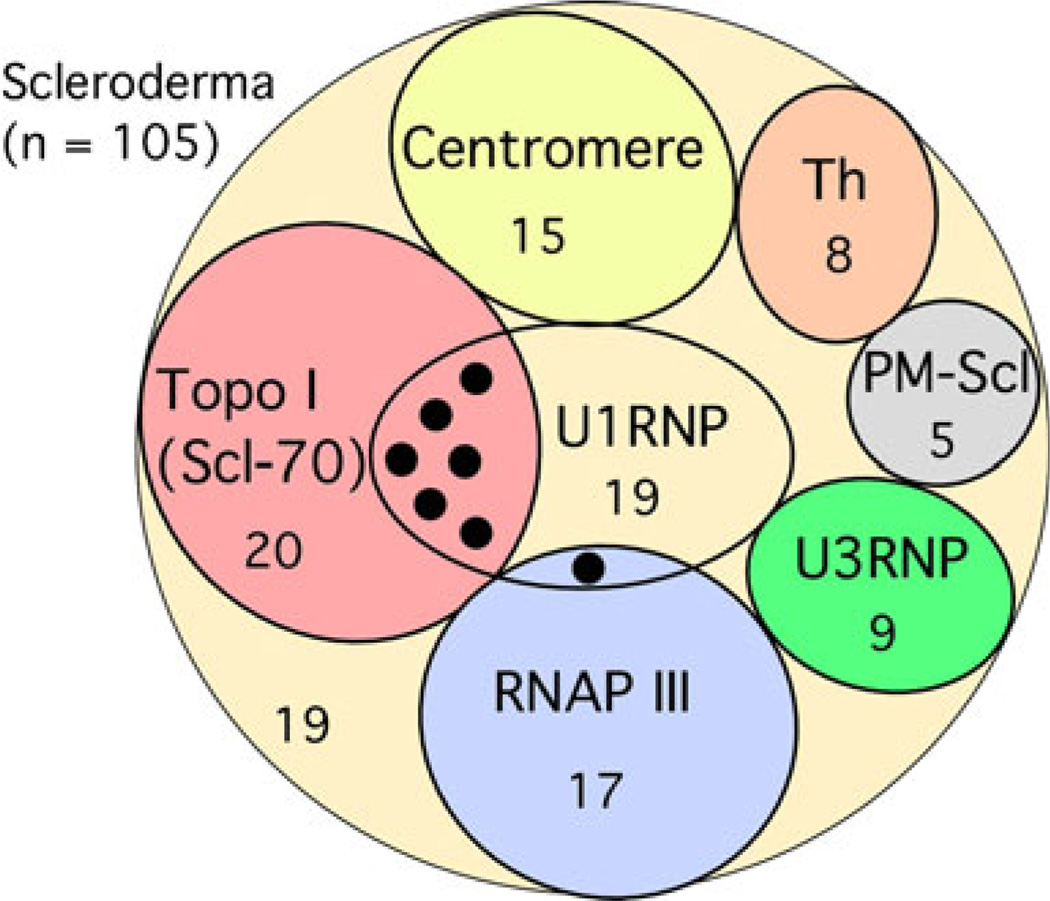
Prevalence of SSc-related autoantibodies comparing Caucasian-, African-, and Latin-Americans is summarized (Table 1). Coexistence of more than one SSc-related autoantibodies was very unusual except for coexistence of anti-U1RNP and anti-topo I antibodies (6 of 20, 30%, p=0.0012 vs all other SSc-related autoantibodies, Fisher’s exact test), consistent with previous literatures [3, 12]. Anti-U1/U2RNP antibodies with unusual U2RNP dominant reactivity were found in one anti-RNAP III-positive patient (Fig. 2).
Fig. 2.
Rare coexistence of scleroderma-related autoantibodies. Pattern of distribution of SSc-related autoantibodies is shown. Black circle indicates the cases of anti-U1RNP positives that coexist with anti-topo I (six cases) or anti-RNAPIII (one case)
Prevalence of autoantibodies in patients with scleroderma classified by race and gender
Since gender may also affect autoantibody specificities, gender difference in each race is analyzed (Table 2). Anti-topo I was positive in all three male African-American patients (p=0.04 vs Caucasian male, p=0.004 vs Caucasian female). Anti-U3RNP, anti-Th, and anti-PM-Scl (Caucasians only) were found only in female, and all except one (1 of 18) anti-U1RNP were also female. Anti-U3RNP was the most common specificity in African-American female (35% vs 3% in Caucasian female, p=0.0005, p=0.03 vs Caucasian male), and anti-U1RNP was also more common in African-American (35% vs 16% in Caucasians). ILD (Caucasians vs African-Americans, p=0.07; Caucasian female vs African-American female, p=0.07) and PH may be more prevalent in African-Americans while renal crisis appeared to be more common in Caucasians, even if they did not reach statistical significance.
Table 2.
Prevalence of autoantibodies and clinical manifestations in different race and gender
| Race | Caucasian American | African-American | Latin American | ||
|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Female |
| N | 62 | 13 | 20 | 3 | 5 |
| Age (mean±SD) | 51.1±12.1 | 58.8±10.3 | 46.1±16.5 | 56.6±6.2 | 41.8±15.4 |
| Topo I | 13%1 (8/62) | 23%2 (3/13) | 25% (5/20) | 100%1,2 (3/3) | 20% (1/5) |
| RNAP III | 18% (11/62) | 23% (3/13) | 10% (2/20) | 0% | 0% |
| Centromere | 16%3 (10/62) | 23% (3/13) | 0%3 | 0% | 40% (2/5) |
| U3RNP | 3%4 (2/62) | 0%5 | 35%4,5 (7/20) | 0% | 0% |
| Th | 11% (7/62) | 0% | 5% (1/20) | 0% | 0% |
| U1RNP | 16%6 (10/62) | 0% | 35%6,7 (7/20) | 33% (1/3) | 40% (2/5) |
| PM-Scl | 8% (5/62) | 0% | 0% | 0% | 0% |
| None of the above | 18% (11/62) | 31% (4/13) | 10% (2/20) | 0% | 0% |
| Proximal scleroderma | 50% (31/62) | 62% (8/13) | 55% (11/20) | 67% (2/3) | 60% (3/5) |
| ILD8 | 37%9 (23/62) | 46% (6/13) | 65%9 (13/20) | 67% (2/3) | 40% (2/5) |
| Renal crisis | 15% (9/62) | 8% (1/13) | 5% (1/20) | 0% (0/3) | 0% |
| Pulmonary hypertension | 15% (10/62) | 23% (3/13) | 25% (5/20) | 33% (1/3) | 0% |
ILD interstitial lung disease
p=0.004;
p=0.04;
p=0.11;
p=0.0005;
p=0.03;
p=0.11;
p=0.04 vs Caucasian;
p=0.03, Caucasian vs African-American;
p=0.07, ILD
Race, autoantibodies, and clinical manifestations
Prevalence of proximal scleroderma (Table 3), ILD (Table 4), renal crisis, and pulmonary hypertension (not shown) in SSc classified by race and autoantibodies was analyzed. In general, association of specific autoantibodies and prevalence of proximal scleroderma was consistent with previous literature [3, 4]: anti-topo I, RNAPIII, and U3RNP with diffuse skin involvement; ACA, anti-Th, and U1RNP with limited skin involvement (Table 3). The only significant difference was the low prevalence of proximal scleroderma in African-American with anti-topo I (38% vs 91% in Caucasian, p=0.04). This was related to a subset of patients in this group: four out of eight had anti-topo I and U1RNP and three out of four of them had no sclerodermatous changes.
Table 3.
Prevalence of proximal scleroderma in scleroderma patients classified by race and autoantibodies
| All | Caucasian American | African-American | Latin American | |
|---|---|---|---|---|
| N | 105a | 75 | 23 | 5 |
| Total | 54% (57/105) | 52% (39/75) | 57% (13/23) | 60% (3/5) |
| Topo I | 70% (14/20) | 91% (10/11)* | 38% (3/8)* | 1/1 |
| RNAP III | 81% (13/16) | 71% (10/14) | 100% (2/2) | NA |
| Centromere | 20% (3/15) | 15% (2/13) | NA | 1/2 |
| U3RNP | 78% (7/9) | 50% (1/2) | 86% (6/7) | NA |
| Th | 38% (3/8) | 43% (3/7) | 0/1 | NA |
| U1RNP | 32% (6/19) | 30% (3/10) | 29% (2/7) | 1/2 |
| PM-Scl | 60% (3/5) | 60% (3/5) | NA | NA |
| None of the above | 61% (11/18) | 60% (9/15) | 1/2 | NA |
NA not applicable
p=0.04
Includes one each of Asian and mixed patient
Table 4.
Prevalence of interstitial lung disease in scleroderma patients classified by race and autoantibodies
| All | Caucasian American | African-American | Latin American | |
|---|---|---|---|---|
| N | 105a | 75 | 23 | 5 |
| Total | 44% (46/105) | 39%* (29/75) | 65%* (15/23) | 40% (2/5) |
| Topo I | 70%** (14/20) | 64% (7/11) | 75% (6/8) | 1/1 |
| RNAP III | 41% (7/17) | 43% (6/14) | 1/2 | NA |
| Centromere | 33%** (5/15) | 31% (4/13) | NA | 1/2 |
| U3RNP | 33% (3/9) | 0% (0/2) | 43% (3/7) | NA |
| Th | 25% (2/8) | 29% (2/7) | 1/1 | NA |
| U1RNP | 53% (10/19) | 40% (4/10) | 86% (6/7) | 0/2 |
| PM-Scl | 20% (1/5) | 20% (1/5) | NA | NA |
| None of the above | 50% (9/18) | 47% (7/15) | 2/2 | NA |
NA not applicable
p=0.06;
p=0.04
Includes one each of Asian and mixed patient
Prevalence of ILD was the highest in anti-topo I-positive patients (p=0.04 vs ACA) and relatively low in anti-RNAPIII patients, consistent with literature (Table 4) [3, 4]. ILD in anti-U1RNP-positive African-American was 86% (six of seven); however, four of them also had anti-topo I, all with ILD.
Scleroderma renal crisis was significantly associated with anti-RNAPIII as documented in literature (p=0.002 vs anti-RNAPIII negative, p=0.02 vs ACA group) [3, 4], seen in 35% in total and 43% in Caucasian patients. PH was seen in 26% of African-Americans and 17% of Caucasians, but association with race or autoantibodies was not statistically significant (not shown).
Discussion
High specificity of certain autoantibodies, such as anti-topo I and anti-RNAPIII, for the diagnosis of SSc is well established [1, 3] as evidenced by many studies and by the inclusion of these autoantibodies in the proposal of classification criteria of SSc [13, 14]. Target autoantigens and their biological functions recognized by SSc-related autoantibodies are well characterized [15]. Significant progress has been made in understanding genetics and immunopathogenesis of autoantibody production in general [16]. Nevertheless, the mechanisms and reasons of association between the production of specific autoantibodies and certain diagnosis or clinical manifestations are still poorly understood, other than the general consensus that both genetic and environmental factors play a role in the production of specific autoantibodies [16, 17].
Data in the present study including rare coexistence of SSc-related autoantibodies; higher prevalence of anti-topo I, U3RNP, and U1RNP in African-American; and higher prevalence of anti-RNAPIII, centromere, Th, and PM-Scl in Caucasians are consistent with previous literature [3]. Association of autoantibodies with limited vs diffuse skin involvement in the present study is also consistent with virtually all other studies regardless the location of the studies or ethnicity of the cohort, further supporting universal association of these autoantibodies with SSc subset: anti-topo I, anti-RNAPIII, anti-U3RNP, and anti-PM-Scl with diffuse cutaneous involvement vs ACA, anti-Th, and anti-U1RNP with limited cutaneous involvement [3, 18].
Race-ANA pattern-specific autoantibodies analysis (Fig. 1) may seem overly simplified since many sera have more than one location in the stained cells. Nevertheless, this may be similar to what many clinicians actually receive as a test report of clinical samples. We and others reported that anti-RNAPI/III sera are seldom reported as nucleolar staining positive despite biological localization of RNAPI to nucleoli and detection of nucleolar staining in many sera of this group by careful examination [4, 9, 19]. As expected and consistent with our previous study, anti-U3RNP (nine of nine), anti-Th (six of eight), and anti-PM-Scl (four of five) had nucleolar dominant pattern [19]. There are fine differences within staining by different antinucleolar antibodies [20]. Also, typically nuclear staining by anti-RNAPIII vs topo I is distinct, the former in moderate size speckled whereas the latter is fine speckled to homogeneous staining. Thus, with reading by an expert, nucleolar and nuclear staining patterns can be further classified and may be correlated with specific autoantibodies as illustrated in the recent articles [20, 21]. However, this is beyond the ability of most clinical laboratories and what we showed in this simplified analysis may be more practical. It is clear that potential autoantibody specificities can be narrowed down very efficiently by considering race and dominant ANA pattern. Since tests for nucleolar antibodies such as anti-U3RNP and anti-Th are not readily available for most clinicians, keeping this chart in mind and carefully examining ANA pattern may be useful.
Coexistence of SSc-related autoantibodies, in particular when tested by immunodiffusion or IP as further confirmed in the present study (Fig. 2; vs studies primarily using ELISA), is uncommon [3]. The only exception is the coexistence of anti-topo I and U1RNP, which has also been described in literature [12].
Low prevalence of proximal scleroderma in African-Americans (Table 2) is somewhat unexpected compared with literature [12]; however, there are several possible explanations. Selection of patients and definition of SSc may be a factor. African-American population in the present study includes a subset of patients who had anti-topo I with U1RNP and RP, pitting scars, ILD but no or limited sclerodermatous changes, since SSc was defined and selected purely based on the ACR SSc classification criteria in the present study. However, cases like these may not be included in other SSc studies. In studies from the University of Pittsburgh, selection of SSc was based on diagnosis by physicians; thus, only 86–88% of their study population fulfill the ACR SSc criteria [12, 22, 23]. In other studies, sclerodactyly was the requirement for selection of study patients [24] or criteria by LeRoy et al. [25] were used [26]. Thus, the selected SSc patients may not include patients with RP with pitting scars and ILD as in our study. This selection bias alone may explain some differences reported in the clinical studies of SSc patients selected based on the different criteria. In a recent large epidemiological study in SSc, bias due to difference in patient recruitment was considered a likely explanation of large variability of SSc presentation between centers or even within a single city [17]. Another consideration is the definition and categorization of patients with MCTD and overlap syndromes [27]. How these groups of patients are handled is not clearly stated in all SSc studies. However, whether the studied SSc cohort includes MCTD or overlap syndromes will significantly affect the prevalence of certain autoantibodies such as anti-U1RNP, PM-Scl, and Ku, which are often seen in SSc patients with overlapping features and clinical analysis [3]. If MCTD or overlap syndromes are defined as a separate category, patients with anti-U1RNP and anti-topo I can be easily classified as MCTD as suggested by reports on detection of anti-topo I in patients with MCTD [28, 29].
Another potential difference in the present study was the production of anti-U3RNP, Th, and PM-Scl only in female patients (Table 2). Increased percentage of male patients in anti-Th-positive SSc in Caucasians reported in some studies [5] was not observed in this study. The number of male SSc patients was small in the present study; nevertheless, heterogeneity in patients’ population within race may be another factor as discussed below. In clinical studies, ethnicity is usually classified into Caucasian, African-American, Latin, Asian, Native American, etc. Although this is probably the best we can do practically, each category of race is quite heterogeneous and is a mixture of people with different ethnic background in different ratios, in particular in the USA. For example, Caucasians in the USA includes people originally from anywhere in Europe and also Jewish population. This population may be quite different from studies performed in a single European country where Caucasian population is relatively homogeneous. This could be a part of the reason for high prevalence of anti-RNAPIII in Caucasians in the USA vs low prevalence in French or Italian studies [5, 30–32]. Of course, differences in environmental factors, which can never be clearly separated, complicate the issue. Caucasians and African Americans in the USA have different ethnic background depending on the location of the study.
In summary, both race and gender impact the prevalence of SSc-related autoantibodies substantially. Based on ANA pattern and race, specific autoantibodies can be predicted and tested efficiently. The data suggest that multiple genetic and/or environmental factors may play a role in the production of specific autoantibodies. This may also help to explain the infrequent coexistence of more than one of the SSc-related autoantibodies in a single patient’s serum.
Acknowledgments
We would like to thank Marlene Sarmiento, Annie Chan, and UF GCRC staff for assistance with clinical data collection. This study was supported by NIH grant R01-AR40391 and M01R00082 from the US Public Health Service and by generous gifts from Lupus Link, Inc. (Daytona Beach, FL, USA) and Mr. Lewis M. Schott to the University of Florida Center for Autoimmune Disease.
Footnotes
Disclosures None
Contributor Information
Malgorzata E. Krzyszczak, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA
Yi Li, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA.
Steven J. Ross, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA Department of Oral Biology, University of Florida, Gainesville, FL 32610-0221, USA.
Angela Ceribelli, Department of Oral Biology, University of Florida, Gainesville, FL 32610-0221, USA.
Edward K. L. Chan, Department of Oral Biology, University of Florida, Gainesville, FL 32610-0221, USA
Michael R. Bubb, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA
Eric S. Sobel, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL, USA.
Westley H. Reeves, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL, USA.
Minoru Satoh, Email: minoru.satoh@medicine.ufl.edu, Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Florida, P.O. Box 100221, Gainesville, FL 32610-0221, USA; Department of Pathology, Immunology, and Laboratory Medicine, University of Florida, Gainesville, FL, USA.
References
- 1.Satoh M, Chan EKL, Sobel ES, Kimpel DL, Yamasaki Y, Narain S, Mansoor R, Reeves WH. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert Rev Clin Immunol. 2007;3:721–738. doi: 10.1586/1744666X.3.5.721. [DOI] [PubMed] [Google Scholar]
- 2.Satoh M, Vazquez-Del Mercado M, Chan EK. Clinical interpretation of antinuclear antibody tests in systemic rheumatic diseases. Mod Rheumatol. 2009;19:219–228. doi: 10.1007/s10165-009-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Steen VD. The many faces of scleroderma. Rheum Dis Clin North Am. 2008;34:1–15. doi: 10.1016/j.rdc.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Ceribelli A, Cavazzana I, Franceschini F, Airo P, Tincani A, Cattaneo R, Pauley BA, Chan EK, Satoh M. Anti-Th/To are common antinucleolar autoantibodies in Italian patients with scleroderma. J Rheumatol. 2010;37:2071–2075. doi: 10.3899/jrheum.100316. [DOI] [PubMed] [Google Scholar]
- 6.Gussin HA, Ignat GP, Varga J, Teodorescu M. Anti-topoisomerase I (anti-Scl-70) antibodies in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:376–383. doi: 10.1002/1529-0131(200102)44:2<376::AID-ANR56>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Hamidou MA, Audrain MA, Masseau A, Agard C, Moreau A. Anti-topoisomerase I antibodies in systemic lupus erythematosus as a marker of severe nephritis. Clin Rheumatol. 2006;25:542–543. doi: 10.1007/s10067-005-0061-9. [DOI] [PubMed] [Google Scholar]
- 8.Mahler M, Silverman ED, Schulte-Pelkum J, Fritzler MJ. Anti-Scl-70 (topo-I) antibodies in SLE: myth or reality? Autoimmun Rev. 2010;9:755–760. doi: 10.1016/j.autrev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Cavazzana I, Angela C, Paolo A, Stefania Z, Angela T, Franco F. Anti-RNA polymerase III antibodies: a marker of systemic sclerosis with rapid onset and skin thickening progression. Autoimmun Rev. 2009;8:580–584. doi: 10.1016/j.autrev.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M, Ajmani AK, Ogasawara T, Langdon JJ, Hirakata M, Wang J, Reeves WH. Autoantibodies to RNA polymerase II are common in systemic lupus erythematosus and overlap syndrome. Specific recognition of the phosphorylated (IIO) form by a subset of human sera. J Clin Invest. 1994;94:1981–1989. doi: 10.1172/JCI117550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuwana M, Kaburaki J, Arnett FC, Howard RF, Medsger TA, Jr, Wright TM. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999;42:465–474. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–1576. [PubMed] [Google Scholar]
- 14.Nadashkevich O, Davis P, Fritzler MJ. A proposal of criteria for the classification of systemic sclerosis. Med Sci Monit. 2004;10:CR615–CR621. [PubMed] [Google Scholar]
- 15.Welting TJ, Raijmakers R, Pruijn GJ. Autoantigenicity of nucleolar complexes. Autoimmun Rev. 2003;2:313–321. doi: 10.1016/s1568-9972(03)00029-6. [DOI] [PubMed] [Google Scholar]
- 16.Harris ML, Rosen A. Autoimmunity in scleroderma: the origin, pathogenetic role, and clinical significance of autoantibodies. Curr Opin Rheumatol. 2003;15:778–784. doi: 10.1097/00002281-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Walker UA, Tyndall A, Czirjak L, Denton CP, Farge-Bancel D, Kowal-Bielecka O, Muller-Ladner U, Matucci-Cerinic M. Geographical variation of disease manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research (EUSTAR) group database. Ann Rheum Dis. 2009;68:856–862. doi: 10.1136/ard.2008.091348. [DOI] [PubMed] [Google Scholar]
- 18.Medsger TA., Jr . Systemic sclerosis (scleroderma): clinical aspects. In: Koopman WJ, editor. Arthritis and allied conditions a textbook of rheumatology. 14th edn. vol 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1590–1624. [Google Scholar]
- 19.Yamasaki Y, Honkanen-Scott M, Hernandez L, Ikeda K, Barker T, Bubb MR, Narain S, Richards HB, Chan EK, Reeves WH, et al. Nucleolar staining cannot be used as a screening test for the scleroderma marker anti-RNA polymerase I/III antibodies. Arthritis Rheum. 2006;54:3051–3056. doi: 10.1002/art.22043. [DOI] [PubMed] [Google Scholar]
- 20.Wiik AS, Hoier-Madsen M, Forslid J, Charles P, Meyrowitsch J. Antinuclear antibodies: a contemporary nomenclature using HEp-2 cells. J Autoimmun. 2010;35:276–290. doi: 10.1016/j.jaut.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Dellavance A, Gallindo C, Soares MG, da Silva NP, Mortara RA, Andrade LE. Redefining the Scl-70 indirect immunofluorescence pattern: autoantibodies to DNA topoisomerase I yield a specific compound immunofluorescence pattern. Rheumatology (Oxford) 2009;48:632–637. doi: 10.1093/rheumatology/kep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okano Y, Medsger TA., Jr Autoantibody to Th ribonucleoprotein (nucleolar 7–2 RNA protein particle) in patients with systemic sclerosis. Arthritis Rheum. 1990;33:1822–1828. doi: 10.1002/art.1780331210. [DOI] [PubMed] [Google Scholar]
- 23.Okano Y, Steen VD, Medsger TAJ. Autoantibody to U3 nucleolar ribonucleoprotein(fibrillarin) in patients with systemic sclerosis. Arthritis Rheum. 1992;35:95–100. doi: 10.1002/art.1780350114. [DOI] [PubMed] [Google Scholar]
- 24.Steen VD, Ziegler GL, Rodnan GP, Medsger TA., Jr Clinical and laboratory associations of anticentromere antibody in patients with progressive systemic sclerosis. Arthritis Rheum. 1984;27:125–131. doi: 10.1002/art.1780270202. [DOI] [PubMed] [Google Scholar]
- 25.LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA, Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 26.Steen VD, Medsger TA., Jr Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 27.Maddison PJ. Mixed connective tissue disease: overlap syndromes. Baillières Best Pract Res Clin Rheumatol. 2000;14:111–124. doi: 10.1053/berh.1999.0080. [DOI] [PubMed] [Google Scholar]
- 28.Jarzabek-Chorzelska M, Blaszczyk M, Jablonska S, Chorzelski T, Kumar V, Beutner EH. Scl 70 antibody—a specific marker of systemic sclerosis. Br J Dermatol. 1986;115:393–401. doi: 10.1111/j.1365-2133.1986.tb06233.x. [DOI] [PubMed] [Google Scholar]
- 29.Cruz M, Mejia G, Lavalle C, Cortes JJ, Reyes PA. Antinuclear antibodies in scleroderma, mixed connective tissue disease and “primary” Raynaud’s phenomenon. Clin Rheumatol. 1988;7:80–86. doi: 10.1007/BF02284061. [DOI] [PubMed] [Google Scholar]
- 30.Meyer OC, Fertig N, Lucas M, Somogyi N, Medsger TA., Jr Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol. 2007;34:104–109. [PubMed] [Google Scholar]
- 31.Codullo V, Cavazzana I, Bonino C, Alpini C, Cavagna L, Cozzi F, Del Papa N, Franceschini F, Guiducci S, Morozzi G, et al. Serologic profile and mortality rates of scleroderma renal crisis in Italy. J Rheumatol. 2009;36:1464–1469. doi: 10.3899/jrheum.080806. [DOI] [PubMed] [Google Scholar]
- 32.Faucher B, Stein P, Granel B, Weiller PJ, Disdier P, Serratrice J, Harle JR, Durand JM, Frances Y, Guis S, et al. Low prevalence of anti-RNA polymerase III antibodies in a French scleroderma population: anti-RNA polymerase III scleroderma. Eur J Intern Med. 2010;21:114–117. doi: 10.1016/j.ejim.2010.01.004. [DOI] [PubMed] [Google Scholar]