Abstract
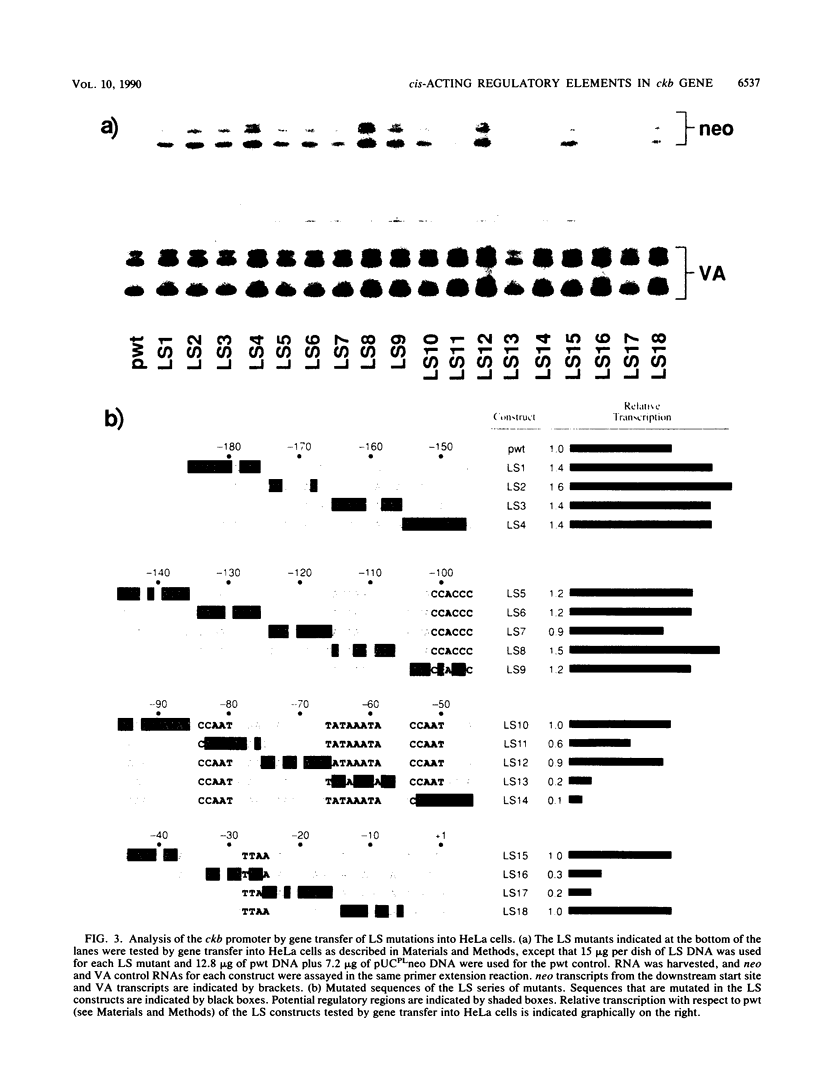
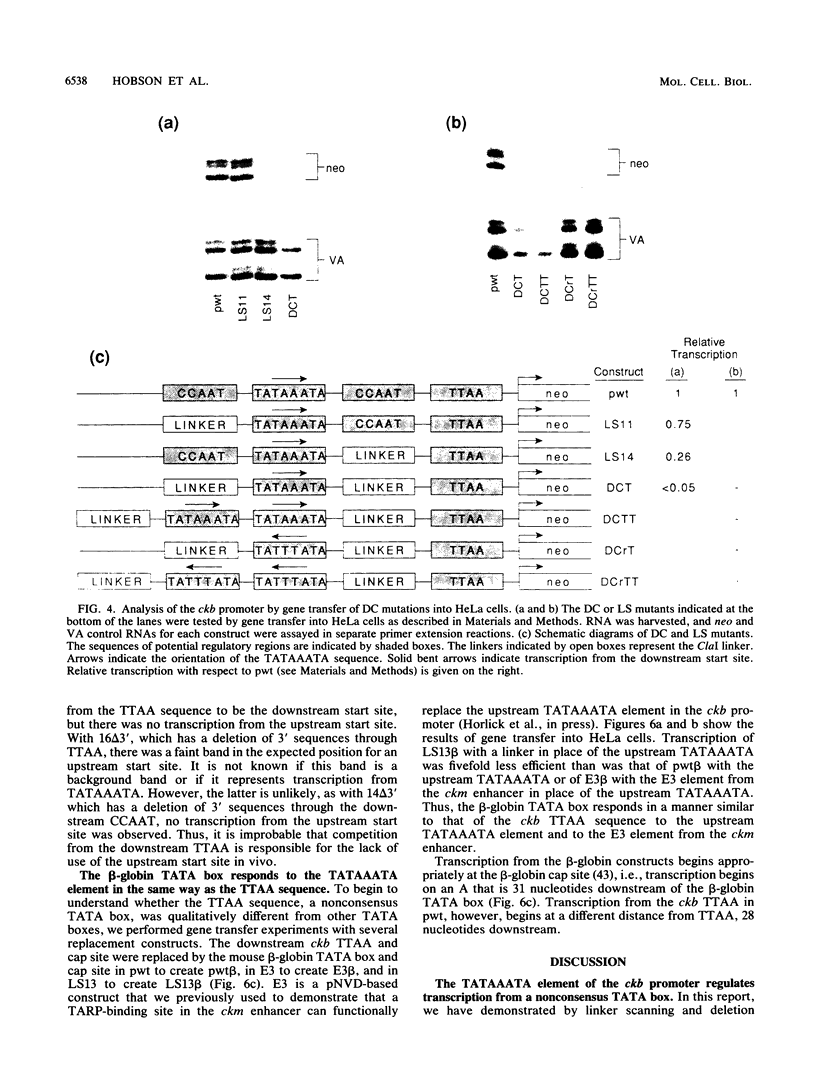
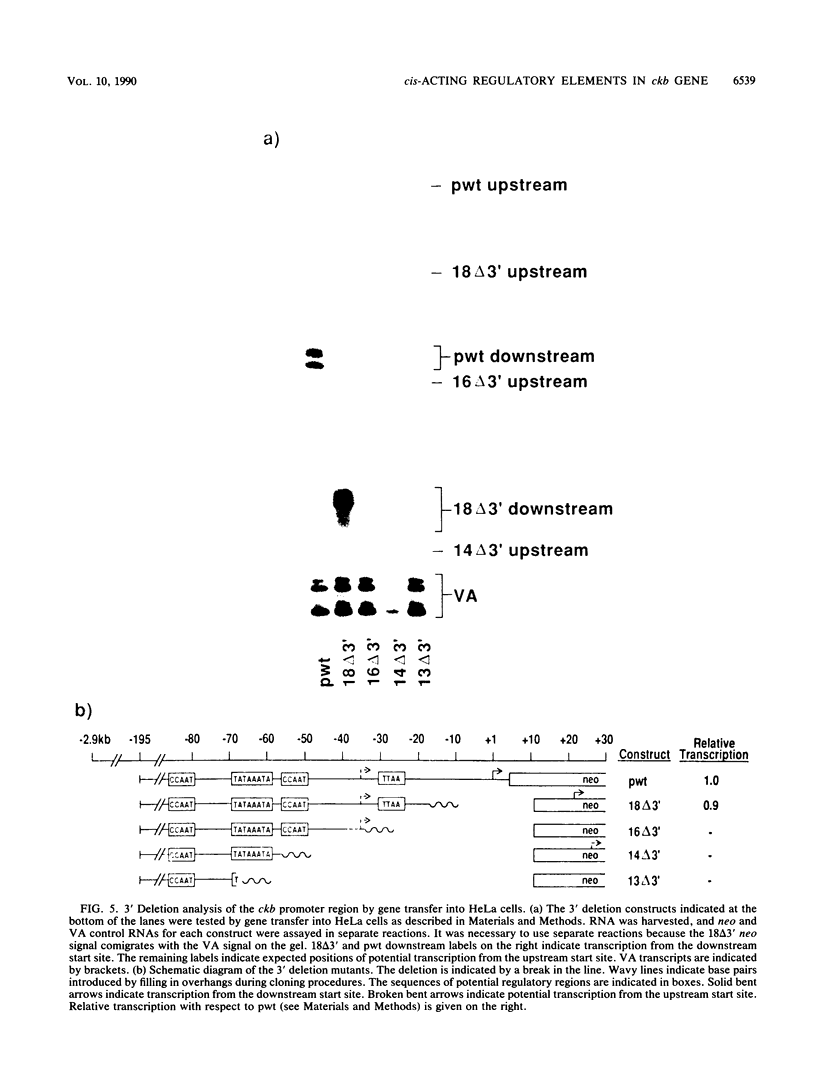
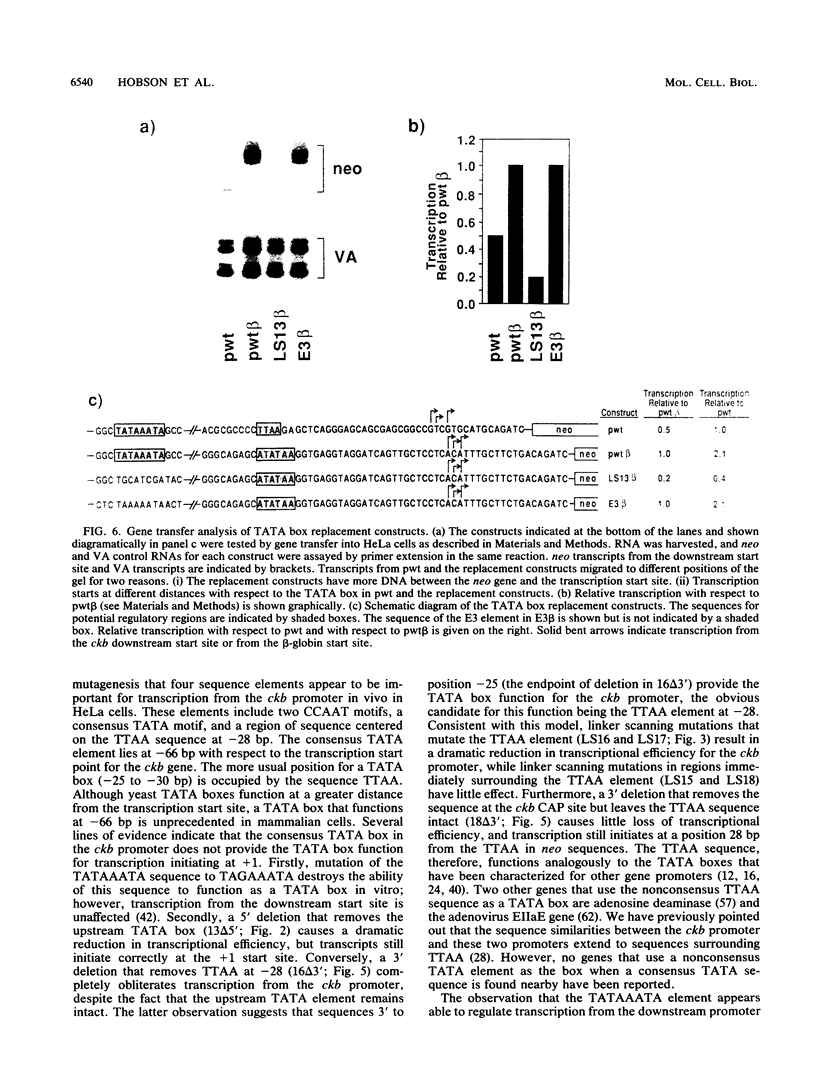
The functional organization of the rat brain creatine kinase (ckb) promoter was analyzed by deletion, linker scanning, and substitution mutagenesis. Mutations were introduced into the ckb promoter of hybrid ckb/neo (neomycin resistance gene) genes, and the mutant genes were expressed transiently in HeLa cells. Expression was assayed by primer extension analysis of neo RNA, which allowed the transcription start sites and the amount of transcription to be determined. Transfections and primer extension reactions were internally controlled by simultaneous analysis of transcription from the adenovirus VA gene located on the same plasmid as the hybrid ckb/neo gene. We demonstrate that 195 bp of the ckb promoter is sufficient for efficient in vivo expression in HeLa cells. A nonconsensus TTAA element at -28 bp appears to provide the TATA box function for the ckb promoter in vivo. Two CCAAT elements, one at -84 bp and the other at -54 bp, and a TATAAA TA element (a consensus TATA box sequence) at -66 bp are required for efficient transcription from the TTAA element. In addition, we present evidence that the consensus beta-globin TATA box responds to the TATAAATA element in the same way as the ckb nonconsensus TTAA element.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Svensson C., Nygård O. A mechanism by which adenovirus virus-associated RNAI controls translation in a transient expression assay. Mol Cell Biol. 1987 Jan;7(1):549–551. doi: 10.1128/mcb.7.1.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcot S. S., Flemington E. K., Deininger P. L. The human thymidine kinase gene promoter. Deletion analysis and specific protein binding. J Biol Chem. 1989 Feb 5;264(4):2343–2349. [PubMed] [Google Scholar]
- Benfield P. A., Graf D., Korolkoff P. N., Hobson G., Pearson M. L. Isolation of four rat creatine kinase genes and identification of multiple potential promoter sequences within the rat brain creatine kinase promoter region. Gene. 1988 Mar 31;63(2):227–243. doi: 10.1016/0378-1119(88)90527-6. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Bienz M., Pelham H. R. Heat shock regulatory elements function as an inducible enhancer in the Xenopus hsp70 gene and when linked to a heterologous promoter. Cell. 1986 Jun 6;45(5):753–760. doi: 10.1016/0092-8674(86)90789-0. [DOI] [PubMed] [Google Scholar]
- Bond-Matthews B., Davidson N. Transcription from each of the Drosophila act5C leader exons is driven by a separate functional promoter. Gene. 1988;62(2):289–300. doi: 10.1016/0378-1119(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Cavallini B., Faus I., Matthes H., Chipoulet J. M., Winsor B., Egly J. M., Chambon P. Cloning of the gene encoding the yeast protein BTF1Y, which can substitute for the human TATA box-binding factor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9803–9807. doi: 10.1073/pnas.86.24.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Mellon P., Maniatis T. Linker scanning mutagenesis of the 5'-flanking region of the mouse beta-major-globin gene: sequence requirements for transcription in erythroid and nonerythroid cells. Mol Cell Biol. 1985 Jun;5(6):1498–1511. doi: 10.1128/mcb.5.6.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Struhl K. Saturation mutagenesis of a yeast his3 "TATA element": genetic evidence for a specific TATA-binding protein. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2691–2695. doi: 10.1073/pnas.85.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Santomenna L. D. Selective induction of chromosomal gene expression by human cytomegalovirus. Virology. 1988 Sep;166(1):217–228. doi: 10.1016/0042-6822(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Corden J., Wasylyk B., Buchwalder A., Sassone-Corsi P., Kedinger C., Chambon P. Promoter sequences of eukaryotic protein-coding genes. Science. 1980 Sep 19;209(4463):1406–1414. doi: 10.1126/science.6251548. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., Stoeckle M. Y., Hanafusa H. Serum and v-src increase the level of a CCAAT-binding factor required for transcription from a retroviral long terminal repeat. Genes Dev. 1990 Feb;4(2):243–254. doi: 10.1101/gad.4.2.243. [DOI] [PubMed] [Google Scholar]
- Fromm M., Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–481. [PubMed] [Google Scholar]
- Gazdar A. F., Zweig M. H., Carney D. N., Van Steirteghen A. C., Baylin S. B., Minna J. D. Levels of creatine kinase and its BB isoenzyme in lung cancer specimens and cultures. Cancer Res. 1981 Jul;41(7):2773–2777. [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golander A., Binderman I., Kaye A. M., Nimrod A., Sömjen D. Stimulation of creatine kinase activity in rat organs by human growth hormone in vivo and in vitro. Endocrinology. 1986 May;118(5):1966–1970. doi: 10.1210/endo-118-5-1966. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Haas R. C., Korenfeld C., Zhang Z. F., Perryman B., Roman D., Strauss A. W. Isolation and characterization of the gene and cDNA encoding human mitochondrial creatine kinase. J Biol Chem. 1989 Feb 15;264(5):2890–2897. [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Isolation of the gene encoding the yeast TATA binding protein TFIID: a gene identical to the SPT15 suppressor of Ty element insertions. Cell. 1989 Sep 22;58(6):1173–1181. doi: 10.1016/0092-8674(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Harbury P. A., Struhl K. Functional distinctions between yeast TATA elements. Mol Cell Biol. 1989 Dec;9(12):5298–5304. doi: 10.1128/mcb.9.12.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson G. M., Mitchell M. T., Molloy G. R., Pearson M. L., Benfield P. A. Identification of a novel TA-rich DNA binding protein that recognizes a TATA sequence within the brain creatine kinase promoter. Nucleic Acids Res. 1988 Sep 26;16(18):8925–8944. doi: 10.1093/nar/16.18.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Wang C. K., Fujii H., Cromlish J. A., Weil P. A., Roeder R. G. Cloning and structure of a yeast gene encoding a general transcription initiation factor TFIID that binds to the TATA box. Nature. 1989 Sep 28;341(6240):299–303. doi: 10.1038/341299a0. [DOI] [PubMed] [Google Scholar]
- Horlick R. A., Hobson G. M., Patterson J. H., Mitchell M. T., Benfield P. A. Brain and muscle creatine kinase genes contain common TA-rich recognition protein-binding regulatory elements. Mol Cell Biol. 1990 Sep;10(9):4826–4836. doi: 10.1128/mcb.10.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., Chamberlain J. S., Buskin J. N., Johnson J. E., Hauschka S. D. Transcriptional regulation of the muscle creatine kinase gene and regulated expression in transfected mouse myoblasts. Mol Cell Biol. 1986 Aug;6(8):2855–2864. doi: 10.1128/mcb.6.8.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Edlund T., Moss J. B., Rutter W. J., Walker M. D. A mutational analysis of the insulin gene transcription control region: expression in beta cells is dependent on two related sequences within the enhancer. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8819–8823. doi: 10.1073/pnas.84.24.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon G. L., Reed G. H. Creatine kinase: structure-activity relationships. Adv Enzymol Relat Areas Mol Biol. 1983;54:367–426. doi: 10.1002/9780470122990.ch6. [DOI] [PubMed] [Google Scholar]
- Knight G. B., Gudas J. M., Pardee A. B. Cell-cycle-specific interaction of nuclear DNA-binding proteins with a CCAAT sequence from the human thymidine kinase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8350–8354. doi: 10.1073/pnas.84.23.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loike J. D., Kozler V. F., Silverstein S. C. Creatine kinase expression and creatine phosphate accumulation are developmentally regulated during differentiation of mouse and human monocytes. J Exp Med. 1984 Mar 1;159(3):746–757. doi: 10.1084/jem.159.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani R., Malgaretti N., Nicolis S., Giglioni B., Comi P., Cappellini N., Bertero M. T., Caligaris-Cappio F., Ottolenghi S. An erythroid specific nuclear factor binding to the proximal CACCC box of the beta-globin gene promoter. Nucleic Acids Res. 1988 May 25;16(10):4299–4313. doi: 10.1093/nar/16.10.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariman E. C., Broers C. A., Claesen C. A., Tesser G. I., Wieringa B. Structure and expression of the human creatine kinase B gene. Genomics. 1987 Oct;1(2):126–137. doi: 10.1016/0888-7543(87)90004-8. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Lane M. D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989 Dec;3(12B):2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- Mitchell M. T., Benfield P. A. Two different RNA polymerase II initiation complexes can assemble on the rat brain creatine kinase promoter. J Biol Chem. 1990 May 15;265(14):8259–8267. [PubMed] [Google Scholar]
- Myers R. M., Tilly K., Maniatis T. Fine structure genetic analysis of a beta-globin promoter. Science. 1986 May 2;232(4750):613–618. doi: 10.1126/science.3457470. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro J. M., Schweinfest C. W., Rajkovic A., Pavlovic J., Jamal S., Dottin R. P., Hart J. T., Kamarck M. E., Rae P. M., Carty M. D. cDNA cloning and mapping of the human creatine kinase M gene to 19q13. Am J Hum Genet. 1987 Feb;40(2):115–125. [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Factors involved in specific transcription by human RNA polymerase II: analysis by a rapid and quantitative in vitro assay. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4394–4398. doi: 10.1073/pnas.82.13.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel J., Zurbriggen B., Wegmann G., Wyss M., Eppenberger H. M., Wallimann T. Native mitochondrial creatine kinase forms octameric structures. I. Isolation of two interconvertible mitochondrial creatine kinase forms, dimeric and octameric mitochondrial creatine kinase: characterization, localization, and structure-function relationships. J Biol Chem. 1988 Nov 15;263(32):16942–16953. [PubMed] [Google Scholar]
- Schüle R., Muller M., Kaltschmidt C., Renkawitz R. Many transcription factors interact synergistically with steroid receptors. Science. 1988 Dec 9;242(4884):1418–1420. doi: 10.1126/science.3201230. [DOI] [PubMed] [Google Scholar]
- Schüle R., Muller M., Otsuka-Murakami H., Renkawitz R. Cooperativity of the glucocorticoid receptor and the CACCC-box binding factor. Nature. 1988 Mar 3;332(6159):87–90. doi: 10.1038/332087a0. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Strijker R., Fritz D. T., Levinson A. D. Adenovirus VAI-RNA regulates gene expression by controlling stability of ribosome-bound RNAs. EMBO J. 1989 Sep;8(9):2669–2675. doi: 10.1002/j.1460-2075.1989.tb08407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strähle U., Schmid W., Schütz G. Synergistic action of the glucocorticoid receptor with transcription factors. EMBO J. 1988 Nov;7(11):3389–3395. doi: 10.1002/j.1460-2075.1988.tb03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga G., Barberis A., Schaffner G., Busslinger M. The -117 mutation in Greek HPFH affects the binding of three nuclear factors to the CCAAT region of the gamma-globin gene. EMBO J. 1988 Oct;7(10):3099–3107. doi: 10.1002/j.1460-2075.1988.tb03176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. C., Kingston R. E. Factor substitution in a human HSP70 gene promoter: TATA-dependent and TATA-independent interactions. Mol Cell Biol. 1990 Jan;10(1):165–175. doi: 10.1128/mcb.10.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdal P., Urdal K., Strømme J. H. Cytoplasmic creatine kinase isoenzymes quantitated in tissue specimens obtained at surgery. Clin Chem. 1983 Feb;29(2):310–313. [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Levy G., Ma T. S., Kerner S. A., Roberts R., Perryman M. B. Human creatine kinase: isolation and sequence analysis of cDNA clones for the B subunit, development of subunit specific probes and determination of gene copy number. Biochem Biophys Res Commun. 1987 May 14;144(3):1116–1127. doi: 10.1016/0006-291x(87)91427-6. [DOI] [PubMed] [Google Scholar]
- Webster N., Jin J. R., Green S., Hollis M., Chambon P. The yeast UASG is a transcriptional enhancer in human HeLa cells in the presence of the GAL4 trans-activator. Cell. 1988 Jan 29;52(2):169–178. doi: 10.1016/0092-8674(88)90505-3. [DOI] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Boeuf H., Kédinger C. E1a inducibility of the adenoviral early E2a promoter is determined by specific combinations of sequence elements. Gene. 1987;58(2-3):243–256. doi: 10.1016/0378-1119(87)90379-9. [DOI] [PubMed] [Google Scholar]