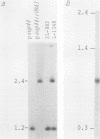
Abstract
The accurate and efficient translation of proteins is of fundamental importance to both bacteria and higher organisms. Most of our knowledge about the control of translational fidelity comes from studies of Escherichia coli. In particular, ram (ribosomal ambiguity) mutations in structural genes of E. coli ribosomal proteins S4 and S5 have been shown to increase translational error frequencies. We describe the first sequence of a ribosomal protein gene that affects translational ambiguity in a eucaryote. We show that the yeast omnipotent suppressor SUP44 encodes the yeast ribosomal protein S4. The gene exists as a single copy without an intron. The SUP44 protein is 26% identical (54% similar) to the well-characterized E. coli S5 ram protein. SUP44 is also 59% identical (78% similar) to mouse protein LLrep3, whose function was previously unknown (D.L. Heller, K.M. Gianda, and L. Leinwand, Mol. Cell. Biol. 8:2797-2803, 1988). The SUP44 suppressor mutation occurs near a region of the protein that corresponds to the known positions of alterations in E. coli S5 ram mutations. This is the first ribosomal protein whose function and sequence have been shown to be conserved between procaryotes and eucaryotes.
Full text
PDF




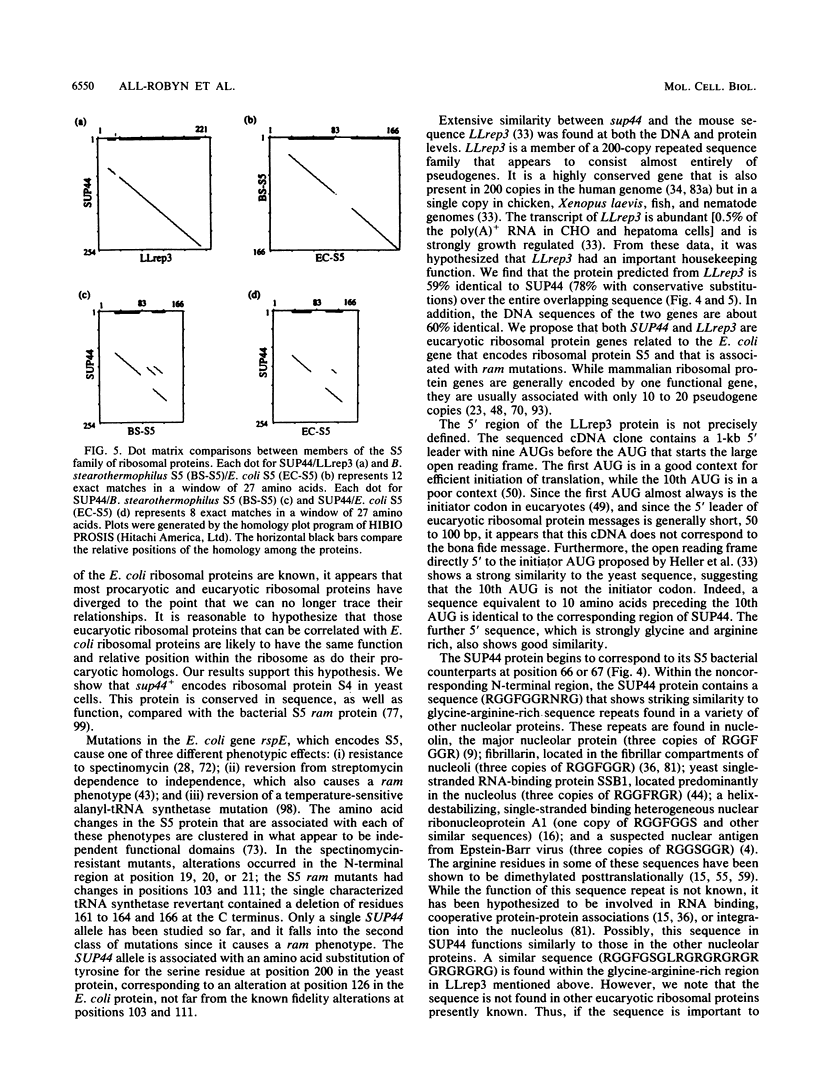



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- All-Robyn J. A., Kelley-Geraghty D., Griffin E., Brown N., Liebman S. W. Isolation of omnipotent suppressors in an [eta+] yeast strain. Genetics. 1990 Mar;124(3):505–514. doi: 10.1093/genetics/124.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. N., Noller H. F. Mutations in ribosomal proteins S4 and S12 influence the higher order structure of 16 S ribosomal RNA. J Mol Biol. 1989 Aug 5;208(3):457–468. doi: 10.1016/0022-2836(89)90509-3. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Elseviers D., Gorini L. Low activity of -galactosidase in frameshift mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1192–1195. doi: 10.1073/pnas.69.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilofsky H. S., Burks C., Fickett J. W., Goad W. B., Lewitter F. I., Rindone W. P., Swindell C. D., Tung C. S. The GenBank genetic sequence databank. Nucleic Acids Res. 1986 Jan 10;14(1):1–4. doi: 10.1093/nar/14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Cabezón T., de Wilde M., Villarroel R., Herzog A. Alteration of ribosomal protein S17 by mutation linked to neamine resistance in Escherichia coli. I. General properties of neaA mutants. J Mol Biol. 1975 Dec 25;99(4):795–806. doi: 10.1016/s0022-2836(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Bourbon H. M., Lapeyre B., Amalric F. Structure of the mouse nucleolin gene. The complete sequence reveals that each RNA binding domain is encoded by two independent exons. J Mol Biol. 1988 Apr 20;200(4):627–638. doi: 10.1016/0022-2836(88)90476-7. [DOI] [PubMed] [Google Scholar]
- Breining P., Piepersberg W. Yeast omnipotent supressor SUP1 (SUP45): nucleotide sequence of the wildtype and a mutant gene. Nucleic Acids Res. 1986 Jul 11;14(13):5187–5197. doi: 10.1093/nar/14.13.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Cabezón T., Herzog A., De Wilde M., Villarroel R., Bollen A. Cooperative control of translational fidelity by ribosomal proteins in Escherichia coli. III. A ram mutation in the structural gene for protein S5 (rpx E). Mol Gen Genet. 1976 Feb 27;144(1):59–62. doi: 10.1007/BF00277305. [DOI] [PubMed] [Google Scholar]
- Capel M. S., Engelman D. M., Freeborn B. R., Kjeldgaard M., Langer J. A., Ramakrishnan V., Schindler D. G., Schneider D. K., Schoenborn B. P., Sillers I. Y. A complete mapping of the proteins in the small ribosomal subunit of Escherichia coli. Science. 1987 Dec 4;238(4832):1403–1406. doi: 10.1126/science.3317832. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Fuxa K. P. The nucleolar protein, B-36, contains a glycine and dimethylarginine-rich sequence conserved in several other nuclear RNA-binding proteins. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1278–1283. doi: 10.1016/s0006-291x(88)81279-8. [DOI] [PubMed] [Google Scholar]
- Cobianchi F., SenGupta D. N., Zmudzka B. Z., Wilson S. H. Structure of rodent helix-destabilizing protein revealed by cDNA cloning. J Biol Chem. 1986 Mar 15;261(8):3536–3543. [PubMed] [Google Scholar]
- Crouzet M., Izgu F., Grant C. M., Tuite M. F. The allosuppressor gene SAL4 encodes a protein important for maintaining translational fidelity in Saccharomyces cerevisiae. Curr Genet. 1988 Dec;14(6):537–543. doi: 10.1007/BF00434078. [DOI] [PubMed] [Google Scholar]
- Crouzet M., Tuite M. F. Genetic control of translational fidelity in yeast: molecular cloning and analysis of the allosuppressor gene SAL3. Mol Gen Genet. 1987 Dec;210(3):581–583. doi: 10.1007/BF00327216. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dequard-Chablat M., Coppin-Raynal E., Picard-Bennoun M., Madjar J. J. At least seven ribosomal proteins are involved in the control of translational accuracy in a eukaryotic organism. J Mol Biol. 1986 Jul 20;190(2):167–175. doi: 10.1016/0022-2836(86)90290-1. [DOI] [PubMed] [Google Scholar]
- Dorsman J. C., Doorenbosch M. M., Maurer C. T., de Winde J. H., Mager W. H., Planta R. J., Grivell L. A. An ARS/silencer binding factor also activates two ribosomal protein genes in yeast. Nucleic Acids Res. 1989 Jul 11;17(13):4917–4923. doi: 10.1093/nar/17.13.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Elder R. T., Loh E. Y., Davis R. W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustice D. C., Wakem L. P., Wilhelm J. M., Sherman F. Altered 40 S ribosomal subunits in omnipotent suppressors of yeast. J Mol Biol. 1986 Mar 20;188(2):207–214. doi: 10.1016/0022-2836(86)90305-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Pearson N. J., Kim C. H., Warner J. R. The genes for fifteen ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10176–10183. [PubMed] [Google Scholar]
- Funatsu G., Nierhaus K., Wittmann-Liebold B. Ribosomal proteins. XXII. Studies on the altered protein S5 from a spectinomycin-resistant mutant of Escherichia coli. J Mol Biol. 1972 Feb 28;64(1):201–209. doi: 10.1016/0022-2836(72)90329-4. [DOI] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini L. Informational suppression. Annu Rev Genet. 1970;4:107–134. doi: 10.1146/annurev.ge.04.120170.000543. [DOI] [PubMed] [Google Scholar]
- Hamil K. G., Nam H. G., Fried H. M. Constitutive transcription of yeast ribosomal protein gene TCM1 is promoted by uncommon cis- and trans-acting elements. Mol Cell Biol. 1988 Oct;8(10):4328–4341. doi: 10.1128/mcb.8.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D. L., Gianola K. M., Leinwand L. A. A highly conserved mouse gene with a propensity to form pseudogenes in mammals. Mol Cell Biol. 1988 Jul;8(7):2797–2803. doi: 10.1128/mcb.8.7.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D., Jackson M., Leinwand L. Organization and expression of non-Alu family interspersed repetitive DNA sequences in the mouse genome. J Mol Biol. 1984 Mar 15;173(4):419–436. doi: 10.1016/0022-2836(84)90389-9. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Kelly J. D., Cohen E. H. Transcription terminates in yeast distal to a control sequence. Cell. 1983 Jun;33(2):607–614. doi: 10.1016/0092-8674(83)90441-5. [DOI] [PubMed] [Google Scholar]
- Henríquez R., Blobel G., Aris J. P. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990 Feb 5;265(4):2209–2215. [PubMed] [Google Scholar]
- Higo K., Otaka E. Isolation and characterization of fourteen ribosomal proteins from small subunits of yeast. Biochemistry. 1979 Sep 18;18(19):4191–4196. doi: 10.1021/bi00586a024. [DOI] [PubMed] [Google Scholar]
- Himmelfarb H. J., Maicas E., Friesen J. D. Isolation of the SUP45 omnipotent suppressor gene of Saccharomyces cerevisiae and characterization of its gene product. Mol Cell Biol. 1985 Apr;5(4):816–822. doi: 10.1128/mcb.5.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A. TUF, the yeast DNA-binding factor specific for UASrpg upstream activating sequences: identification of the protein and its DNA-binding domain. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3648–3652. doi: 10.1073/pnas.84.11.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro J., Ono B. I., Masurekar M., McLaughlin C. S., Sherman F. Altered ribosomal protein S11 from the SUP46 suppressor of yeast. J Mol Biol. 1981 Apr 15;147(3):391–397. doi: 10.1016/0022-2836(81)90491-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Wittmann H. G. Amino acid replacements in proteins S5 and S12 of two Escherichia coli revertants from streptomycin dependence to independence. Mol Gen Genet. 1973 Dec 14;127(1):19–32. doi: 10.1007/BF00267779. [DOI] [PubMed] [Google Scholar]
- Itoh T., Higo K., Otaka E. Isolation and characterization of twenty-three ribosomal proteins from large subunits of yeast. Biochemistry. 1979 Dec 25;18(26):5787–5791. doi: 10.1021/bi00593a007. [DOI] [PubMed] [Google Scholar]
- Jong A. Y., Clark M. W., Gilbert M., Oehm A., Campbell J. L. Saccharomyces cerevisiae SSB1 protein and its relationship to nucleolar RNA-binding proteins. Mol Cell Biol. 1987 Aug;7(8):2947–2955. doi: 10.1128/mcb.7.8.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Shimatake H., Kikuchi A. A yeast gene required for the G1-to-S transition encodes a protein containing an A-kinase target site and GTPase domain. EMBO J. 1988 Apr;7(4):1175–1182. doi: 10.1002/j.1460-2075.1988.tb02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Proteins of the Bacillus stearothermophilus ribosome. The amino acid sequences of proteins S5 and L30. J Biol Chem. 1984 Jan 25;259(2):1051–1055. [PubMed] [Google Scholar]
- Kirsebom L. A., Isaksson L. A. Involvement of ribosomal protein L7/L12 in control of translational accuracy. Proc Natl Acad Sci U S A. 1985 Feb;82(3):717–721. doi: 10.1073/pnas.82.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Meyuhas O. A multigene family of intron lacking and containing genes, encoding for mouse ribosomal protein L7. Nucleic Acids Res. 1984 May 11;12(9):3763–3776. doi: 10.1093/nar/12.9.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the "scanning model" for initiation of protein synthesis in eucaryotes. Cell. 1980 Nov;22(1 Pt 1):7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Nishikawa K., Takahashi S., Ooi T. Correspondence of homologies in amino acid sequence and tertiary structure of protein molecules. Biochim Biophys Acta. 1982 Feb 18;701(2):242–252. doi: 10.1016/0167-4838(82)90120-0. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Takahashi S., Nishikawa K., Ooi T. Homology in protein sequences expressed by correlation coefficients. J Theor Biol. 1981 Jul 21;91(2):347–361. doi: 10.1016/0022-5193(81)90237-x. [DOI] [PubMed] [Google Scholar]
- Kushnirov V. V., Ter-Avanesyan M. D., Telckov M. V., Surguchov A. P., Smirnov V. N., Inge-Vechtomov S. G. Nucleotide sequence of the SUP2 (SUP35) gene of Saccharomyces cerevisiae. Gene. 1988 Jun 15;66(1):45–54. doi: 10.1016/0378-1119(88)90223-5. [DOI] [PubMed] [Google Scholar]
- Kühberger R., Piepersberg W., Petzet A., Buckel P., Böck A. Alteration of ribosomal protein L6 in gentamicin-resistant strains of Escherichia coli. Effects on fidelity of protein synthesis. Biochemistry. 1979 Jan 9;18(1):187–193. doi: 10.1021/bi00568a028. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Amalric F., Ghaffari S. H., Rao S. V., Dumbar T. S., Olson M. O. Protein and cDNA sequence of a glycine-rich, dimethylarginine-containing region located near the carboxyl-terminal end of nucleolin (C23 and 100 kDa). J Biol Chem. 1986 Jul 15;261(20):9167–9173. [PubMed] [Google Scholar]
- Leer R. J., Van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. Conserved sequences upstream of yeast ribosomal protein genes. Curr Genet. 1985;9(4):273–277. doi: 10.1007/BF00419955. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Cook R. G., Ahn Y. S., Yeoman L. C., Busch H. Clustering of glycine and NG,NG-dimethylarginine in nucleolar protein C23. Biochemistry. 1985 Oct 22;24(22):6025–6028. doi: 10.1021/bi00343a001. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Masurekar M., Palmer E., Ono B. I., Wilhelm J. M., Sherman F. Misreading of the ribosomal suppressor SUP46 due to an altered 40 S subunit in yeast. J Mol Biol. 1981 Apr 15;147(3):381–390. doi: 10.1016/0022-2836(81)90490-3. [DOI] [PubMed] [Google Scholar]
- Moore P. B. The ribosome returns. Nature. 1988 Jan 21;331(6153):223–227. doi: 10.1038/331223a0. [DOI] [PubMed] [Google Scholar]
- Ono B., Tanaka M., Awano I., Okamoto F., Satoh R., Yamagishi N., Ishino-Arao Y. Two new loci that give rise to dominant omnipotent suppressors in Saccharomyces cerevisiae. Curr Genet. 1989 Dec;16(5-6):323–330. doi: 10.1007/BF00340710. [DOI] [PubMed] [Google Scholar]
- Otaka E., Higo K., Osawa S. Isolation of seventeen proteins and amino-terminal amino acid sequences of eight proteins from cytoplasmic ribosomes of yeast. Biochemistry. 1982 Sep 14;21(19):4545–4550. doi: 10.1021/bi00262a005. [DOI] [PubMed] [Google Scholar]
- Otaka E., Suzuki K., Hashimoto T. Examination of protein sequence homologies. VII. The complementary molecular coevolution of ribosomal proteins equivalent to Escherichia coli L7/L12 and L10. Protein Seq Data Anal. 1990 Mar;3(1):11–19. [PubMed] [Google Scholar]
- Peled-Yalif E., Cohen-Binder I., Meyuhas O. Isolation and characterization of four mouse ribosomal-protein-L18 genes that appear to be processed pseudogenes. Gene. 1984 Jul-Aug;29(1-2):157–166. doi: 10.1016/0378-1119(84)90176-8. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Böck A., Wittmann H. G. Effect of different mutations in ribosomal protein S5 of Escherichia coli on translational fidelity. Mol Gen Genet. 1975 Sep 29;140(2):91–100. doi: 10.1007/BF00329777. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Böck A., Yaguchi M., Wittmann H. G. Genetic position and amino acid replacements of several mutations in ribosomal protein S5 from Escherichia coli. Mol Gen Genet. 1975 Dec 30;143(1):43–52. doi: 10.1007/BF00269419. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Woolford J. L., Jr Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986 Feb;6(2):674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T., Tollervey D., Kern H., Frank R., Hurt E. C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989 Dec 20;8(13):4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Tuohy T. M., Mosurski K. R. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986 Jul 11;14(13):5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slynn G., Jenner D., Potts W., Elvin P., Morten J. E., Markham A. F. Human cDNA sequence homologous to the mouse LLRep3 gene family. Nucleic Acids Res. 1990 Feb 11;18(3):681–681. doi: 10.1093/nar/18.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapio S., Kurland C. G. Mutant EF-Tu increases missense error in vitro. Mol Gen Genet. 1986 Oct;205(1):186–188. doi: 10.1007/BF02428051. [DOI] [PubMed] [Google Scholar]
- Teem J. L., Abovich N., Kaufer N. F., Schwindinger W. F., Warner J. R., Levy A., Woolford J., Leer R. J., van Raamsdonk-Duin M. M., Mager W. H. A comparison of yeast ribosomal protein gene DNA sequences. Nucleic Acids Res. 1984 Nov 26;12(22):8295–8312. doi: 10.1093/nar/12.22.8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C. EFTu provides an internal kinetic standard for translational accuracy. Trends Biochem Sci. 1988 Mar;13(3):91–93. doi: 10.1016/0968-0004(88)90047-3. [DOI] [PubMed] [Google Scholar]
- Vijgenboom E., Vink T., Kraal B., Bosch L. Mutants of the elongation factor EF-Tu, a new class of nonsense suppressors. EMBO J. 1985 Apr;4(4):1049–1052. doi: 10.1002/j.1460-2075.1985.tb03737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Petes T. D. Mitotic and meiotic gene conversion of Ty elements and other insertions in Saccharomyces cerevisiae. Genetics. 1989 Aug;122(4):759–772. doi: 10.1093/genetics/122.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakem L. P., Sherman F. Isolation and characterization of omnipotent suppressors in the yeast Saccharomyces cerevisiae. Genetics. 1990 Mar;124(3):515–522. doi: 10.1093/genetics/124.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R. Synthesis of ribosomes in Saccharomyces cerevisiae. Microbiol Rev. 1989 Jun;53(2):256–271. doi: 10.1128/mr.53.2.256-271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Perry R. P. Characterization of the expressed gene and several processed pseudogenes for the mouse ribosomal protein L30 gene family. Mol Cell Biol. 1984 Nov;4(11):2518–2528. doi: 10.1128/mcb.4.11.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C. M., Liebman S. W. Integration of an aberrant retrotransposon in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Sep;9(9):4096–4098. doi: 10.1128/mcb.9.9.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. G., Culbertson M. R. SUF12 suppressor protein of yeast. A fusion protein related to the EF-1 family of elongation factors. J Mol Biol. 1988 Feb 20;199(4):559–573. doi: 10.1016/0022-2836(88)90301-4. [DOI] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Greuer B. The primary structure of protein S5 from the small subunit of the Escherichia coli ribosome. FEBS Lett. 1978 Nov 1;95(1):91–98. doi: 10.1016/0014-5793(78)80059-3. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöffler G. Altered S5 and S20 ribosomal proteins in revertants of an alanyl-tRNA synthetase mutant of Escherichia coli. Mol Gen Genet. 1974;134(3):225–236. doi: 10.1007/BF00267717. [DOI] [PubMed] [Google Scholar]
- Woudt L. P., Smit A. B., Mager W. H., Planta R. J. Conserved sequence elements upstream of the gene encoding yeast ribosomal protein L25 are involved in transcription activation. EMBO J. 1986 May;5(5):1037–1040. doi: 10.1002/j.1460-2075.1986.tb04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]