Abstract
Study Objectives:
Empirical findings on the prospective link between obstructive sleep apnea (OSA) and subsequent depression are mixed. This nationwide, population-based study thus aimed at assessing the risk of depressive disorder within the first year following a diagnosis with OSA. Gender effects were further examined.
Design:
Cohort study.
Setting:
Taiwan.
Patients:
This study used data from the Longitudinal Health Insurance Database 2000. A total of 2,818 patients diagnosed with OSA between 2002 and 2008 were evaluated, and 14,090 matched non-OSA enrollees used as a comparison cohort.
Measurements and Results:
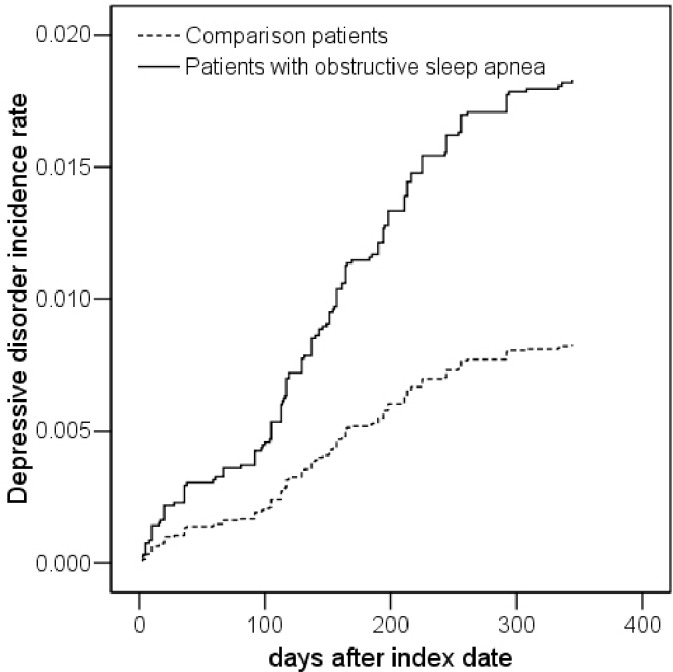
Each patient was followed for one year to identify subsequent depressive disorder. We found that during the one-year follow-up, the incidence of depressive disorder per thousand person-years was about twice as high among patients with OSA (18.10, 95% CI = 13.62-23.61) as those without OSA (8.23, 95% CI = 6.83-9.84). The Cox proportional hazards model revealed that patients with OSA were independently associated with a 2.18 times (95% CI = 1.55-3.08) increased risk of subsequent depressive disorder within a year, compared to those without OSA. As epidemiological studies have consistently documented an increased risk for depression in women, we hypothesized and confirmed higher risks of depressive disorder among female patients with OSA (2.72, 95% CI = 1.68-4.40) than their male counterparts (1.81, 95% CI = 1.09-3.01).
Conclusion:
A prospective link between OSA and subsequent depressive disorder within one year was confirmed by the current study. The risk was particularly evident among women. Regular psychiatric screening among patients with OSA is suggested to prompt the timely detection of depression.
Commentary:
A commentary on this article appears in this issue on page 425.
Citation:
Chen YH; Keller JK; Kang JH; Hsieh HJ; Lin HC. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med 2013;9(5):417-423.
Keywords: Obstructive sleep apnea, depressive disorder, prospective link
Obstructive sleep apnea (OSA), the most common subtype of sleep breathing disorder, is characterized by sleep-related decreases (hypopneas) or pauses (apneas) in respiration.1 The prevalence of OSA increases with age and is higher among males than females. Among the population between 30 to 65 years of age, 24% of men and 9% of women have been demonstrated to suffer from OSA.2
Depression is prevalent in people with OSA, ranging from 5% to 63% in clinical and community studies.3 In a large and retrospective review study, a significantly higher prevalence of depression was identified in people with OSA (21.8%) compared to those without OSA (9.43%).4 While this higher prevalence of depression among OSA points toward an underlying association, some OSA and depressive symptoms may overlap (e.g., fatigue, loss of interest, poor concentration),5 thus making it difficult to determine whether OSA contributes to the following occurrence of depression.
Although prior studies have addressed the link between OSA and depression, results in the literature are mixed. Some studies, including two large-scale studies,6,7 have reported no association between the disorders, while one longitudinal study reported an increased risk of consequent depression that exhibited a dose-response relationship with OSA severity.8 Two retrospective studies also observed OSA to be linked with depression.9,10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Depression is prevalently observed in people with obstructive sleep apnea (OSA). However, although prior studies have addressed the link between OSA and depression, the results in the literature are mixed.
Study Impact: A prospective link between OSA and subsequent depressive disorder within one year was confirmed by the current study. Regular psychiatric screening among patients with OSA is suggested to prompt the timely detection of depression.
Inconsistencies in previous findings may be attributable to inadequate control of confounders (e.g., obesity, hypertension, diabetes, alcohol consumption), biases (e.g., self-reported data), and other measurement issues (e.g., different study populations, questionnaires, and scales).1,3,11 Based upon the heterogeneity of these data and numerous confounding factors, follow-up studies of patient populations have been suggested to better address the link between OSA and depression.1 In addition, while males are more frequently diagnosed with OSA,2 higher rates of depression have been found in female patients with OSA.12 Previous findings have rarely examined the modifying effects of gender, as most studies are unable to enroll sufficient numbers of both males and females to report sex-stratified risks of consequent depression.11 We hypothesized that risks of depressive disorder (DD) among female patients with OSA are higher than their male counterparts.
Thus, the objective of this nationwide population-based study was to assess the risk of DD in one year following a diagnosis of OSA. The association between OSA and DD was further be examined by sex.
METHODS
Database
We retrieved the data from the Longitudinal Health Insurance Database 2000 (LHID2000), which is derived from Taiwan's Bureau of National Health Insurance (NHI) records and released by the Taiwan National Health Research Institute annually. The LHID2000 includes the registration files and medical claims data for the reimbursement of 1,000,000 beneficiaries under the NHI program. These 1,000,000 beneficiaries were randomly selected from the year 2000 Registry of Beneficiaries (n = 23.72 million) of the NHI by the Taiwan National Health Research Institute. Prior studies have validated the completeness and accuracy of the claims data of NHI research database.13,14 Hundreds of researchers have employed the data from the Taiwan NHI to perform and publish their studies in peer-reviewed journals. In particular, 3 recent studies have employed the LHID2000 to explore the relationships between OSA and psoriasis, autoimmune diseases, and sudden hearing loss.15–17
The LHID2000 consists of de-identified secondary data released to the public for research purposes and was therefore exempted from full review, following consultation with the Taipei Medical University's Institutional Review Board.
Study Sample
This retrospective cohort study included a study cohort and a comparison cohort. For the study cohort, we first identified individuals who had been diagnosed with OSA (ICD-9-CM codes 327.23, 780.51, 780.53, or 780.57) after receiving polysomnography during ambulatory care visits between January 1, 2002, and December 31, 2008 (n = 3,292). Since administrative datasets are often criticized for poor diagnostic validity, this study included only patients who had received OSA diagnoses following polysomnography to better ensure diagnostic validity. To include only newly diagnosed cases and to avoid the potential effects of chronicity, we excluded those individuals who had received a diagnosis of OSA prior to 2002 (n = 212). As OSA in children is distinct from OSA in adults,18,19 we further excluded individuals younger than 18 years (n = 103) to increase the homogeneity of our study sample. Finally, we assigned the date of their first ambulatory care visit in which they received a diagnosis of OSA following polysomnography as the index date. We excluded those individuals who had received a diagnosis of DD (ICD-9-CM codes 296.2, 296.3, 300.4, and 311) schizophrenia, or bipolar disorder prior to their index date (n = 159). As a result, a total of 2,818 individuals with OSA were included in the study cohort.
For the selection of the comparison cohort, we likewise extracted the individuals from the LHID2000. We first excluded all the individuals who had ever been diagnosed with OSA since the initiation of the NHI program (from 1995 to 2009) from our consideration. Then we randomly selected 14,090 beneficiaries (5 for each patient in the study cohort) matched with the study cohort in sex, age (18-34, 35-39, 40-44, 45-49, 50-54,55-59, 60-64, 65-59, and > 69), urbanization level (5 levels, with 1 referring to the most urbanized and 5 referring to the least), and year of index date, using the SAS program proc SurveySelect (SAS System for Windows, Version 8.2). For the study cohort, the index date was defined as the year in which the cases received their first diagnosis of OSA; index date for the comparison cohort was simply a matched year in which the comparison individuals had used medical services. For the comparison cohort, we assigned their first use of medical care occurring in the year of index as their index date. In addition, we matched the variable of urbanization level of the patient's residence to help control for error variables, namely unmeasured neighborhood socioeconomic characteristics between the study cohort and comparison cohort. According to a prior study, all 359 cities/towns in Taiwan were stratified into 5 groups ranked by urbanization level.16 We further ensured that patients selected for the comparison cohort had never been diagnosed with DD, schizophrenia, or bipolar disorder prior to their index date.
We individually followed up each sampled individual (n = 16,998) for a one-year period starting from their index date to identify those individuals who subsequently received a diagnosis of DD (ICD-9-CM codes 296.2, 296.3, 300.4, and 311).
Statistical Analysis
The SAS statistical package (SAS System for Windows, Version 8.2, Cary NC, USA) was used to perform all statistical analyses in this study. We used χ2 tests to compare differences in monthly income, geographic region, and prevalence of hypertension, diabetes, coronary heart disease (CHD), hyperlipidemia, obesity, and alcohol abuse/alcohol dependency syndrome between the study cohort and the comparison cohort. We calculated the one-year DD-free survival rates by the Kaplan-Meier method, with the log-rank test also being used to examine differences in DD-free survival rates between cohorts. Stratified Cox proportional hazards regressions (stratified on sex, age group, urbanization level, and index date) were performed to compute the risk for DD during the one-year follow-up period between the study cohort and the comparison cohort. In this study, we have also examined the proportional hazards assumption and found this assumption to be satisfied because the survival curves for both strata (individuals in the study cohort and comparison cohort) had hazard functions that were proportional over time. A two-sided p-value < 0.05 was considered statistically significant for this study.
RESULTS
The distribution of demographic characteristics and comorbidities between the study cohort and the comparison cohort is shown in Table 1. Of the total of 16,908 individuals, the mean age was 46.4 years (SD 15.7 years); only one-third were females. It also shows that individuals with OSA had a higher prevalence of hypertension (p < 0.001), diabetes (p < 0.001), CHD (p < 0.001), hyperlipidemia (p < 0.001), obesity (p < 0.001), and insomnia (p < 0.001) than comparison individuals. However, no significant difference in the prevalence of alcohol abuse/alcohol dependency syndrome (p = 0.391) and restless legs syndrome (p = 0.527) was observed between the study cohort and the comparison cohort.
Table 1.
Demographic characteristics for the sampled Taiwanese patients stratified by the presence/absence of obstructive sleep apnea, 2002-2008 (n = 16,908)

Table 2 presents the incidence of DD during the one-year follow-up period after the index date for the sampled individuals. The incidence of DD during the one-year follow-up period was 18.10 (95% CI = 13.62-23.61) and 8.23 (95% CI = 6.83-9.84) for individuals with and without OSA, respectively. The log-rank test revealed that individuals with OSA had significantly lower one-year DD-free survival rates than comparison individuals. Figure 1 compares the DD incidence rates between these 2 cohorts.
Table 2.
Hazard ratios of depressive disorder among the sample patients during the one-year follow-up periods (n = 16,908)
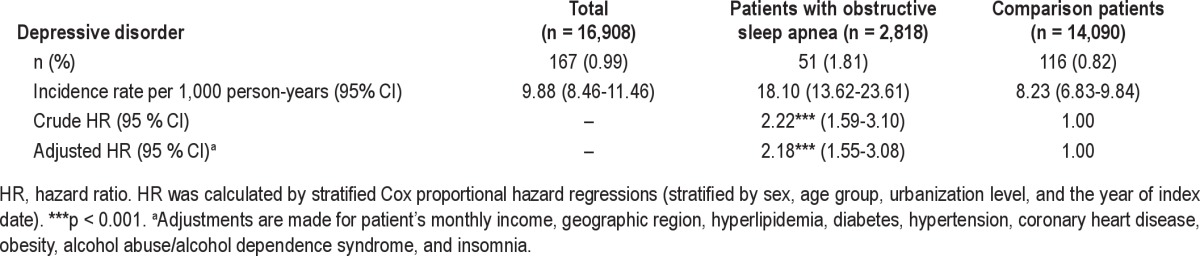
Figure 1. Depressive disorder-free survival rates for patients with obstructive sleep apnea and comparison group in Taiwan.
These are the incidence rates of depressive disorder among patients with and without obstructive sleep apnea during the one-year follow-up periods.
Table 2 also presents the hazard ratio (HR) of DD between the study and comparison cohort. After adjusting for monthly income, geographic region, hypertension, diabetes, CHD, hyperlipidemia, obesity, and alcohol abuse/alcohol dependency syndrome, stratified Cox proportional hazards regressions (stratified on sex, age group, urbanization level, and the year of index date) revealed that the HR for DD among individuals with OSA was 2.18 (95% CI = 1.55-3.08, p < 0.001) times that of comparison individuals.
As the interaction between OSA and sex reached a level of significance (p < 0.1) sufficient to suggest the inclusion of sex in the model, we further present the HR of DD stratified by sex in Table 3. We found that among females, the adjusted hazard of DD during the one-year follow-up period was 2.72 (95% CI = 1.68-4.40) for individuals with OSA when compared with the comparison cohort. However, among males, the adjusted HR of DD among OSA individuals was only 1.81 (95% CI = 1.09-3.01) times that of matched comparison individuals.
Table 3.
Hazard ratios of depressive disorder by gender among the sample patients during the one-year follow-up periods
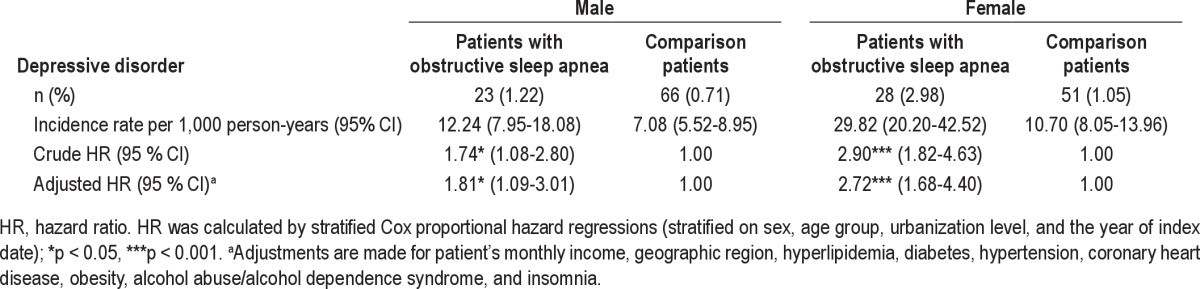
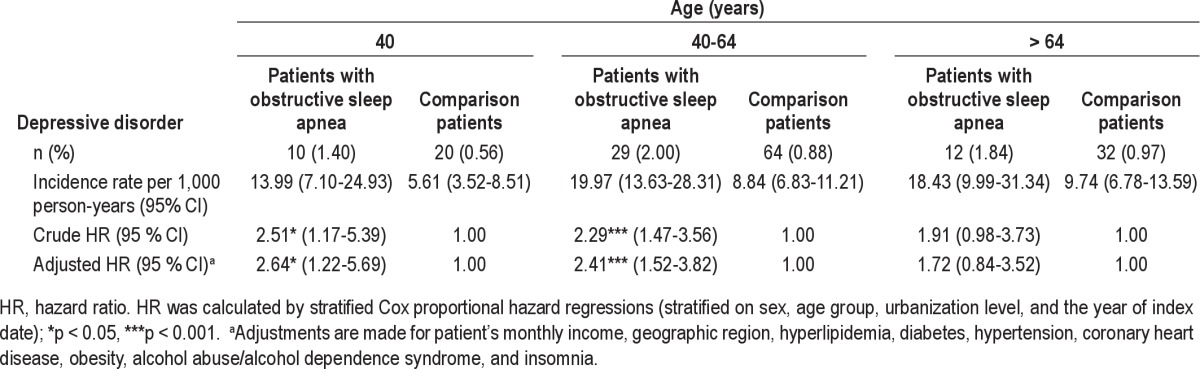
Table 4 further presents the HR of DD stratified by age group. We found that among patients < 40 and 40-64 years old, the adjusted HR of DD during the one-year follow-up period was 2.64 (1.22-5.69) and 2.41 (95% CI = 1.52-3.82), respectively, for individuals with OSA when compared with the comparison cohort. However, among patients older than 64 years, there was no significant relationship between DD and OSA.
Table 4.
Hazard ratios of depressive disorder by age among the sample patients during the one-year follow-up periods
DISCUSSION
We found that during a one-year follow-up, the incidence of DD per thousand person-years was about twice as high among patients with OSA than as those without OSA. Within one year following their diagnosis, patients with OSA were also independently associated with a 2.18 times increased risk of consequent DD, after taking confounders into consideration, including monthly income, geographic region, hypertension, diabetes, CHD, hyperlipidemia, obesity, and alcohol abuse/ alcohol dependency syndrome. After further examining the modifying effects of gender, higher risks of consequent DD occurrence were observed among female patients with OSA than their male counterparts. While increased risks of subsequent DD were observed for patients younger than 40 and 40-64 years old with OSA, no significant relationship between DD and OSA was observed among those with OSA older than 64 years.
Many previous studies exploring the link between OSA and DD have been cross-sectional or retrospective. For example, Aloia et al. conducted a retrospective study and recruited 93 patients from sleep clinics and found apnea severity (percent of sleep time < 90% oxygen saturation) to contribute to depressive symptomatology in OSA.9 Another study conducted on this association was a population-based prospective study with 1,408 community participants designed to assess OSA as a longitudinal predictor of depression. Compared with those without OSA, the odds of developing depression during a 4-year interval were increased by 1.6-, 2.0-, and 2.6-fold for participants with minimal, mild, and moderate or worse OSA, respectively.8 Nevertheless, a null association between OSA and DD was observed in two retrospective studies.7,20 Methodological variation (e.g., different populations, study designs, disease definitions, and assessment instruments) or limitations (e.g., inappropriate consideration of confounding effects) may render comparison between investigations difficult. After considering confounding factors, we confirmed a prospective link between OSA and subsequent DD within the first year following OSA diagnosis in this nationwide population-based study.
Regarding the modifying effects of gender, Enright et al. examined 5,201 community adults aged 65 years and older and observed apneas to be associated with depression in women but not in men.10 Consistent with previous findings,12,21,22 our large-scale study identified women as having higher risks of subsequent DD within the first year following OSA diagnosis. This might reflect a more general finding that depression is more prevalent in women than in men.23
The underlying mechanisms explaining the association between OSA and DD are not clearly delineated. Yet, a biological plausibility exists. First, sleep fragmentation or oxygen desatu-ration during sleep in OSA patients may impact the presentation of mood symptoms, although the results regarding this possibility are mixed. In a randomized controlled trial, hypoxia in OSA was shown to potentially associate with depression, as a significant reduction of depressive symptoms was observed for patients with OSA receiving oxygen therapy.24 Recent preliminary imaging data also suggested that hypoxemia linked with OSA might play a part in affecting mood.1 On the other hand, sleep fragmentation, mainly causing excessive daytime sleepiness in OSA was proposed to contribute to the depressive symptomatology of OSA.25 Nevertheless, depression was also not found to be associated with either sleep fragmentation or hypoxia in OSA.26 This issue will need to be further clarified in the future with studies utilizing larger sample sizes and the appropriate consideration of confounders (e.g., body mass index, hypertension).
Second, it is possible that there is a shared signaling pathway possibly involving proinflammatory markers, neurotransmitters, or undisclosed underlying factors between the two conditions. While OSA was associated with increased levels of IL-6 and tumor necrosis factor,27 an immune response implicating proinflammatory cytokines IL-1, IL-6, and interferons was observed among patients with DD.28 Several excitatory and inhibitory neurotransmitters, such as serotonin, norepinephrine, and γ-aminobutyric acid (GABA), may also be involved in both the sleep/wake cycle and mood regulation. Shared common risk factors (e.g., obesity, cardiovascular disease, metabolic syndrome) are another plausible explanation. Finally, as depression was prevalently observed in patients with chronic medical diseases,29 OSA may decrease patients' quality of life, further leading to other chronic diseases including depression.11,30
Our study contributes to the emerging findings regarding the association between OSA and DD. As comorbid DD was found to exacerbate OSA and to have a negative impact on self-management and treatment adherence of chronic medial illness such as OSA,31,32 it is possible that prompt detection and appropriate treatment of DD can aid in the management of OSA. We suggest that clinicians should be more aware of the frequently observed link between OSA and DD. Since patients with OSA might not voluntarily voice mood symptoms in the context of a sleep evaluation, regular screening and monitoring of psychiatric condition among patients with OSA are needed, especially among women. Proper and timely referral for assessment and treatment of mood symptoms, not just the treatment of OSA itself, might assist in promoting patients' well-being and reducing the detrimental health consequences that might follow.
Our study has several strengths. Important confounders were carefully addressed. Both OSA and DD have independently been reported to associate with metabolic syndrome and cardiovascular disease.33,34 Potential confounding factors (e.g., obesity, hypertension, diabetes) that may impact the connection between OSA and DD were taken into consideration in our analysis. In addition, as consideration of the modifying effects of gender was felt to be of great importance in elucidating the prospective link between OSA and depression,11 we used a nationwide population-based dataset with ample sample size to clarify this issue. Finally, established clinical diagnostic criteria were used for the identification of DD and OSA, with the diagnosis of the latter stipulating the confirmatory results of polysomnography to ensure for diagnostic validity. Furthermore, in Taiwan a diagnosis of DD can only be made by psychiatrists.
However, four limitations merit attention. First, the LHID2000 database represents patients who had sought treatment for OSA and DD. OSA is a highly prevalent but underdiagnosed illness.3 While the diagnosis of DD is based on DSM-IV criteria,35 certain factors, such as socioeconomic status and social stigma might lead some psychiatric patients to decline healthcare services. Moreover, the symptoms of these illnesses are often overlooked, potentially leading to underappreciation, underdiagnosis, and undertreatment.36,37 Secondly, although our study only included newly diagnosed cases to avoid potential effects of chronicity, it should be kept in mind that some of them may not be newly developed OSA cases. Patients might have had undetected OSA for years before being diagnosed. Thirdly, the subsequent risk of depression could not be analyzed or compared between patients with treated and untreated OSA in our study. As treatment for OSA is not covered under the National Health Insurance Program, this information is unavailable in our claims dataset. However, to the best of our knowledge most OSA patients are left untreated. Currently, the most effective treatments for OSA are devices (e.g., continuous positive airway pressure [CPAP]) that may be uncomfortable to use. Therefore, because of out-of-pocket costs and difficulties becoming accustomed to using these devices, most patients are left untreated. Finally, referral bias should be of concern for studies investigating clinical populations. It is possible that the frequent medical consultation of patients with OSA might prompt referrals and boost the detection of DD. Meanwhile, due to the overlap of symptoms in OSA and DD, we are not able to rule out the possibility that symptoms of DD might be attributed to a prior diagnosis of OSA and work to reduce further identification of DD.
A prospective link between OSA and subsequent DD within the first year following OSA diagnosis was confirmed in our study. Further studies should seek to elucidate the underlying pathophysiological mechanisms for this link. Studies on the effect of appropriate management of OSA on the subsequent risk of DD might help yield strategies for more effective prevention and intervention programs.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, Taiwan and managed by the National Health Research Institutes. The interpretations and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Research Institutes.
REFERENCES
- 1.Schroder CM, O'Hara R. Depression and obstructive sleep apnea (OSA) Ann Gen Psychiatry. 2005;4:13. doi: 10.1186/1744-859X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive sleep apnea and depression: a review. Innov Clin Neurosci. 2011;8:17–25. [PMC free article] [PubMed] [Google Scholar]
- 4.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 5.Sforza E, de Saint Hilaire Z, Pelissolo A, Rochat T, Ibanez V. Personality, anxiety and mood traits in patients with sleep-related breathing disorders: effect of reduced daytime alertness. Sleep Med. 2002;3:139–45. doi: 10.1016/s1389-9457(01)00128-9. [DOI] [PubMed] [Google Scholar]
- 6.Phillips BA, Berry DT, Lipke-Molby TC. Sleep-disordered breathing in healthy, aged persons. Fifth and final year follow-up. Chest. 1996;110:654–8. doi: 10.1378/chest.110.3.654. [DOI] [PubMed] [Google Scholar]
- 7.Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest. 1998;114:697–703. doi: 10.1378/chest.114.3.697. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 9.Aloia MS, Arnedt JT, Smith L, Skrekas J, Stanchina M, Millman RP. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005;6:115–21. doi: 10.1016/j.sleep.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Enright PL, Newman AB, Wahl PW, Manolio TA, Haponik EF, Boyle PJ. Prevalence and correlates of snoring and observed apneas in 5,201 older adults. Sleep. 1996;19:531–8. doi: 10.1093/sleep/19.7.531. [DOI] [PubMed] [Google Scholar]
- 11.Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. 2009;13:437–44. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–14. [PubMed] [Google Scholar]
- 13.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–42. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 14.Lin HC, Xirasagar S, Chen CH, Hwang YT. Physician's case volume of intensive care unit pneumonia admissions and in-hospital mortality. Am J Respir Crit Care Med. 2008;177:989–94. doi: 10.1164/rccm.200706-813OC. [DOI] [PubMed] [Google Scholar]
- 15.Kang JH, Lin HC. Obstructive sleep apnea and the risk of autoimmune diseases: A longitudinal population-based study. Sleep Med. 2012;13:583–8. doi: 10.1016/j.sleep.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Sheu JJ, Wu CS, Lin HC. Association between obstructive sleep apnea and sudden sensorineural hearing loss: a population-based case-control study. Arch Otolaryngol Head Neck Surg. 2012;138:55–9. doi: 10.1001/archoto.2011.227. [DOI] [PubMed] [Google Scholar]
- 17.Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285–9. doi: 10.1016/j.sleep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Carroll JL, McLoughlin GM. Diagnostic criteria for obstructive sleep apnea in children. Pediatr Pulmonol. 1992;14:71–4. doi: 10.1002/ppul.1950140202. [DOI] [PubMed] [Google Scholar]
- 19.Gozal D. Obstructive sleep apnea in children: implications for the developing central nervous system. Semin Pediatr Neurol. 2008;15:100–6. doi: 10.1016/j.spen.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCall WV, Harding D, O'Donovan C. Correlates of depressive symptoms in patients with obstructive sleep apnea. J Clin Sleep Med. 2006;2:424–6. [PubMed] [Google Scholar]
- 22.Wahner-Roedler DL, Olson EJ, Narayanan S, et al. Gender-specific differences in a patient population with obstructive sleep apnea-hypopnea syndrome. Gend Med. 2007;4:329–38. doi: 10.1016/s1550-8579(07)80062-3. [DOI] [PubMed] [Google Scholar]
- 23.Weissman MM, Klerman GL. Sex differences in the epidemiology of depression. Arch Gen Psychiatry. 1977;34:98–111. doi: 10.1001/archpsyc.1977.01770130100011. [DOI] [PubMed] [Google Scholar]
- 24.Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 25.Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94:1023–7. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- 26.Bardwell WA, Berry CC, Ancoli-Israel S, Dimsdale JE. Psychological correlates of sleep apnea. J Psychosom Res. 1999;47:583–96. doi: 10.1016/s0022-3999(99)00062-8. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hyper-cytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 28.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29:147–55. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335–49. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 31.Evans DL, Charney DS, Lewis L, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–89. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Akashiba T, Kawahara S, Akahoshi T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–5. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 33.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0. [DOI] [PubMed] [Google Scholar]
- 34.Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4:261–72. [PMC free article] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. [Google Scholar]
- 36.Hossain JL, Shapiro CM. The prevalence, cost implications, and management of sleep disorders: an overview. Sleep Breath. 2002;6:85–102. doi: 10.1007/s11325-002-0085-1. [DOI] [PubMed] [Google Scholar]
- 37.Kleinberg A, Aluoja A, Vasar V. Help-seeking for emotional problems in major depression: findings of the 2006 Estonian Health Survey. Community Ment Health J. 2012 Feb 4; doi: 10.1007/s10597-012-9499-9. [epub ahead of print] [DOI] [PubMed] [Google Scholar]


