Abstract
Introduction:
Few studies have compared the effect of surgical and conservative weight loss strategies on obstructive sleep apnea (OSA). We hypothesized that Roux-en-Y gastric bypass (RYGB) would be more effective than intensive lifestyle intervention (ILI) at reducing the prevalence and severity of OSA (apnea-hypopnea-index [AHI] ≥ 5 events/hour).
Methods:
A total of 133 morbidly obese subjects (93 females) were treated with either a 1-year ILI-program (n = 59) or RYGB (n = 74) and underwent repeated sleep recordings with a portable somnograph (Embletta).
Results:
Participants had a mean (SD) age of 44.7(10.8) years, BMI 45.1(5.7) kg/m2, and AHI 17.1(21.4) events/hour. Eighty-four patients (63%) had OSA. The average weight loss was 8% in the ILI-group and 30% in the RYGB-group (p < 0.001). The mean (95%CI) AHI reduced in both treatment groups, although significantly more in the RYGB-group (AHI change -6.0 [ILI] vs -13.1 [RYGB]), between group difference 7.2 (1.3, 13.0), p = 0.017. Twenty-nine RYGB-patients (66%) had remission of OSA, compared to 16 ILI-patients (40%), p = 0.028. At follow-up, after adjusting for age, gender, and baseline AHI, the RYGB-patients had significantly lower adjusted odds for OSA than the ILI-patients—OR (95% CI) 0.33 (0.14, 0.81), p = 0.015. After further adjustment for BMI change, treatment group difference was no longer statistically significant—OR (95% CI) 1.31 (0.32, 5.35), p = 0.709.
Conclusion:
Our study demonstrates that RYGB was more effective than ILI at reducing the prevalence and severity of OSA. However, our analysis also suggests that weight loss, rather than the surgical procedure per se, explains the beneficial effects.
Citation:
Fredheim JM; Rollheim J; Sandbu R; Hofsø D; Omland T; Røislien J; Hjelmesaeth J. Obstructive sleep apnea after weight loss: a clinical trial comparing gastric bypass and intensive lifestyle intervention. J Clin Sleep Med 2013;9(5):427-432.
Keywords: General, bariatric surgery, obstructive sleep apnea, weight loss
Obstructive sleep apnea (OSA) is a commonly unrecognized condition with an especially high prevalence among morbidly obese subjects.1,2 OSA is characterized by apneas and hypopneas due to the collapse of upper airways and is defined by ≥ 5 apneas or hypopneas per hour during sleep, as measured by the apnea-hypopnea index (AHI). Mild OSA is defined as 5-15 episodes of apneas or hypopneas per hour, moderate OSA 15-30, and severe OSA ≥ 30. A meta-analysis from 2009 concluded that continuous positive airway pressure (CPAP) is a cost-effective treatment of OSA and that it reduces daytime sleepiness compared to placebo or usual care.3 Accordingly, CPAP is the first-line treatment and is recommended for all patients with moderate to severe OSA (AHI ≥ 15).4
Obesity, older age, male sex, and heredity are well-established risk factors for OSA,5 with obesity being the single most important modifiable risk factor. In cases of obesity, fat deposits narrow the upper airways, and abdominal fat masses decrease the tracheal tension, both increasing the collapsibility of the upper airways. If untreated, OSA is associated with increased risk of diabetes, cardiovascular disease, driving accidents, and all-cause mortality.6
BRIEF SUMMARY
Current Knowledge/Study Rationale: Weight reduction reduces the severity of OSA in obese subjects, but the relative efficacy of lifestyle modifications versus bariatric surgery remains to be established. We aimed to compare the effectiveness of lifestyle modification and bariatric surgery in terms of reducing AHI and the severity of OSA.
Study Impact: Gastric bypass surgery was more effective than intensive lifestyle intervention at reducing the prevalence and severity of OSA in a population of treatment seeking morbidly obese patients, and the beneficial effect of surgery seemed to be mediated by weight loss. Weight reduction should have a high priority in the treatment of obstructive sleep apnea in morbidly obese patients.
The prevalence of OSA in the Wisconsin Sleep Cohort study was 9% among women and 24% among men.7 Recently, we demonstrated a much higher prevalence of OSA among the morbidly obese: 55% in women and 80% in men.2
Despite the close relationship between OSA and obesity, only a few recently published randomized controlled trials have assessed the effect of weight loss on obstructive sleep apnea.8–10 Tuomilehto et al. examined the effect of a very low calorie diet followed by lifestyle counseling for one year in overweight and obese patients with mild OSA.8 The intervention group had significantly lower odds of having OSA at follow-up than the control group: OR 0.24 (95% CI 0.08, 0.72). Isolated physical activity without weight loss may also have a positive effect on OSA.11 Exercise leads to more muscles and less fat; the lower pharyngeal pressure from diminished fat deposits might decrease the severity of OSA.
In the Sleep AHEAD study, intensive lifestyle changes in overweight and obese patients with type 2 diabetes and mild to severe OSA were associated with a significant improvement in AHI.9 Finally, Johansson et al. reported that in obese men with moderate to severe OSA, a very low energy 9-week diet improved OSA, particularly severe OSA, compared to a control group who adhered to their usual diet.10
A recent meta-analysis of 12 surgical studies including 342 patients argued that patients undergoing bariatric surgery should not expect to be cured of OSA after surgically induced weight loss. Accordingly, some surgically treated patients will still be in need of continued CPAP treatment.12 In another meta-analysis the authors were unable to conclude whether surgically induced weight loss was associated with greater improvements in OSA than conservative treatment.13 Further, it remains unknown whether RYGB may influence the severity of OSA through a mechanism other than weight loss. To summarize, weight reduction reduces the severity of OSA, but the relative efficacy of lifestyle modifications versus bariatric surgery in terms of reducing AHI remains to be established.
Our main hypothesis was that Roux-en-Y gastric bypass (RYGB) would be more effective than intensive lifestyle intervention (ILI) at reducing the prevalence and severity of OSA. To address this issue, we performed sleep recordings both before the start of each intervention type and one year after.
METHODS
Study Design
This study is part of a non-randomized, controlled clinical trial; the MOBIL-study (Morbid Obesity treatment, Bariatric surgery versus Intensive Lifestyle intervention Study) (ClinicalTrials.gov number NCT00273104). The design and setting of the study has previously been described in detail.14 A brief summary of materials and methods is given below.
Setting
The study was conducted at the Morbid Obesity Centre (MOC) under the auspices of Vestfold Hospital Trust, a regional tertiary care center in Health Region South East, Norway. The intensive lifestyle intervention programme was carried out at Evjeklinikken, a center specializing in obesity treatment.
All eligible patients underwent a thorough (up to 6 month) assessment by a multidisciplinary team consisting of an internist, a dietician, a physiotherapist, and a trained “obesity” nurse. During the first visit, the internist established a detailed medical history, checked previous diagnostic workups, performed a physical examination, and briefly informed the patients of further investigations and treatment alternatives. At the second visit the doctor reiterated this message, providing complete information about the possible risks and benefits of an operation and also encouraged the patients to incorporate their own values and preferences in the decision-making process. If no contraindication against surgery existed, the patient and the physician together agreed upon the most appropriate choice of therapy, either surgical or conservative.15
Participants
The patients were recruited from the MOC and the study approved by the Regional Ethics Committee (ClinicalTrials.gov-registry trial number NCT00273104). All patients provided written informed consent. All patients were morbidly obese, i.e., BMI ≥ 40 kg/m2 or BMI ≥ 35 kg/m2 with at least one obesity related comorbidity.16
A total of 139 patients completed the MOBIL study—63 in the ILI-group and 76 in the RYGB-group. After the exclusion of 6 patients with missing sleep registrations, (4 in the ILI-group and 2 in the RYGB group), 133 patients (93 females), 59 in the ILI group and 74 in the RYGB group were eligible for inclusion.
Intervention
The patients in the ILI-group underwent a 1-year lifestyle program at a rehabilitation center (Evjeklinikken, Norway). This program comprised 4 intermittent stays, one lasting 2 weeks and 3 lasting 1 week each (total, 7 weeks). The program included organized physical activity (3-4 h), psychosocially oriented interventions, and individual consultations with a medical doctor, nutritionist, physiotherapist, and trained nurse. Those leading the counseling interviews were trained in motivational interviewing, a client-centered counseling style that aims to invoke behavioral changes. The patients also took part in group sessions focusing on the emotional aspects of sedentary behavior and classroom lessons on topics related to nutrition, physical activity, and comorbidities. Patients were encouraged to follow the guidelines of the Norwegian National Council of Nutrition, which recommend that the daily intake of protein, fat, and carbohydrate, should account for 10% to 20%, < 30%, and 50% to 60% of energy consumed, respectively.14 When at home, patients were encouraged to self-monitor their lifestyle habits (e.g., by keeping a physical activity diary and a food diary) and visit their general practitioner for weight monitoring. They were contacted by telephone every 2 weeks. The treatment aim was ≥ 10% weight loss. Vitamin supplements were not prescribed.
Roux-en-Y gastric bypass was performed laparoscopically with a gastric pouch of about 25 mL.14 In order to reduce liver size, surgical patients consumed a low-calorie diet (900 kcal) for 3 weeks preoperatively.
Variables
An apnea event was defined as ≥ 90% reduction in baseline nasal air flow lasting ≥ 10 seconds. Hypopnea events were defined as a 50% to 90% decrease in pre-event nasal air flow lasting ≥ 10 sec accompanied by ≥ 3% drop in oxygen saturation and/or signs of awakening or increased stress. Both supine and non-supine values of AHI were recorded, given that these might have clinical implications. Oxygen desaturation index (ODI) was defined as the number of episodes with ≥ 3% drop in oxygen saturation per hour. Oxygen saturation (SpO2), both mean and lowest value through the night, was measured by a finger pulse oximeter.
The primary outcome variables were changes in the prevalence and severity of OSA in both treatment groups. OSA was addressed as both a continuous (AHI) and categorical variable (OSA; yes/no, if yes: mild, moderate, severe). Other outcome variables were resolution of OSA (AHI cutoff 5) and improvement of moderate or severe OSA (AHI ≥ 15) to mild or no OSA. OSA was categorized as mild (AHI 5-14), moderate (AHI 15-29), or severe (AHI ≥ 30). Scoring rules were in accordance with the 2007 American Academy of Sleep Medicine (AASM) manual for the scoring of sleep.17
The main explanatory variable was treatment group. Other possible explanatory or confounding variables were age, gender, anthropometric measures (BMI, weight, and neck circumference), and baseline AHI.
Data Sources/Measurement
Portable monitors were used for somnography such that each patient could be monitored in their natural sleeping habitat with their everyday pre-bed rituals. Portable sleep diagnostic systems like the Embletta have a high sensitivity and are considered reliable in the diagnosis of OSA, as there are few false positive results compared to polysomnography.18,19
For the sleep registrations, we used Embletta, a portable multi-channel recorder consisting of a nasal cannula, 2 piezoelectric belts, a finger pulse oximeter, and a body position detector. To monitor respiratory movements, 2 piezoelectric belts were placed around the thorax and abdomen. To avoid interrater variation, Embletta recordings were manually scored by the same person (JMF).
Patients received both written and oral instructions regarding equipment usage. Patients equipped themselves, and registrations were manually scored the following day. Treatment was provided according to current guidelines.4 Patients already using CPAP had a one-week washout period prior to the sleep registration where they did not use the machine. A case report form (CRF) was completed for all patients.
Statistical Methods
Data are given as either mean (SD) or proportions (%) unless stated otherwise. All patients (n = 133) were included in the analysis of the change in prevalence of OSA, while only patients with OSA at baseline (n = 84) were included in the analysis of changes in the severity of OSA. Skewed data were transformed using natural logarithms. Between-group comparisons at baseline were analyzed using independent samples t-test for continuous variables and Fisher exact test for categorical variables. Within-group comparisons were performed using paired samples t-test for continuous variables and McNemar test for dichotomized variables. Between-group differences in outcome variables were assessed using t-test, Fisher exact test, and multiple logistic regression analyses with predefined explanatory variables.
In the first logistic regression model, the effect of treatment choice on the prevalence of OSA was adjusted for established confounding factors; baseline AHI, age, and gender. In a second analysis we further adjusted for BMI change.
In supplementary analyses, BMI change was replaced with either weight change or neck circumference. AHI cutoffs at both 5 and 15 events/h were used in the regression analyses.
To address the issue of multicollinearity, we assessed the variance inflation factor (VIF) in the logistic regression models. Statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline Characteristics
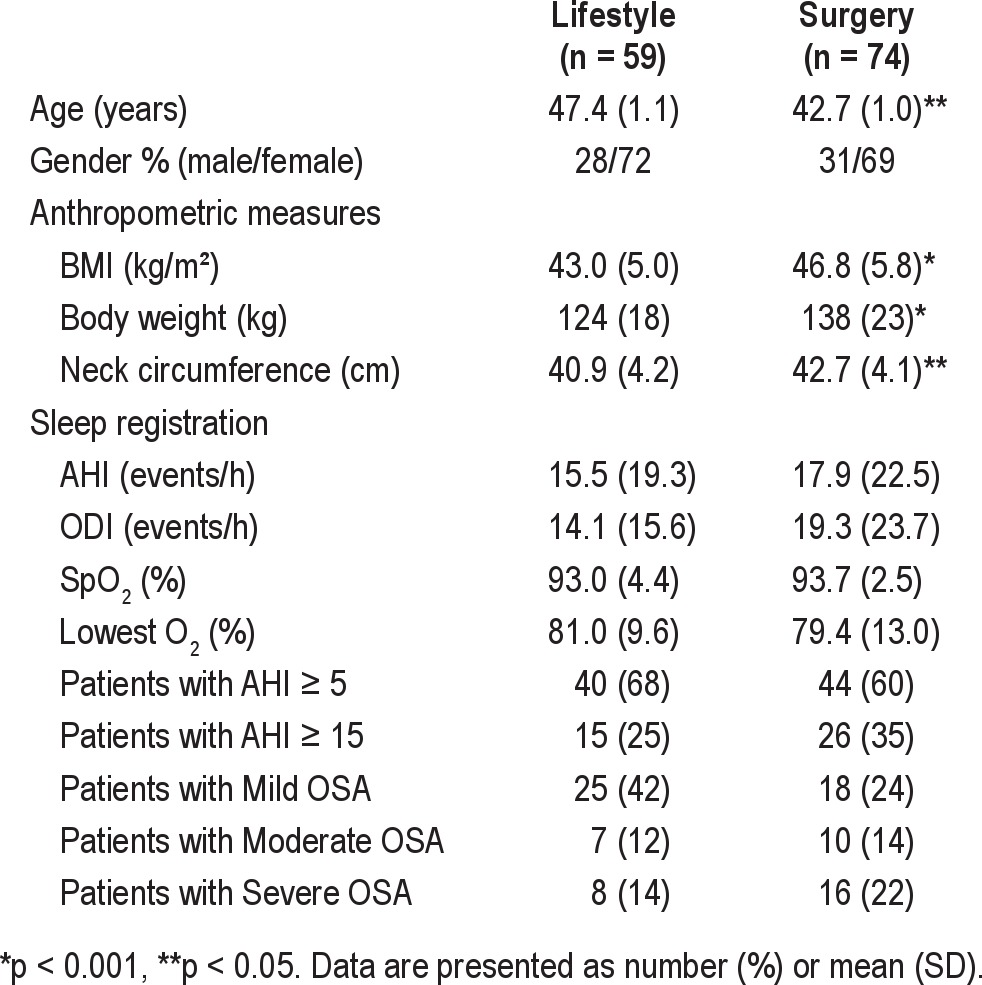
Baseline demographic and anthropometric characteristics of the 133 morbidly obese patients are listed in Table 1. A total of 84 patients (63%) had OSA (32% mild, 13% moderate, and 18% severe). The prevalence of mild, moderate, and severe OSA did not differ significantly between groups (p = 0.159). The mean AHI, ODI, SpO2, and lowest SpO2 did not differ significantly between the 2 intervention groups at baseline (all p > 0.16). The RYGB patients were significantly heavier and younger than the ILI patients.14
Table 1.
Baseline demographics, anthropometric characteristics, and sleep registration data in 133 morbidly obese subjects by treatment group (intensive lifestyle intervention or Roux-en-Y gastric bypass surgery)
Effect of the Interventions
Compared with baseline, the prevalence of OSA was significantly lower after treatment in both the ILI-group (46% vs 68%) and the RYGB-group (20% vs 60%) (Figure 1). In addition, a significant number of patients changed from an AHI ≥ 15 events/h (requiring CPAP) at baseline to AHI < 15 (not requiring CPAP) at follow-up: 19 subjects (73%) in the RYGB group and 8 subjects (53%) in the ILI group, within-group difference, p < 0.001 and 0.039, respectively, between-group difference p = 0.306 (Figure 1).
Figure 1. Percentage of patients with specified AHI at baseline and after 1-year of treatment.
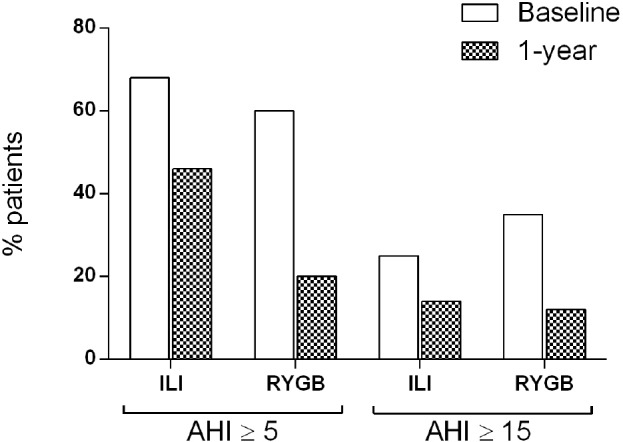
Proportion of patients with an apnea-hypopnea index (AHI) ≥ 5 events/h and AHI ≥ 15 events/h, respectively, in 133 morbidly obese patients before (baseline) and after (1-year) treatment through an intensive lifestyle intervention (ILI) program or Roux-en-Y gastric bypass (RYGB) surgery.
Among the 84 patients with OSA at baseline OSA severity reduced in both groups, although significantly more in the RYGB group than in the ILI group (Table 2). AHI reduced significantly more in the RYGB than in the ILI group, mean between group difference (95% CI) 7.2 (4.4, 21.2) events/h, p = 0.003. BMI-change correlated significantly with the change in AHI, r = 0.273, p = 0.001. Mean SpO2 improved in both treatment groups, but was significantly better in the RYGB group. Snoring improved in both groups, but there was no significant difference between groups.
Table 2.
Sleep registration and anthropometric characteristics in 84 morbidly obese patients with OSA (AHI ≥ 5) at baseline and 1-year follow-up, according to treatment group (intensive lifestyle intervention or Roux-en-Y gastric bypass surgery)

At follow-up, remission of OSA (AHI < 5 events/h) was registered in 29 of 44 (66%) RYGB patients and 16 of 40 (40%) ILI patients, between group difference p = 0.028. Another 5 (13%) ILI patients and 7 (16%) RYGB patients improved to a less severe level of OSA at follow-up (Figure 2). A total of 3 patients in the ILI group and one in the RYGB group deteriorated from no OSA to mild OSA. One RYGB patient and two ILI patients with mild OSA deteriorated to moderate OSA, and one patient in the ILI group deteriorated from moderate to severe OSA. AHI was reduced, with 0.93 events/h per kg weight reduction in the ILI group and 0.52 in the RYGB group, mean between-group difference (95% CI) 0.41 (-0.6, 1.4), p = 0.405.
Figure 2. Change in OSA category after treatment.

Percentage distribution of change in OSA category (none, mild, moderate, or severe) of 84 morbidly obese OSA patients treated with either intensive lifestyle intervention (ILI, 40 patients) or Roux-en-Y gastric bypass surgery (RYGB, 44 patients).
Predictors of OSA at One Year
Treatment choice was an independent predictor of the presence of OSA (AHI ≥ 5 events/h) at one year after adjusting for age, gender, and baseline AHI. The surgical patients had a 67% lower odds of OSA at one year (OR [95% CI] 0.33 [0.14, 0.81], p = 0.015). After adding BMI change to this model treatment group assignment was no longer significant: OR (95% CI) 1.31 (0.32, 5.35), p = 0.709. There was no significant interaction between gender and treatment group in a multivariate logistic regression model (data not shown).
DISCUSSION
Our findings suggest that gastric bypass surgery is more effective than intensive lifestyle intervention at reducing the prevalence and severity of OSA in morbidly obese patients.
Although the proportion of patients with remission of OSA was higher in the RYGB group than in the ILI group, it should be noted that 2 of 5 patients in the ILI group had remission of OSA (compared to 2 of 3 in the RYGB group). The beneficial effect of ILI on remission of OSA should not therefore be underestimated.
To the best of our knowledge, no prospective clinical trial has compared the effects of bariatric surgery and intensive lifestyle intervention on the severity or resolution of OSA. Our findings are, however, in accordance with previous observational studies addressing the effect of bariatric surgery20–22 and lifestyle intervention.8,9 In accordance with our findings, a recent meta-analysis of 12 studies focusing on the effects of bariatric surgery reported an average 71% reduction in AHI events.12 The ILI patients reduced their weight by approximately 10% and their AHI by approximately 40%. This also corresponds well with the findings of a study that found a 50% reduction in AHI when patients reduced their body weight by 10% to 15%.23
Although the beneficial effect on OSA was more pronounced in the RYGB group, multivariate analyses indicated that this effect seemed to be mediated by weight loss rather than treatment choice. It is widely accepted that weight loss leads to improvement in OSA24; comparing different methods of weight reduction and their effects on OSA might therefore be useful in the determination of appropriate treatment options. Body composition and the distribution of muscles and fat deposits also play a role in the pathogenesis of OSA, not only body weight.
We have previously published data demonstrating a greater increase in the physical activity levels of the lifestyle group than the surgery group.14 We cannot exclude the possibility of an independent effect of physical activity on sleep apnea, but, unfortunately, our study was not powered to address this hypothesis. The majority of patients with absolute indication for CPAP (AHI ≥ 15) at baseline improved to mild/no OSA at follow-up—73% in the RYGB group and 53% in the ILI group. The apparent lack of significant difference between groups might be explained by the relatively low number of patients (type II error). Nonetheless, CPAP is an effective treatment option, but many patients struggle with the use of the machine. Compliance is at best moderate, and prescription of the device is considered by some patients to be stigmatizing. Even though CPAP use helps improve quality of life,25 one should not disregard the many reservations patients might have. The positive effects of CPAP cessation, often overlooked, ought to be further investigated. Our results indicate that both RYGB and ILI may reduce the need for CPAP-treatment in morbidly obese patients.
The strengths of the present study include the prospective design and the relatively high number of patients in two comparable groups. Although the RYGB subjects were slightly heavier and younger, the two treatment groups were not significantly different in terms of baseline AHI and gender. This study has a number of limitations. Firstly, it suffers from a lack of randomization, which was after ethical and legal consultations considered unethical.14 Our center is a public health care provider; according to national guidelines patients should be offered either conservative or surgical therapy in an obesity clinic based on the multidisciplinary team model.15 Randomization to surgery would therefore be obsolete if the patient both wanted and qualified for a lifestyle intervention program, and vice versa. Second, as the intensive lifestyle intervention was delivered in the form of a residential program this could limit the generalizability of the results. Further, compliance with respect to physical activity and CPAP usage was not registered. Intakes of alcohol and sedative drugs were not addressed.
The subjects were mainly Caucasians, limiting the generaliz-ability of our findings to other ethnicities. We used a portable sleep recorder, which is the most widely used device in Scandinavia. Consequently, we could not score sleep stages and had to rely on a sleep diary and time in bed to define when patients slept.
CONCLUSION
Gastric bypass surgery was more effective than intensive lifestyle intervention at reducing the prevalence and severity of OSA in a population of treatment seeking morbidly obese patients. The beneficial effect of surgical treatment seemed to be mediated by weight loss rather than RYGB per se. Intensive lifestyle intervention was also associated with significant improvements in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Shah N, Roux F. The relationship of obesity and obstructive sleep apnea. Clin Chest Med. 2009;30:455–65. vii. doi: 10.1016/j.ccm.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Fredheim JM, Rollheim J, Omland T, et al. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13:iii–xiv. 1. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 4.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 5.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–93. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 8.Tuomilehto HP, Seppa JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 9.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson K, Neovius M, Lagerros YT, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline CE, Crowley EP, Ewing GB, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34:1631–40. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax. 2012;67:442–9. doi: 10.1136/thx.2010.151225. [DOI] [PubMed] [Google Scholar]
- 14.Hofso D, Nordstrand N, Johnson LK, et al. Obesity related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass and intensive lifestyle intervention. Eur J Endocrinol. 2010;163:735–45. doi: 10.1530/EJE-10-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakobsen GS, Hofso D, Roislien J, Sandbu R, Hjelmesaeth J. Morbidly obese patients--who undergoes bariatric surgery? Obes Surg. 2010;20:1142–8. doi: 10.1007/s11695-009-0053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de KS, Husler R, Banic A, Constantinescu MA. Body contouring surgery following bariatric surgery and dietetically induced massive weight reduction: a risk analysis. Obes Surg. 2009;19:553–9. doi: 10.1007/s11695-008-9659-8. [DOI] [PubMed] [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Ng SS, Chan TO, To KW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnoea syndrome (OSAS) Respirology. 2010;15:336–42. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 19.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Respir J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
- 20.Ramalingam G, Anton CK. Our 1-year experience in laparoscopic sleeve gastrectomy. Obes Surg. 2011;21:1828–33. doi: 10.1007/s11695-011-0484-0. [DOI] [PubMed] [Google Scholar]
- 21.Charuzi I, Lavie P, Peiser J, Peled R. Bariatric surgery in morbidly obese sleep-apnea patients: short- and long-term follow-up. Am J Clin Nutr. 1992;55:594S–596S. doi: 10.1093/ajcn/55.2.594s. [DOI] [PubMed] [Google Scholar]
- 22.Valencia-Flores M, Orea A, Herrera M, et al. Effect of bariatric surgery on obstructive sleep apnea and hypopnea syndrome, electrocardiogram, and pulmonary arterial pressure. Obes Surg. 2004;14:755–62. doi: 10.1381/0960892041590773. [DOI] [PubMed] [Google Scholar]
- 23.Smith PL, Gold AR, Meyers DA, Haponik EF, Bleecker ER. Weight loss in mildly to moderately obese patients with obstructive sleep apnea. Ann Intern Med. 1985;103:850–5. doi: 10.7326/0003-4819-103-6-850. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16:563–9. doi: 10.1007/s11325-011-0543-8. [DOI] [PubMed] [Google Scholar]


