Abstract
Study Objectives:
To study the possible predictive value of drug-induced sleep endoscopy (DISE) in assessing therapeutic response to implanted upper airway stimulation (UAS) for obstructive sleep apnea (OSA).
Methods:
During DISE, artificial sleep is induced by midazolam and/or propofol, and the pharyngeal collapse patterns are visualized using a flexible fiberoptic nasopharyngoscope. The level (palate, oropharynx, tongue base, hypopharynx/epiglottis), the direction (anteroposterior, concentric, lateral), and the degree of collapse (none, partial, or complete) were scored in a standard fashion.
Results:
We report on the correlation between DISE results and therapy response in 21 OSA patients (apnea-hypopnea index [AHI] 38.5 ± 11.8/h; body mass index [BMI] 28 ± 2 kg/m2, age 55 ± 11 y, 20 male/1 female) who underwent DISE before implantation of a UAS system. Statistical analysis revealed a significantly better outcome with UAS in patients (n = 16) without palatal complete concentric collapse (CCC), reducing AHI from 37.6 ± 11.4/h at baseline to 11.1 ± 12.0/h with UAS (p < 0.001). No statistical difference was noted in AHI or BMI at baseline between the patients with and without palatal CCC. In addition, no predictive value was found for the other DISE collapse patterns documented.
Conclusions:
The absence of palatal CCC during DISE may predict therapeutic success with implanted UAS therapy. DISE can be recommended as a patient selection tool for implanted UAS to treat OSA.
Citation:
Vanderveken OM; Maurer JT; Hohenhorst W; Ha-mans E; Lin HS; Vroegop AV; Anders C; de Vries N; Van de Heyning PH. Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013;9(5):433-438.
Keywords: Electrical stimulation, hypoglossal nerve, neuromodulation, obstructive sleep apnea hypopnea syndrome, prediction, sleep disordered breathing, snoring
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse during sleep, causing hypoxemia and sleep fragmentation that lead to daytime sleepiness and increased risk of cardiovascular incidents, motor vehicle, and occupational accidents. 1–3 The gold standard for the treatment of OSA is continuous positive airway pressure (CPAP).4,5 It has been demonstrated that successful CPAP treatment improves systemic hypertension and prolongs survival.6 However, the clinical effectiveness of CPAP is often limited by low patient acceptance, poor tolerance, and suboptimal compliance.7 Therefore, non-CPAP alternatives for the treatment of sleep disordered breathing, such as oral appliance therapy with custom-made titratable mandibular advancement devices, surgery, or upper airway stimulation (UAS) have gained growing interes t.8–13 UAS therapy, which uses electrical stimulation of the hypoglossal nerve, has been previously reported to be safe and efficacious in a select group of OSA patients who cannot or will not use CPAP as primary treatme nt.13–17 In non-selected OSA patients undergoing UAS therapy, a large interindividual difference in response to stimulation is obser ved.13,14 A recent study by Van de Heyning et al. examined a set of factors predictive for therapy response to UAS.13
BRIEF SUMMARY
Current Knowledge/Study Rationale: Upper airway stimulation (UAS) using respiratory-paced electrical stimulation of the hypoglossal nerve is a safe and efficacious non-CPAP therapy in a well-selected group of patients with moderate to severe obstructive sleep apnea (OSA). Patient baseline characteristics and the pattern and sites of upper airway obstruction are suggested to be predictors of UAS treatment success.
Study Impact: In this study, the predictive value of drug-induced sleep-endoscopy (DISE) was assessed towards UAS treatment success in 21 OSA patients. The results demonstrate a strong correlation between the absence of a complete circular collapse at the level of the palate as documented during DISE and a successful treatment outcome with UAS; based on these results, DISE can be recommended as a patient selection tool for implanted UAS to treat OSA.
Drug-induced sleep endoscopy (DISE) is increasingly being performed, offering the possibility of dynamic upper airway evaluation during artificial sleep as a promising technique for selecting the proper non-CPAP treatment for OSA patients.18,19
The aim of this study was to perform a detailed assessment of the possible predictive value of DISE in the evaluation of therapeutic response to implanted UAS therapy for OSA. Some of the results of this study have been previously reported in the form of an abstract.20
METHODS
We report on OSA patients who underwent a DISE before UAS system implantation.13 Patients with moderate to severe OSA (apnea-hypopnea index [AHI] ≥ 15/h sleep) and BMI < 35 kg/m2 were selected for UAS system implantation if they failed or were intolerant of CPAP treatment. Exclusion criteria included chronic obstructive pulmonary disease, New York Heart Association class III or IV congestive heart failure, neuromuscular diseases, or prior upper airway surgeries not related to OSA. The trial was approved by the institutional review boards or ethics committees at all participating centers, and informed consent was obtained from all study subjects.
Polysomnography
An 18-channel in-laboratory polysomnography examination was conducted according to the American Academy of Sleep Medicine (AASM) guidelines.21 Hypopneas were scored according to the AASM 2007 Rule 4a: a nasal pressure drop ≥ 30% of baseline, duration > 10 sec, ≥ 4% desaturation from baseline, and ≥ 90% of the event duration must meet the amplitude reduction criteria for hypopnea.
Drug-Induced Sleep Endoscopy (DISE)
The DISE procedure was performed by an ENT surgeon in a semi-dark and silent operating room with the patient in supine position.22,23 Continuous monitoring of cardiac rhythms and oxygen saturation was provided.22 Unconscious sedation was induced by intravenous administration of midazolam with a bolus injection of 1.5 mg and/or with propofol using a target-controlled infusion system at a target of 2.0 to 3.0 mcg/mL. During DISE, the level (palate, oropharynx, tongue base, hypopharynx/epiglottis), the direction (anteroposterior [AP], concentric, lateral), and degree of upper airway collapse (none, partial, or complete) were scored in a standard fashion.22–26 The palate is defined as the particular portion of the upper airway at the level of the soft palate and uvula, while the oropharynx is defined by the pharyngeal region at the levels of the tonsils (above the tongue base). The tongue base is defined as the retroglossal area, and the hypopharynx is defined as the upper airway region below the tongue base, including of the tip of the epiglottis. The collapse patterns were assessed during inspiration. All ENT surgeons who performed DISE in the present study were experienced with this procedure, and the DISE videos were assessed by the ENT surgeon who performed the procedure. The mean duration of the procedure was 25 ± 18 minutes.
Upper Airway Stimulation (UAS) System
The UAS system consists of a respiration sensor, a programmable implanted pulse generator, and stimulating electrodes (Inspire Upper Airway Stimulation therapy, Inspire Medical Systems, Minneapolis, MN, USA). The Inspire II Upper Airway Stimulation (UAS) system (Inspire Medical Systems, Maple Grove, MN) consisted of a respiration sensor, programmable implanted pulse generator (IPG), and stimulating electrodes. The sensor detected respiratory efforts from the chest that were analyzed by the IPG. From the sensor signal, the IPG predicted the onset of inspiration, delivering stimulation pulses between the end of expiration and the beginning of the next expiratory phase of each respiratory cycle. The electrical pulses were applied to the hypoglossal nerve through platinum/iridium electrodes. The patient was given a programming device capable of initiating and terminating the UAS therapy. The operative technique of the implantation of the UAS system used in this study has been described in detail previously.13,27
Definition of Treatment Success
The present study used the criteria established by Sher et al. to define treatment success as AHI < 20/h after treatment and an AHI reduction ≥ 50% as compared to baseline.28 Additionally, success rates were assessed for a success definition of AHI < 15/h sleep. To assess daytime sleepiness, patients filled out the Epworth Sleepiness Scale (ESS).29
Statistical Analysis
The pre-implantation AHI was compared to AHI 6 months after implantation. Statistical analysis was performed using MATLAB (The Mathworks, Natick, MA, USA) and Excel (Microsoft Corp, Redmond, WA, USA). A Wilcoxon signed-rank test was used to compare the pre-implantation AHI to the post-implantation AHI. Differences were considered statistically significant if the p-value was less than 0.05. Results were presented as means and standard deviations.
RESULTS
Subjects
DISE videos were recorded for 21 patients with an established diagnosis of moderate to severe OSA before the implantation of the UAS system. Patients were predominantly male, with an average age of 55 ± 11 years, a baseline AHI of 38.5 ± 11.8/h, and a BMI of 28 ± 2 kg/m2 (Table 1).
Table 1.
Patient demographics, including baseline differences between patients with and without complete concentric collapse (CCC) at the level of the palate

DISE Analysis
An overview of the distribution of the levels of upper airway collapse for all patients included in this study based on DISE scoring is provided in Figure 1 and Table 2. The majority of patients (91%) had multilevel collapse, predominantly at the palatal and tongue base levels and rarely at the oropharyngeal and hypopharynx/epiglottis levels (Figure 1). The most common upper airway collapse patterns noted in this study were AP collapse at the levels of the palate (76.2%) and the tongue base (71.4%) (Table 2).
Figure 1. Venn diagram showing the percentages per upper airway level including the percentages of overlap between the different levels in case of multi-level collapse.
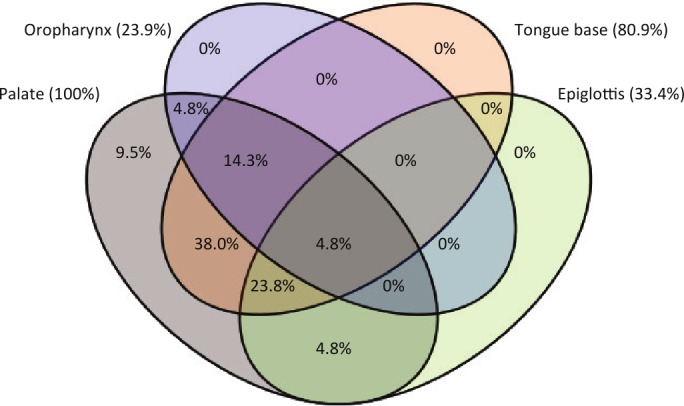
Table 2.
Overview of the distribution of the levels of upper airway collapse including the corresponding direction of upper airway collapse based on DISE scoring (n = 21)

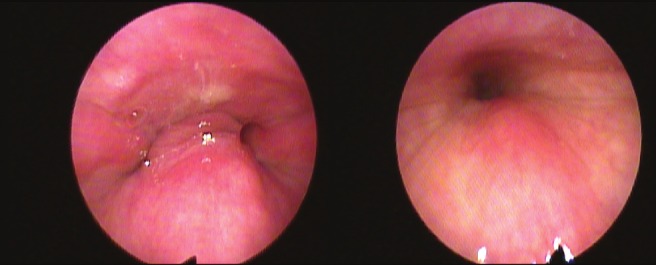
Sixteen patients were categorized as having predominant AP palatal collapse, and 5 were categorized as having complete concentric collapse (CCC) at the palatal level (Figure 2). There was no significant difference in baseline AHI, BMI, or age between patients with and without palatal CCC (Table 1).
Figure 2. Example of anteroposterior (left) versus concentric (right) collapse at the level of the palate during DISE.
In this patient group, 19 of 21 patients had multilevel collapse. All patients had at least a collapse at the level of the palate (Figure 1), whereas tongue base, hypopharynx/epiglottis, and oropharynx collapse were noted in 80.9%, 33.4%, and 23.9% of patients, respectively (Figure 1). Conversely, no patients had tongue base collapse without palatal collapse (Figure 1). The most common combination of multilevel collapse was the combination of AP palatal and AP tongue base collapse without epiglottis collapse, which occurred in 33% of the patients.
UAS Effect on Various Collapse Types
Patients with palatal CCC did not have a significant change in AHI with UAS 6 months after implantation, as baseline AHI was 41.5 ± 13.8/h and AHI with UAS was 48.1 ± 18.7/h, (p = 0.44; Figure 2). The patients without palatal CCC had a significant improvement in AHI with UAS despite multilevel collapse at the palate and tongue base. For this subset of patients, AHI went from 37.6 ± 11.4/h at baseline to 11.6 ± 11.7/h with UAS (p < 0.001; Figure 2). Thirteen patients with both palatal AP and tongue base AP collapse had a significant improvement in the AHI, decreasing from 38.0 ± 10.3 at baseline to 13.6 ± 12.1 with UAS (p < 0.001).
Treatment Success Analysis
The overall UAS treatment success rate for all 21 patients included in this study using Sher's criteria was 62% (13/21). Treatment success in the subset of patients without CCC collapse at the level of the palate was 81% (13/16), while treatment success could not be achieved in any patient with CCC collapse at the level of the palate in this study (0/5). When assessing the success rates specifically for AHI < 15, overall success would be achieved in 11/21 (52.4%) patients. In patients without palatal CCC success would be achieved in 11/16 patients (68.8%); in patients with palatal CCC this would be 0/5 (0%). There was no significant difference in BMI between baseline and 6 months in either group. Overall, ESS improved significantly from baseline 8.2 ± 5.0 to 6.4 ± 4.3 (p = 0.02; n = 18).
DISCUSSION
This study evaluates DISE as a patient selection tool for implanted UAS therapy to treat OSA. The results of this study indicate that the absence of CCC at the level of the palate as documented during DISE may predict therapeutic success for OSA patients with implanted UAS therapy. These finding are highly relevant to the field, as previous studies have indicated that the application of hypoglossal nerve stimulation in non-selected OSA patients leads to high interindividual variation in therapeutic effectiveness.13,15–17,30 The role of DISE in patient selection for implanted UAS therapy was recently addressed; however, the present study population consists of 5 additional subjects and provides specific demographics of the various combinations of multilevel upper airway collapse in this population. Patients with palatal CCC did not have a significant change in AHI with UAS 6 months after implantation (AHI 41.5 ± 13.8/h at baseline versus 48.1 ± 18.7/h with UAS), suggesting that the tongue protrusion resulting from the UAS therapy is not sufficient to overcome the airway obstruction in patients with CCC at the level of the palate. The results of this study also suggest that the stimulation at the base of tongue can potentially overcome AP obstruction not only at the base of tongue level but also at the palatal level. A statistically significant reduction in AHI was seen in patients with both AP palatal and tongue base collapse, suggesting that UAS can resolve multilevel collapse. One possible explanation might be that an AP palatal collapse is due to a tongue base obstruction pushing back the soft palate. In this concept, UAS is able to maintain airway patency as the tongue base is directly hindered from obstruction which keeps the palate in an anterior position. These results showing that palatal CCC prohibits therapeutic success with UAS might indicate that an AP movement induced by hypoglossal nerve stimulation cannot resolve a concentric collapse of the upper airway. Thus, the actual effects of upper airway muscle activation on upper airway shape are dependent on both the upper airway region and cross-sectional area.31 Further research on DISE as a patient selection tool for implanted UAS may focus on upper airway behavior during UAS as assessed during DISE. In a recent study by Goding et al., cross-table fluoroscopic images were obtained during hypoglossal nerve stimulation in 26 subjects while two-dimensional changes in the AP dimensions of both the retropalatal and the retrolingual airway spaces were recorded.32 The results of this fluoroscopy study indicate that during hypoglossal nerve stimulation, an opening of the upper airway at the level of the palate occurs in a majority of cases, confirming the beneficial effect of hypoglossal nerve stimulation on the AP upper airway dimensions.32
There is great interest in the prospective prediction of treatment outcome of non-CPAP options such as surgery and oral appliance therapy.22,33 DISE provides an alternative method of studying the upper airway in OSA patients while performing a fiberoptic endoscopy during sedation. The lack of uniformity in the methods used for sedation during DISE as well as the fact that a consensus on DISE scoring systems has not yet been established, are clear limitations to this study.22,23,34,35 Recent studies that address the test-retest and the intra- and interob-server variability in DISE scoring indicate that the reliability of both are moderate to fair, and that inter-observer agreement is higher in ENT surgeons who are experienced with DISE.36–39 The limitations of this study also include the fact that DISE was performed only in the supine position, whereas upper airway collapse patterns should ideally be assessed in both the supine and non-supine position40.
It is well known that the probability of a multilevel collapse is significantly associated with the severity of OSA, as higher AHI values are correlated with a higher percentage of multilevel collapse.25,35,41 This finding might explain the high prevalence of multilevel collapse in our study (91%) given the relatively high overall baseline AHI of 38.5 ± 11.8/h. Upper airway collapse at the level of the palate was the most common level of collapse in this study, with collapse at the level of the tongue base being the second most common (Figure 1). Again, these findings are in line with previous studies.35,41
Two recent studies have shown a correlation between a patient's BMI and the therapeutic response to UAS.13,32 In addition, a baseline AHI ≤ 50/h turned out to be a predictor of UAS therapy response.13
Although the number of patients included in this study was relatively low, a clinically and statistically significant difference in AHI was detected between the two groups of OSA patients (those with versus those without palatal CCC; Figure 3). According to Sher's criteria, treatment success in the patients without palatal CCC was 81%, while no patients with CCC at the level of the palate could be treated successfully with UAS. The correlation between the absence of CCC at the level of the palate and treatment success with UAS turned out to be independent from baseline AHI and the patient's BMI (Table 1). Given that both parameters were previously described as predictors of therapeutic outcome with hypoglossal nerve stimulation for OSA, the fact that the absence of palatal CCC remains highly predictive independent from AHI and BMI certainly adds to the power of these findings.
Figure 3. Apnea-hypopnea index (AHI) in patients with (black color) and without (white color) complete concentric collapse (CCC) at the level of the palate 6 months after UAS implantation during stimulation as compared to baseline without UAS; the different grayscales represent the distinction between normal nocturnal breathing (AHI < 5/h sleep), mild OSA (AHI 5-15/h), moderate OSA (AHI 15-30/h), and severe OSA (AHI > 30/h).
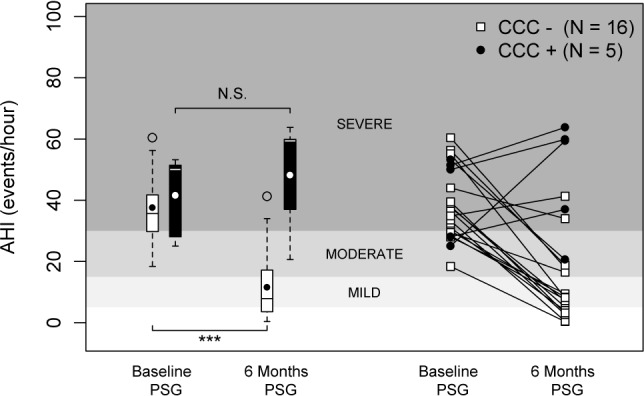
Left panel: boxplots showing the 75th and 25th percentiles by the upper and lower margins, the mean values by the closed circle, and the median values by the horizontal line. Whiskers represent the maximum value (top) and the minimum value (bottom) of the dataset; this range includes all data except the outliers. Outliers are represented by an open circle. Right panel: the individual patient response data are plotted in line graphs, with the white squares being the AHI values in patients without CCC and the black circles being the AHI values in patients with CCC. N.S., not significant; ***p < 0.001.
In conclusion, based on the results of the reported study, DISE can be recommended as a patient selection tool for implanted UAS to treat OSA. Further analysis of the predictive value of DISE in assessing therapeutic response to UAS therapy needs to be performed in larger multicenter trials that are currently ongoing.
DISCLOSURE STATEMENT
This study was supported by Inspire Medical Systems, Inc. Mr. Vanderveken is co-investigator for a study supported by Inspire Medical Systems Inc. Dr. Maurer is co-investigator for a study supported by Inspire Medical Systems Inc. He had paid speaking engagements with Inspire Medical Systems Inc. (USA), GlaxoSmithKline (Germany), Weinmann (Germany), Olympus (Germany), ResMed (Germany), Neuwirth (Germany), Medtronic (USA) and Heinen & Löwenstein (Germany). Dr. Maurer received paid speaking engagement and surgical cadaver training for beginners. Mr. Hohenhorst is investigator for Inspire Medical Systems Inc. Mr. Hamans is a consultant for Philips Healthcare. Mr. Lin has participated as a co-investigator in clinical trials sponsored by Inspire Medical Systems Inc. and has served as a consultant for Inspire Medical Systems Inc. Dr. Anders is co-investigator for a study supported by Inspire Medical Systems Inc. He had paid speaking engagements with Inspire Medicial Systems Inc. (USA), GlaxoSmithKline (Germany), Weinmann (Germany), Olympus (Germany), ResMed (Germany), Neuwirth (Germany), Medtronic (USA) and Heinen & Löwenstein (Germany). He has also received paid speaking engagement and surgical cadaver training for beginners. Dr. de Vries is medical advisor for MSD, ReVENT, NightBalance; investigator for Inspire Medical Systems Inc.; consultant for Philips; and has stock options in ReVENT. Dr. Van de Heyning is an investigator for Inspire Medical Systems Inc. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AP
anteroposterior
- AHI
apnea-hypopnea index
- BMI
body mass index
- CCC
complete concentric collapse
- CPAP
continuous positive airway pressure
- DISE
drug-induced sleep endoscopy
- OSA
obstructive sleep apnea
- UAS
upper airway stimulation
REFERENCES
- 1.Kuna ST, Sant'Ambrogio G. Pathophysiology of upper airway closure during sleep. JAMA. 1991;266:1384–9. [PubMed] [Google Scholar]
- 2.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164:2031–5. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 3.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. 2006;29:375–80. doi: 10.1093/sleep/29.3.375. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 6.He J, Kryger MH, Zorick FJ, Conway W, Roth T. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 7.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34:105–10. doi: 10.1093/sleep/34.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep. 2006;29:240–3. doi: 10.1093/sleep/29.2.240. [DOI] [PubMed] [Google Scholar]
- 9.de Almeida FR. Complexity and efficacy of mandibular advancement splints: understanding their mode of action. J Clin Sleep Med. 2011;7:447–8. doi: 10.5664/JCSM.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lettieri CJ, Paolino N, Eliasson AH, Shah AA, Holley AB. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7:439–45. doi: 10.5664/JCSM.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. 2008;178:197–202. doi: 10.1164/rccm.200701-114OC. [DOI] [PubMed] [Google Scholar]
- 13.Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122:1626–33. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 14.Oliven A. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr Opin Pulm Med. 2011;17:419–24. doi: 10.1097/MCP.0b013e32834b7e65. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AR, Barnes M, Hillman D, et al. Acute upper airway responses to hypoglossal nerve stimulation during sleep in obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:420–6. doi: 10.1164/rccm.201109-1614OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14:299–305. doi: 10.1016/j.smrv.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–86. doi: 10.5665/sleep.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichler C, Sommer JU, Stuck BA, Hormann K, Maurer JT. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath. 2013;17:63–8. doi: 10.1007/s11325-012-0647-9. [DOI] [PubMed] [Google Scholar]
- 19.Vanderveken OM. Drug-induced sleep endoscopy (DISE) for non-CPAP treatment selection in patients with sleep-disordered breathing. Sleep Breath. 2013;17:13–4. doi: 10.1007/s11325-012-0671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanderveken OM, Maurer JT, Hohenhorst W, et al. The evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. Sleep. 2012;35:A144. doi: 10.5664/jcsm.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. for the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules,terminology and technical specifications. [Google Scholar]
- 22.Vanderveken OM, Vroegop AVM, Van de Heyning PH, Braem MJ. Drug-induced sleep endoscopy completed with a simulation bite approach for the prediction of the outcome of treatment of obstructive sleep apnea with mandibular repositioning appliances. Oper Tech Otolaryngol. 2011;22:175–82. [Google Scholar]
- 23.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–6. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 24.Vroegop AVMT, Vanderveken OM, Dieltjens M, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2012. http://dx.doi.org/10.1111/jsr.12008. [DOI] [PubMed]
- 25.Hamans E, Meeus O, Boudewyns A, Saldien V, Verbraecken J, Van de Heyning P. Outcome of sleep endoscopy in obstructive sleep apnoea: the Antwerp experience. B-ENT. 2010;6:97–103. [PubMed] [Google Scholar]
- 26.Hewitt RJ, Dasgupta A, Singh A, Dutta C, Kotecha BT. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol. 2009;266:691–7. doi: 10.1007/s00405-008-0831-5. [DOI] [PubMed] [Google Scholar]
- 27.Maurer JT, Van de Heyning P, Lin HS, et al. Operative technique of upper airway stimulation: an implantable treatment of obstructive sleep apnea. Oper Tech Otolaryngol Head Neck Surg. 2012;23:227–33. [Google Scholar]
- 28.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–23. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- 31.Kuna ST. Regional effects of selective pharyngeal muscle activation on airway shape. Am J Respir Crit Care Med. 2004;169:1063–9. doi: 10.1164/rccm.200309-1283OC. [DOI] [PubMed] [Google Scholar]
- 32.Goding GS, Jr., Tesfayesus W, Kezirian EJ. Hypoglossal nerve stimulation and airway changes under fluoroscopy. Otolaryngol Head Neck Surg. 2012;146:1017–22. doi: 10.1177/0194599812436472. [DOI] [PubMed] [Google Scholar]
- 33.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg. 2006;132:206–13. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 34.Johal A, Battagel JM, Kotecha BT. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with mandibular advancement splints in obstructive sleep apnoea. Eur J Orthod. 2005;27:607–14. doi: 10.1093/ejo/cji063. [DOI] [PubMed] [Google Scholar]
- 35.Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011;121:2710–6. doi: 10.1002/lary.22369. [DOI] [PubMed] [Google Scholar]
- 36.Vroegop AV, Vanderveken OM, Wouters K, et al. Variation in observer agreement in drug-induced sleep endoscopy. Sleep. 2012;35:A143. [Google Scholar]
- 37.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Inter-rater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg. 2010;136:393–7. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 38.Berry S, Roblin G, Williams A, Watkins A, Whittet HB. Validity of sleep nasendoscopy in the investigation of sleep related breathing disorders. Laryngoscope. 2005;115:538–40. doi: 10.1097/01.mlg.0000157849.16649.6e. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Bruno K, Goldberg AN, McCulloch CE, Kezirian EJ. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg. 2009;140:646–51. doi: 10.1016/j.otohns.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–83. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vroegop AV, Hamans E, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1249 cases. Sleep. 2012;35:A137. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]


