Abstract
Study Objective:
To determine whether pretreatment with zaleplon immediately before CPAP titration improves 1-month CPAP adherence in subjects newly diagnosed with OSA.
Methods:
Prospective, randomized, double-blinded, placebo-controlled trial of a single dose of zaleplon 10 mg or matching placebo at the start of CPAP titration during laboratory-based, split-night polysomnography (PSG). Baseline sleep symptoms were assessed with the Functional Outcomes of Sleep Questionnaire (FOSQ) and Epworth Sleepiness Scale (ESS). CPAP usage and change in symptom questionnaire responses were assessed at 1-month follow-up.
Results:
One hundred thirty-four newly diagnosed OSA patients undergoing their initial split-night PSG (49.8 ± 11.3 years old with an apnea-hypopnea index of 16.5 (7, 32) [median (interquartile range)] were randomized to zaleplon (n = 73) or placebo (n = 63). Complete follow-up data were available in 83 subjects (44 zaleplon group; 39 placebo group). CPAP was used for 6.5 (5, 7) h/day with zaleplon versus 6.5 (5, 8) h/ day with placebo (p = 0.64). Improvements in FOSQ and ESS scores did not differ between the two groups.
Conclusion:
A single dose of zaleplon at the start of a split-night CPAP titration does not result in superior CPAP adherence or improvement in symptoms at 1-month compared to placebo. Our data show that zaleplon is safe and is associated with shorter sleep latency during CPAP titration, but it does not translate into improved short-term CPAP adherence.
Citation:
Park JG; Olson EJ; Morgenthaler TI. Impact of zaleplon on continuous positive airway pressure therapy compliance. J Clin Sleep Med 2013;9(5):439-444.
Keywords: Continuous positive airway pressure, compliance, zaleplon, obstructive sleep apnea
Obstructive sleep apnea (OSA), is a chronic disease that affects at least 2% to 4% of middle aged adults1 and is associated with significant morbidity and mortality.2–7 Among medical, surgical, and appliance options for treating OSA, continuous positive airway pressure (CPAP) is the treatment of choice for most patients.8 Increasing CPAP use is associated with dose-dependent improvements in several clinical outcomes.9 However, a significant number of patients struggle to consistently adhere to CPAP therapy.10
Drake et al. reported that individuals whose sleep improved during the CPAP titration demonstrated two hours of increased nightly compliance, even after correcting for disease severity at initial polysomnography.11 Several investigators examined whether a short course of hypnotic therapy coincident with the start of CPAP therapy would improve compliance, but they found conflicting results. Bradshaw et al. reported that zolpidem after titration, but commencing with the first 14 days of CPAP did not improve compliance compared to placebo or standardized therapy.12 In contrast, Lettieri et al. found that eszopiclone for the first 14 nights of CPAP was associated with greater long-term compliance than placebo.13 Their group, in a retrospective study, also found an association between hypnotic (mostly zolpidem, and to lesser extent eszopiclone) use during CPAP titration and improved compliance at 4-6 weeks.14
Although encouraging, we felt that the conflicting outcomes of the prospective studies and the retrospective nature of the latter study needed further clarification. Thus, we report on a prospective, randomized, double-blinded, placebo-controlled investigation to determine whether administration of a single dose of hypnotic during the CPAP titration in the sleep laboratory would improve CPAP compliance at 1 month. We hypothesized the hypnotic would improve the initial CPAP experience, and thus CPAP adherence would be greater. The hypnotic employed in this study was zaleplon, a short-acting nonbenzodiazepine hypnotic that is a benzodiazepine receptor agonist.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Compliance with continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea remains suboptimal, and usage patterns appear heavily influenced by early CPAP experience. We hoped to improve compliance by improving the very first night's sleep on CPAP by using zaleplon during the titration study.
Study Impact: Our study did not show improvement in CPAP compliance with the use of zaleplon during CPAP titration. A very high level of compliance in our control group and similar quality of sleep during CPAP titration between the zaleplon and control group suggests that other factors are more important in determining CPAP compliance.
METHODS
After approval by our institutional review board, subjects referred to our multidisciplinary accredited sleep facility for suspected OSA between October 2004 and March 2006 were approached for enrollment. Inclusion and exclusion criteria are listed in Table 1. All subjects underwent an evaluation by a board-certified sleep specialist, were thought to likely have OSA, and were tested via a laboratory-based, technologist-attended polysomnogram (PSG). Prior to PSG, each subject viewed an educational video describing OSA, PSG, and CPAP, followed by a personalized CPAP interface sizing session with a polysomnographic technologist. Subjects were allowed to try on different interfaces with CPAP at 5 cm H2O. Participants completed the Functional Outcomes of Sleep Questionnaire (FOSQ),15 a 30-item questionnaire designed to assess 5 areas of functional outcomes affected by sleep disorders: general productivity, social outcomes, activity level, vigilance, and intimate relationships.
Table 1.
Inclusion and exclusion criteria for study participants

PSGs were performed using a digital polygraph (NCI Lamont Medical, Inc., Madison, WI, or Bio-Logic Systems Corp., Mundelein, IL). The following parameters were recorded: electroencephalography (Fz-Cz; Cz-Oz; C4-M1 or C3-M2); electrooculography (right outer canthus-Fpz; Left outer canthus-Fpz); submental and anterior tibialis electromyography; snoring by laryngeal microphone; electrocardiography; pulse oximetry; and respiratory effort (thoracic, abdominal, and summated inductive plethysmography). Airflow was analyzed by nasal pressure transducer (standard at the time of this study). Obstructive apnea was defined as cessation of airflow ≥ 10 sec despite respiratory effort. Hypopnea was defined by ≥ 30% reduction in airflow ≥ 10 sec accompanied by ≥ 4% drop in oxyhemoglobin saturation. Sleep stages and arousals were manually scored by registered polysomnographic technologists using contemporary standards at the time of the study.16,17 OSA was defined by an apnea-hypopnea index (AHI) ≥ 5.
All PSGs were performed in a split-night fashion. The initial (i.e., diagnostic) phase was continued until a minimum total sleep time of 120 min occurred and REM sleep was noted. If REM did not occur, diagnostic portion was stopped at 02:30 if the total sleep time was a minimum of 120 minutes. Subjects with OSA were randomized (using nQuery Advisor 4.0 software) to receive zaleplon 10 mg or an identical-appearing placebo, followed immediately by commencement of the CPAP titration during the second portion of the PSG. CPAP was started at 5 cm H2O (4 cm H2O if the subject was uncomfortable with the initial CPAP setting) through an interface of the subject's choosing and gradually increased in increments of 1 cm H2O until disordered breathing events were eliminated. At the conclusion of the polysomnogram, subjects used a visual analog scale to rate their initial experience with the PSG and CPAP.
A board-certified sleep specialist reviewed all PSGs. For those prescribed CPAP, a standardized education packet regarding the use and care of CPAP equipment supplemented the sleep specialists' education regarding CPAP use, and follow-up visit was scheduled. The follow-up visit target was 1 month, but varied because of differences in time necessary to secure CPAP (CPAP was obtained by subjects through a durable medical equipment vendor of their choosing) and travel distance to our center. All subjects were also encouraged to call our sleep center nurse phone line if they experienced any CPAP problems. At the follow-up visit, CPAP use information was downloaded from their device and subjects completed a second FOSQ. If subjects missed their return appointment, they were contacted by telephone to rescheduled a visit or were sent a mail package asking them to return a second FOSQ and their downloaded CPAP information (via their local vendor) using an enclosed self-stamped envelope. All patients, physicians, technologists, and nurses remained blinded with respect to zaleplon vs. placebo pretreatment throughout the study.
STATISTICS
A retrospective analysis of 274 OSA patients treated with CPAP in our sleep facility and who had downloaded compliance data revealed a mean CPAP use of 5.6 h (± 1.9) per night (non-published data gathered as part of our center's database). Assuming the same standard deviation in our study groups and using α = 0.05, we calculated that 63 patients per study group would provide 80% power to detect a difference in nightly average CPAP use of 60 min (18% improvement) (nQuery Advisor 4.0 software) between groups. We used an intention-to-treat analysis for the pre- and post-CPAP data using a nonparametric analysis of variance (ANOVA). Similarly, nonparametric ANOVA was used to analyze the difference between groups at the time of follow-up regarding compliance and FOSQ. Non-normally distributed data are expressed as median and interquartile range (IQR), 25th and 75th percentiles. Where noted, mean ± standard deviation is used for some parametric data.
RESULTS
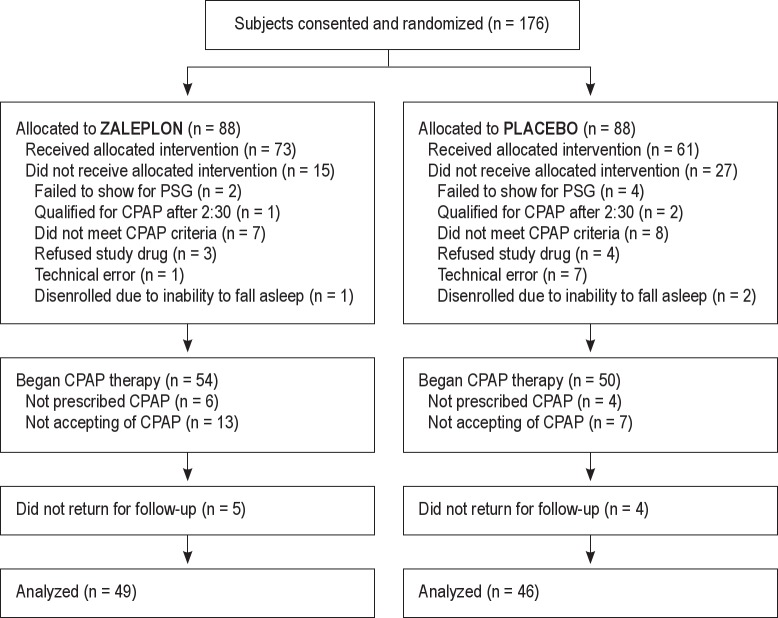
One hundred seventy-six eligible subjects were enrolled and randomly assigned to receive either placebo or zaleplon during split-night PSG (Figure 1). After enrollment, 42 subjects (27 in the placebo group, 15 in the zaleplon group) did not receive the assigned treatment for various reasons (Figure 1). As a result, 61 subjects comprised the placebo group and 73 subjects the zaleplon group.
Figure 1. Subject flow diagram.
Table 2 provides data on baseline demographics and the diagnostic portion of PSG for the 2 groups. Placebo group had slightly greater percentage of women and was slightly younger. There were no significant differences in body mass index, Epworth Sleepiness Scale (ESS) score, or FOSQ scores. There were no significant differences in various diagnostic PSG parameters, including AHI and minimum oxyhemoglobin saturation (Table 2).
Table 2.
Baseline characteristics of study subject and diagnostic polysomnogram data

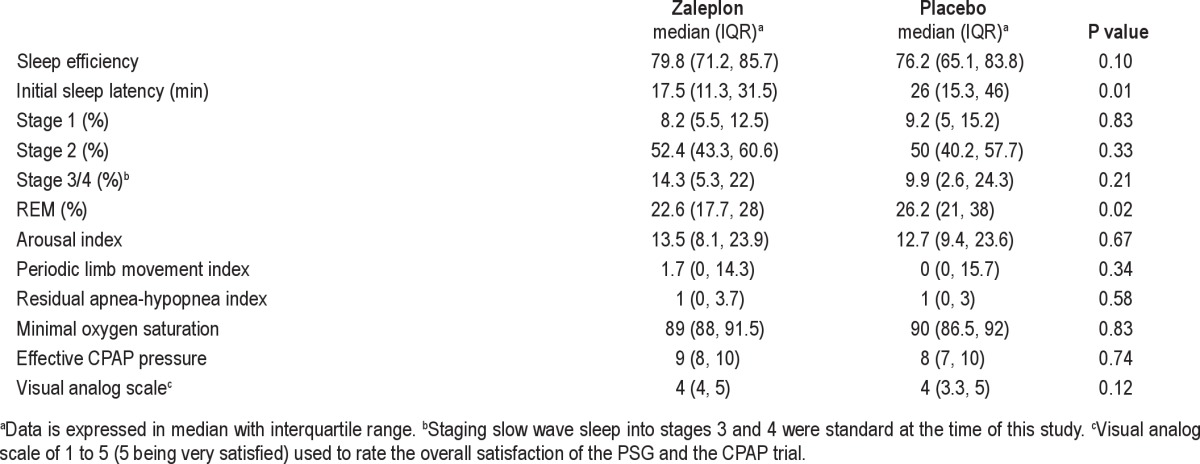
During CPAP titration, zaleplon use was associated with shorter initial sleep latency and decreased REM sleep compared to placebo (Table 3). There were no differences in the effective CPAP pressures, AHI for the entire titration, or the arousal index. Furthermore, there was no difference in overall subject satisfaction with the PSG and CPAP as determined by their responses on the visual analog scale (Table 3). All studies were completed without any adverse events and all CPAP titrations were considered “adequate” or greater per published clinical guidelines for CPAP titration.18
Table 3.
Effect of zaleplon on polysomnogram values during CPAP titration
CPAP was not prescribed in 10 subjects (4 in the placebo group, 6 in the zaleplon group), because of the very mild nature of their OSA (mean AHI 5.9 ± 5.6 in these 10 subjects). An additional 20 subjects (7 in the placebo group, 13 in zaleplon group) refused CPAP therapy. Those who declined therapy were younger (median age of 41 vs 51 [p = 0.019]) and had lower BMI (median BMI of 31.5 vs 35.7 [p = 0.009]), but did not differ in ESS, FOSQ, VAS, or AHI (data not shown). Nine subjects (4 in the placebo group; 5 in the zaleplon group) did not return for follow-up; they were not available by telephone and did not return a mail request for follow-up information (Figure 1). There were no significant differences in the age, BMI, FOSQ, ESS, or AHI between those who did and did not return for follow up (data not shown).
Ultimately, follow-up information was available in 46 subjects in the placebo group and 49 subjects in the zaleplon group, with 39 placebo group patients and 44 zaleplon group patients having CPAP devices with downloadable compliance capability (Table 4). Among these groups, no subjects discontinued CPAP therapy during the follow-up period. Using an intention-to-treat analysis, CPAP compliance and symptomatic improvements were not statistically different between the two groups (Table 4). ESS and FOSQ scores improved similarly in both groups (Table 5).
Table 4.
Intention-to-treat analysis

Table 5.
Outcome variables at follow-up

Overall, ESS score, AHI during the diagnostic test, and sleep efficiency during the CPAP titration was not associated with daily CPAP usage at follow-up (p = 1.0, 0.85, and 0.84, respectively).
DISCUSSION
Our study demonstrated that zaleplon administered just before CPAP titration during split-night PSG improved initial sleep latency without affecting minimum oxygen saturation or resultant CPAP pressure. Contrary to our hypothesis, use of zaleplon did not result improvement in sleep efficiency or arousal indices, and thus perception of sleep quality during the PSG did not differ between the two groups. Although CPAP adherence was relatively high in both groups, use of zaleplon did not result in increased compliance. Improvements in OSA-related symptoms as measured by FOSQ and ESS were also similar in the zaleplon and placebo groups.
Our data conflict with Lettieri's work involving the use of a hypnotic during CPAP titration. A retrospective assessment of 400 consecutive patients prescribed CPAP for OSA showed that of multiple parameters assessed, only age and use of a hypnotic (typically zolpidem) during the CPAP titration were associated with better short-term CPAP compliance.14 This association was significant for patients less than 50 years old, but not with older patients. The retrospective nature of their study did not allow control for selection bias (they only included patients who returned for follow-up; patients using hypnotic-sedative medications prior to PSG were excluded) or medication effects (they could not identify which patients received which hypnotic and could not verify use or non-use in 15% of their patients). In a prospective study, Lettieri and his colleagues also demonstrated that pre-treatment with eszopiclone prior to full-night attended polysomnography for CPAP titration improved CPAP compliance for those patients returning for follow-up.21 However, about one-sixth of their patients did not return, and the improvement in compliance was not demonstrated using an intention-to-treat analysis.
There are several potential explanations for the conflicting results. Twenty-nine percent of successfully randomized patients did not complete the study (77% of these subjects were not prescribed or declined CPAP), leaving our study potentially underpowered to detect a difference in CPAP compliance with zaleplon. In addition, the average rating of the initial CPAP experience in both groups was “very good” (“4” on a 5-point scale with “5” being “very satisfied”), making it challenging to discern an additive contribution of zaleplon when the control group was highly satisfied.
We also used a different hypnotic. Zaleplon is a pyrazolopyrimidine hypnotic/sedative that is unrelated to benzodiazepines but acts on the same GABA-A receptor site as the benzodiazepines. Zaleplon also possesses some anxiolytic effects.22,23 We opted for use zaleplon because of its short-acting hypnotic effect in an effort to mitigate any morning hangover that might result from administration in the middle of split-night PSG. Other studies have used related but longer acting nonbenzodiazepine, benzodiazepine receptor agonists, zolpidem, and eszoplicone. Multiple regression analysis of Drake's study correlated improved CPAP compliance only with objective improvement in sleep efficiency, not with participants' subjective improvement in their sleep quality.11 In our study, zaleplon improved initial sleep latency but did not affect other sleep parameters. This lack of objective improvement in sleep efficiency may also account for lack of improvement in CPAP compliance.
It is probably also important to note that the improvement in CPAP compliance in Drake's study was from 4.09 to 6.12 hours per night.11 Lettieri's prospective study using eszopiclone at the beginning of CPAP titration showed improvement from 3.9 to 4.8 hours per night of CPAP use.21 A prospective use of short-term eszopiclone use improved CPAP use from 2.4 to 3.6 hours.13 Other interventions that improved CPAP compliance were in cases where baseline or placebo group use was under 5 hours (Table 6). Our placebo group's average compliance was already 6.5 h/night. These results imply that the benefit of adding hypnotic in improving CPAP compliance may be limited to those whose compliance is less than 5 hours. Thus, the benefit of adding zolpidem or eszopiclone for full-night CPAP titration studies may hinge on baseline CPAP compliance of the patient population. For example, if CPAP compliance of patients with certain risk factors might be predicted to be less than 5 hours, adding hypnotic such as zolpidem or ezopiclone prior to CPAP titration may result in improved CPAP compliance at follow-up.
Table 6.
Review of interventions' impact on CPAP compliance

Our study may have been limited by the split-night format of our PSG. It is possible that approximately 4 hours of CPAP titration may have been insufficient for our subjects to fully subjectively experience the benefit of CPAP, especially considering some of that time was spent in less than optimum CPAP pressure. With a full-night CPAP titration study, more of the night may be spent at adequate CPAP pressure that may further improve subjective rating. However, given that our subjects felt the titration portion was at least “very good” (rating of 4 on 5-point scale), the split-night format seems less likely to be a major contributing factor. Furthermore, there were several studies looking at the impact of split-night studies that did not find any negative impact on CPAP compliance.24
CONCLUSION
In our double-blinded, placebo-controlled, randomized study, the use of zaleplon at the beginning of CPAP titration during split-night study improved initial sleep latency, but did not yield improvements in CPAP compliance. While there was a trend towards improved sleep efficiency, it did not reach statistical significance. Our study showed that use of zaleplon did not result in systematic changes to AHI, oxygenation, or CPAP titration pressures, and it may therefore be used safely in patients without contraindications during attended polysomnography, and because of shorter sleep latency, might allow for longer PSG recordings. However, benefit for CPAP treatment adherence may only accrue to patients in whom anticipated CPAP adherence is low.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Morgenthaler has received research support from ResMed Corporation. Dr. Park has received research support from Dymedix Corporation and lecture fees from the American Physician Institute. The other author has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Ross A. Dierkhising for his statistical support. We also thank Terri Weaver for giving us the permission to use FOSQ in our research.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death.[see comment] N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 6.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort.[see comment] Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–19. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 11.Drake CL, Day R, Hudgel D, et al. Sleep during titration predicts continuous positive airway pressure compliance. Sleep. 2003;26:308–11. doi: 10.1093/sleep/26.3.308. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw DA, Ruff GA, Murphy DP. An oral hypnotic medication does not improve continuous positive airway pressure compliance in men with obstructive sleep apnea. Chest. 2006;130:1369–76. doi: 10.1378/chest.130.5.1369. [DOI] [PubMed] [Google Scholar]
- 13.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 14.Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term continuous positive airway pressure compliance. Chest. 2009;135:704–9. doi: 10.1378/chest.08-2182. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 16.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Washington, DC: US Department of Health, Education, and Welfare, Public Health Service; 1968. [Google Scholar]
- 18.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Lettieri CJ, Eliasson AH, Andrada T, Khramtsov A, Kristo DA. Does zolpidem enhance the yield of polysomnography? J Clin Sleep Med. 2005;1:129–31. [PubMed] [Google Scholar]
- 20.Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo-controlled trial. Sleep. 2008;31:1310–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Lettieri CJ, Collen JF, Eliasson AH, Quast TM. Sedative use during continuous positive airway pressure titration improves subsequent compliance: a randomized, double-blind, placebo-controlled trial. Chest. 2009;136:1263–8. doi: 10.1378/chest.09-0811. [DOI] [PubMed] [Google Scholar]
- 22.Zaleplon. Am J Health Syst Pharm. 2000;57:430–2. doi: 10.1093/ajhp/57.5.430. [DOI] [PubMed] [Google Scholar]
- 23.Lobarinas E, Falk J. Comparison of benzodiazepines and the non-benzodiaze-pine agents zolpidem and zaleplon with respect to anxiolytic action as measured by increases in hypertonic NaCl-solution drinking in rats. Psychopharmacology (Berl) 2000;149:176–80. doi: 10.1007/s002139900354. [DOI] [PubMed] [Google Scholar]
- 24.Collen J, Holley A, Lettieri C, Shah A, Roop S. The impact of split-night versus traditional sleep studies on CPAP compliance. Sleep Breath. 2010;14:93–9. doi: 10.1007/s11325-009-0294-y. [DOI] [PubMed] [Google Scholar]
- 25.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30:635–40. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 26.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballard RD, Gay PC, Strollo PJ., Jr Interventions to improve compliance in sleep apnea patients previously non-compliant with continous positive airway pressure. J Clin Sleep Med. 2007;3:706–12. [PMC free article] [PubMed] [Google Scholar]