Abstract
Study Objectives:
Obstructive sleep apnea (OSA) has been associated with an increased risk of motor vehicle crashes. This driving risk can be reduced (≥ 50%) by treatment with continuous positive airway pressure (CPAP). However residual excessive daytime sleepiness (EDS) can persist for some patients who regularly use CPAP. The current study was designed to assess the effect of armodafinil on simulated driving performance and subsequent CPAP treatment compliance in newly diagnosed OSA patients with EDS during a 2-week “waiting period” prior to initiation of CPAP.
Methods:
Sixty-nine newly diagnosed OSA patients, awaiting CPAP therapy, were randomized (1:1) to placebo or armodafinil (150 mg/day) treatment. Simulated driving tests and self-report measures were completed at baseline, after 2 weeks of drug treatment, and following 6 weeks of CPAP treatment. CPAP compliance was evaluated at the end of 6 weeks of CPAP.
Results:
Compared to placebo, armodafinil improved simulated driving safety performance in OSA patients awaiting CPAP therapy (p = 0.03). Improvement was seen in lane position deviation (p = 0.002) and number of lane excursions (p = 0.02). Improvement was also observed on measures of sleepiness using the Epworth Sleepiness Scale (ESS) and sleep related quality of life. Following 6 weeks of CPAP, there was also significant improvement observed on multiple measures of simulated driving performance. CPAP compliance did not differ between armodafinil-treated and placebo-treated patients (p = 0.80).
Conclusions:
Armodafinil was found to improve simulated driving performance in OSA patients with EDS prior to initiation of CPAP. Treatment with armodafinil showed no effect on subsequent CPAP compliance.
Citation:
Kay GG; Feldman N. Effects of armodafinil on simulated driving and self-report measures in obstructive sleep apnea patients prior to treatment with continuous positive airway pressure. J Clin Sleep Med 2013;9(5):445-454.
Keywords: CPAP, OSA, driving simulation, ESS, armodafinil
Obstructive sleep apnea syndrome (OSA) is the most common medical disorder causing excessive daytime sleepiness (EDS).1 Untreated individuals with OSA have an increased risk of motor vehicle crashes. This increased risk of crashes was first recognized in the 1980s and has since been reported by multiple investigators.2,3 In this regard, a meta-analysis has shown a 3.71-fold increase in the relative risk for motor vehicle crashes for individuals with untreated OSA.4
Sleepiness related crashes generally result from falling asleep while driving or from impairment of the cognitive, perceptual, or motor abilities essential to the complex task of driving. The impact of sleepiness on driving performance has been demonstrated by driving simulator studies investigating the effects of sleep deprivation and CNS sedatives.5 In general, these studies have demonstrated that drivers with OSA perform worse than matched controls on driving simulators.6 Using the AusEd driving simulator, investigators found that OSA patients performed worse than controls on 4 of 5 outcomes: lane position variability, crash frequency, and performance on measures of divided attention.7 Researchers using the STI driving simulator found poor simulated driving performance in OSA patients was related to EEG evidence of attention lapses.8 Lane position variability was the most sensitive measure for assessing and quantifying impairment. In a study investigating simulated versus real driving that evaluated the effects of fatigue and sleepiness, the investigators concluded that for some variables (e.g., fatigue and lane crossings) the two methodologies were comparable.9
BRIEF SUMMARY
Current knowledge/Study Rationale: This study investigated a strategy for improving driving safety in OSA patients during the interval between the first contact of the clinician with the patient and the initiation of CPAP. The study was designed to assess the effect of armodafinil on simulated driving performance prior to the initiation of CPAP treatment and to determine the impact of this treatment on subsequent CPAP compliance.
Study Impact: Results demonstrate that 2 weeks of treatment with armodafinil improved simulated driving performance prior to the initiation of CPAP therapy and had no impact on subsequent CPAP compliance. The study also provided evidence of the marked improvement in driving performance following 6 weeks of treatment with CPAP (after discontinuation of the drug treatment phase of the study).
It is now well recognized that the risk of motor vehicle crashes for OSA patients is significantly decreased following treatment with nasal continuous positive airway pressure (CPAP).10 It has been estimated that there is at least a 50% reduction in crash risk.2,11 Improved driving safety is also reflected in better driving performance on simulated driving tests following CPAP treatment.12–15 However, some OSA patients, in spite of receiving therapeutic CPAP, continue to experience EDS. For these individuals, modafinil has been shown to be an effective treatment for residual EDS.16,17
Investigators have also examined the effect of modafinil on the simulated driving performance of OSA patients. It has been reported that speed deviation was reduced by 14% following treatment with modafinil (200 mg) in partially sleep-deprived OSA patients.18 Another study investigated the effect of modafinil (300 mg) on sleep-restricted normal adults.19 For these individuals, modafinil was found to reduce lane position deviation, off- road incidents, and reaction time on a divided attention task. More recently, investigators have evaluated the effects of modafinil (200 mg) on the treatment of residual EDS in OSA patients following acute withdrawal from CPAP. In the preliminary study, improvements in driving performance were reported following treatment with modafinil; however, these findings did not reach statistical significance when compared to placebo treatment.20 When the same research group conducted a larger crossover study in the same patient population following acutely interrupted CPAP therapy, they were able to show that modafinil indeed prevented the decline in simulated driving performance, neurocognitive performance, and subjective sleepiness compared to placebo treatment.21
The current study was designed to assess the effects of 150 mg of armodafinil (the R-enantiomer of modafinil) on simulated driving performance in newly diagnosed OSA patients with excessive daytime sleepiness (ESS ≥ 12) during a 2-week period prior to initiating CPAP therapy. We chose an ESS ≥ 12 to obtain a study population with more clinically significant illness that might put them at risk for motor vehicle accidents. Unlike those who participated in the studies conducted by Williams et al.,20,21 our patients were CPAP naïve and studied for a different purpose. In clinical practice, patients newly diagnosed with OSA often must wait for weeks before initiating CPAP therapy. Delays can be the result of scheduling, waiting for insurance approval, or other causes. The obvious concern is that these patients are at higher risk for driving related accidents.2,3 In this regard, practitioners have asked whether it would be advisable to start a newly diagnosed OSA patient on a stimulant medication approved for treatment of residual EDS (e.g., armodafinil) while they are awaiting initiation of CPAP treatment.22 However, an additional concern is that prior use of stimulant treatment might negatively impact subsequent CPAP compliance. Therefore, in addition to determining the effect of armodafinil on simulated driving performance, the present study was designed to assess whether treatment with armodafinil, prior to initiation of CPAP, would affect subsequent CPAP compliance. Finally, the present study was also designed to assess simulated driving performance following 6 weeks of CPAP therapy.
METHODS
Patients
The study was advertised in local newspapers and was described in a local television news story. A total of 69 previously untreated OSA patients were enrolled in the study. Male and female subjects eligible for participation were 21-64 years of age, with a diagnosis of OSA confirmed by nocturnal polysomnogram (PSG) (apnea-hypopnea index [AHI] > 15), and with excessive daytime sleepiness (ESS ≥ 12). All subjects were newly diagnosed and awaiting CPAP therapy. Subjects were required to have a valid driver's license and to have been actively engaged in driving for the past 3 years. Exclusion criteria included any unstable medical condition, circadian rhythm disorder, restless leg syndrome, narcolepsy, other significant sleep disorders, irregular sleep schedules, use of sedating antihistamines, selective serotonin reuptake inhibitors, muscle relaxants or hypnotics, consumption of more than 600 mg of caffeine per day, alcohol abuse, simulator sickness, and medical conditions or use of medications contraindicated for use of armodafinil.23 Subjects were admitted and randomized without regard for their driving history.
Study Design
The study design is shown in Figure 1. This was a double-blind, placebo-controlled, randomized, single-site study. The study was reviewed and approved by an institutional review board. All subjects gave written informed consent. This study was conducted in compliance with Good Clinical Practice, according to the International Conference on Harmonization Tripartite Guideline.
Figure 1. Study Design and Subject Disposition.
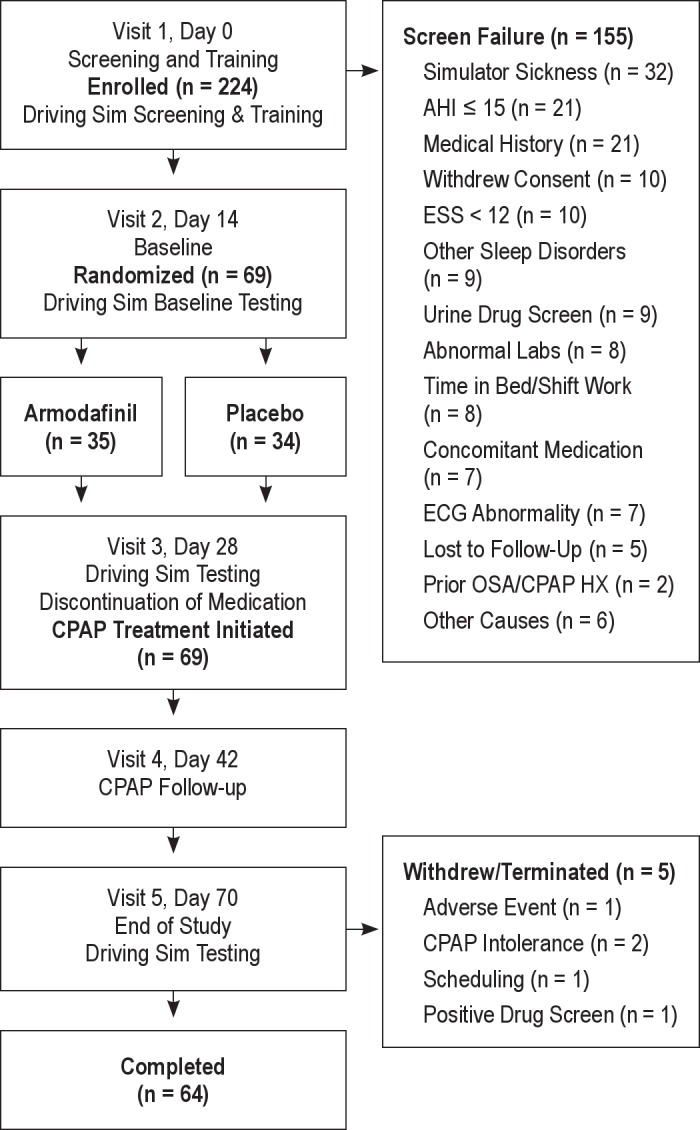
There were two phases to this study. In the first 2-week phase, subjects with OSA and EDS were randomized (1:1) to treatment with armodafinil (150 mg) or placebo. In the second phase, all subjects completing the first phase were treated for 6 weeks with CPAP.
During the 2-week screening period, the diagnosis of OSA was confirmed by nocturnal polysomnogram (AHI > 5) and subjects had to demonstrate at least moderate EDS (ESS ≥ 12). During the screening period subjects were given a brief introduction to the driving simulator (approximately 10 min). Upon completion of this screening drive, subjects were orally administered the Simulator Sickness Questionnaire (SSQ).24 Subjects with scores > 20 on the SSQ Nausea, Disorientation, Oculomotor, or Total scale were excluded. Subjects who passed the screening were shown an instructional orientation slideshow. This was followed by a 20-min training scenario, which provided additional standardized instructions for the scenarios used in the study. This was followed by an additional 20-min practice driving session.
Eligible subjects returned for their baseline visit (Visit 2). During the baseline visit, all study measures were administered. The driving simulation test was administered at approximately 10:00 and consisted of a 20-min vigilance driving scenario (VIG), a 20-min urban scenario (URB), and a 40-min country vigilance scenario (CV). Prior to beginning the driving simulation test, subjects completed a 10-min warm-up drive to reac-quaint them with the driving controls.
Study medication was dispensed following completion of baseline testing. Subjects were randomly assigned on a 1:1 basis to receive armodafinil or matching placebo once daily in the morning (i.e., before 08:00 and 30 min prior to breakfast). Subjects were titrated to a fixed dose of 150 mg of armodafinil: 50 mg of armodafinil for the first 2 days of dosing, 100 mg of armodafinil for the second 2 days of dosing, and 150 mg of armodafinil for the remainder of the dosing period (10 to 24 days). A computer-generated randomization schedule was prepared using SAS Version 9.1.3 PROC PLAN.
Following the 14-day dosing period, subjects returned for Visit 3 procedures. A final dose of the study medication was dispensed at the clinic. Armodafinil dosing compliance was monitored by “pill count” on Visit 3 of the study (see Figure 1). Self-report measures (ESS, Functional Outcomes of Sleep Questionnaire [FOSQ], and Medical Outcomes Study 6-item Cognitive Functioning Scale [MOS-CF6]) were completed prior to beginning the driving tasks. In addition, subjects completed a 10-min warm-up drive. At approximately 10:00am, the subjects drove the 3 scenarios. Subjects returned on the evening of the same day or on the following day for a nocturnal polysomno-gram with CPAP titration and were given instructions on proper use of CPAP. Two weeks following initiation of CPAP, subjects returned to the clinic for a follow-up clinic appointment (Visit 4), which addressed compliance and further instruction on proper use of CPAP as needed.
The final testing visit (Visit 5) was conducted after 4 additional weeks (6 weeks total) of CPAP. Subjects completed the same testing procedures that were completed at the baseline visit and following discontinuation of the study medication.
After completion of driving simulator testing, subjects were administered a battery of neuropsychological tests including measures of vigilance, psychomotor functioning, memory, and executive functions, including the computer-based cognitive test (CogScreen) used in the Apnea Positive Pressure Long-term Efficacy Study (APPLES).25 Results of the effects of armodafinil and CPAP on these cognitive measures will be reported separately.
Adverse events were monitored throughout the study, with severity (mild, moderate, or severe) and relationship to study medication rated by the investigator. Concomitant medications were recorded. Physical examinations (screening and end of study or final visit), vital sign measurements, and standard hematologic laboratory tests and chemistries were performed.
Cognitive Research Corporation Driving Simulator (CRCDS)
The CRCDS is a PC-based driving simulator which incorporates the Systems Technologies Inc. STISIM (Model 100W) software, three 21-inch LCD monitors to provide a wide field of view (105°), and a full-size steering wheel and pedals (ECCI Trackstar 6000GT). The CRCDS complies with current regulatory guidelines (U.S. FDA 21 CFR Part 11), which specify data integrity and system validation requirements. Two equivalent CRCDS simulators were used to conduct the study. The STISIM software used in the CRCDS has previously been used in studies of stimulant effects on driving performance26 and to study the effects of obstructive sleep apnea.8
The specific driving scenarios chosen for the study were designed to be sensitive to the known driving difficulties of untreated patients with OSA and are comparable to those used in prior studies of OSA patients. The subjects began by driving the VIG scenario, a 20-min scenario consisting of a 2-lane, rural highway with rolling hills, occasional oncoming traffic, a single crash likely event, and a secondary (divided-attention) vigilance task. For this task, subjects were instructed to rapidly press a button on the steering wheel when an infrequently presented target stimulus appeared in boxes at the upper left and right sides of the screen. The second scenario was the 20-min URB scenario. This scenario has considerably more traffic, pedestrians, and 3 crash likely events. The final drive was the CV scenario, a 40-min drive consisting of a 2-lane, rural highway with curves and hills but no sharp turns or stops, minimal on-coming traffic, no crash likely events, and a set speed limit (55 miles per hour). Driving data for the CV scenario was grouped into five 8-min time blocks (Epochs 1-5) to evaluate the effect of time-on-task.27
Self-Report Measures
The self-report measures selected for the study are among the most commonly used measures to assess outcome in sleep research, and included the Epworth Sleepiness Scale (ESS),28 the Functional Outcomes of Sleep Questionnaire (FOSQ),29 and the Medical Outcomes Study 6-item Cognitive Functioning Scale (MOS-CF6).30
CPAP Compliance
The Smart Card installed in the CPAP machine (Resmed Elite) was used to provide a measure of CPAP treatment compliance (i.e., mean hours at pressure). Compliance data were obtained for the final 2 weeks of the 6-week period of CPAP treatment.
Statistical Analysis
The primary and secondary efficacy endpoints were conducted on the modified intent-to-treat (mITT) population, defined as all randomized subjects who missed no more than 3 doses of armodafinil and who completed all assessments at end of treatment with armodafinil or placebo (Visit 3). All analyses were prespecified in a Statistical Analysis Plan. Baseline characteristics were assessed for the two medication treatment groups.
The primary efficacy analysis was conducted on the Driving Safety Score (DSS)26 at Visit 3 and compared to baseline (Visit 2) using an analysis of covariance (ANCOVA) with treatment, center (i.e., research site), and treatment × center as fixed effects, and baseline score as the covariate. The DSS is expressed as the mean z-score derived from predefined safety related critical elements (i.e. Total Tickets URB, Total Collisions CV, percent distance exceeded speed tolerance [ES Distance CV], number of times out of driving lane [Out of Lane CV], percent of time exceeded speed tolerance [ES Time CV], number of times over-cornering [Excessive Ay CV], and lane position deviation [Lane Deviation CV; also referred to as standard deviation of lateral position SDLP]).
Recent research on OSA and driving suggested the need to also examine a modified Driving Safety Score (mDSS),26 which was specified a priori. The mDSS is based upon performance on the CV scenario and consists of the following elements; total collisions, time to first collision, number of times out of driving lane (Out of Lane), and lane position deviation during the final 8 min of the scenario (Lane Deviation E5).
The driving related secondary efficacy variables included the components of the DSS and mDSS, lane position deviation by epoch, average speed by epoch and overall, speed deviation by epoch and overall, total crashes by epoch and overall, and out of lane score by epoch and overall. For the URB scenario, additional secondary endpoints included lane position deviation, speed deviation for the construction zone, total collisions outside the construction zone, and total crashes in the construction zone. For the VIG scenario, additional secondary endpoints included out of lane score, total collisions, divided attention correct responses, divided attention omission errors, divided attention commission errors, divided attention reaction time, and total tickets. Driving simulator scores were obtained for each of the 3 driving scenarios at Visit 2 (baseline), following 2 weeks of armodafinil or placebo (Visit 3), and following 6 weeks of CPAP (Visit 5).
Results for the ESS and other self-report measures were analyzed using appropriate nonparametric methods. For categorical secondary efficacy variables, a Cochran-Mantel-Haenszel (CMH) Type 2 (ANOVA mean score) statistic using center as stratum was used for treatment comparison of ordinal variables, while a CMH Type I statistic was used for treatment comparison of nominal variables.
Treatment groups were compared on the measure of CPAP compliance (mean hours at pressure during weeks 5 and 6 of CPAP treatment). Treatment groups were also compared with respect to dropout rates to determine if the use of a pharmacologic agent as a “bridging” therapy affects the rate at which patients present for PSG/titration.
RESULTS
Clinical Population
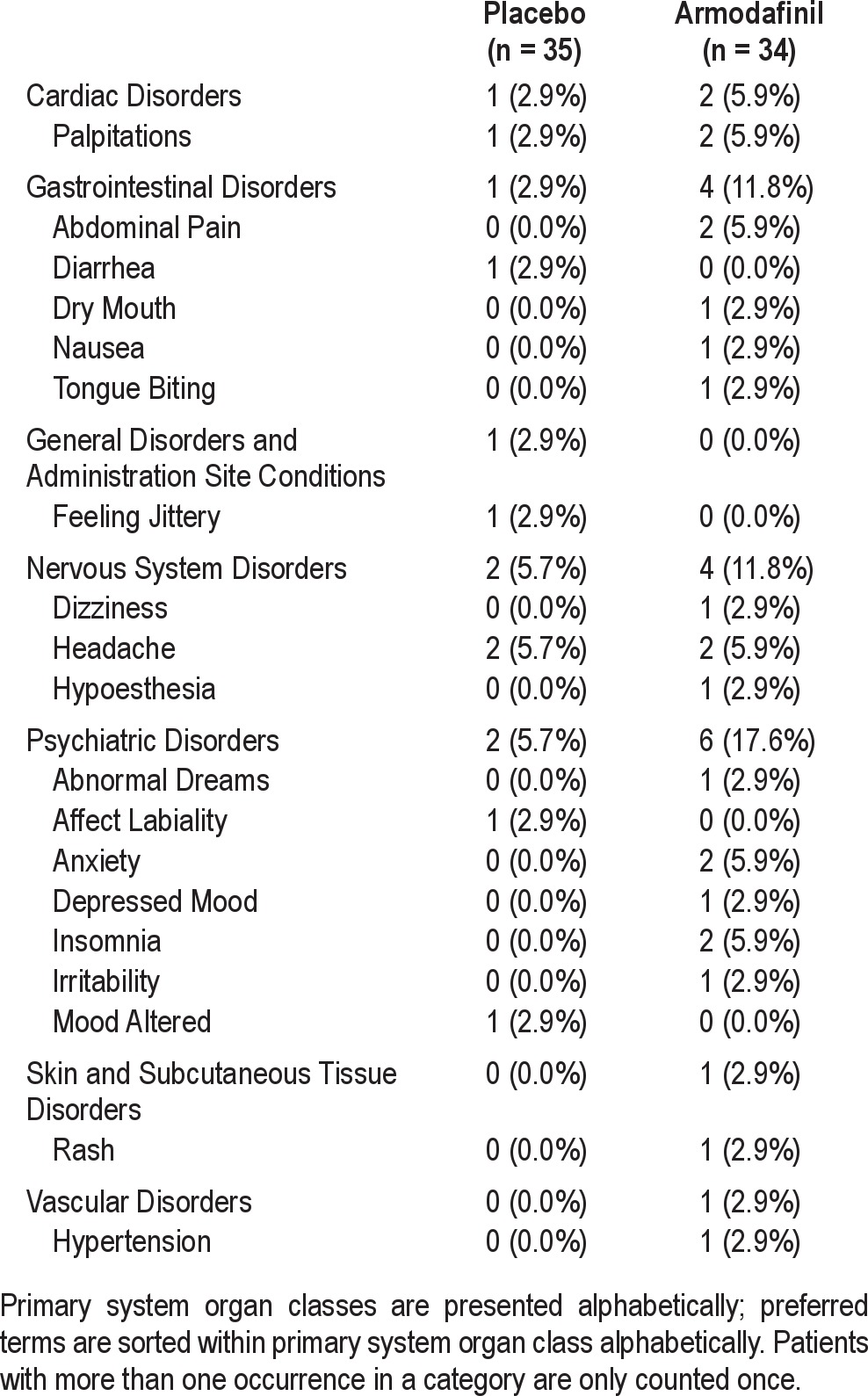
Two hundred twenty-four (224) participants were screened, and 69 were randomized. The most common cause for screen failure was simulator sickness (n = 32; 14.3% of the subjects who were screened), followed by failure to meet the PSG criteria (i.e., AHI ≤ 15; n = 21; 9.4%), failure to meet the medical history criteria (n = 21, 9.4%), low ESS score (n = 10; 4.5%), withdrawal of consent (n = 10; 4.5%), and positive urine drug screen (n = 9; 4.0%). One subject was excluded from the efficacy analysis (the mITT population) due to unwillingness to perform the driving simulation test at the end of Period 1. The same subject withdrew from the study at the beginning of Period 2 due to a work schedule conflict. Four other subjects discontinued during Period 2; one withdrew due to a treatment unrelated adverse event (cataract worsening); one for a positive urine drug screen, and 2 subjects could not tolerate CPAP. In the mITT population, 34 subjects were randomized to each of the 2 treatments. Treatment related adverse events are reported in Table 1.
Table 1.
Treatment-related, treatment-emergent adverse events by system organ class (mITT)
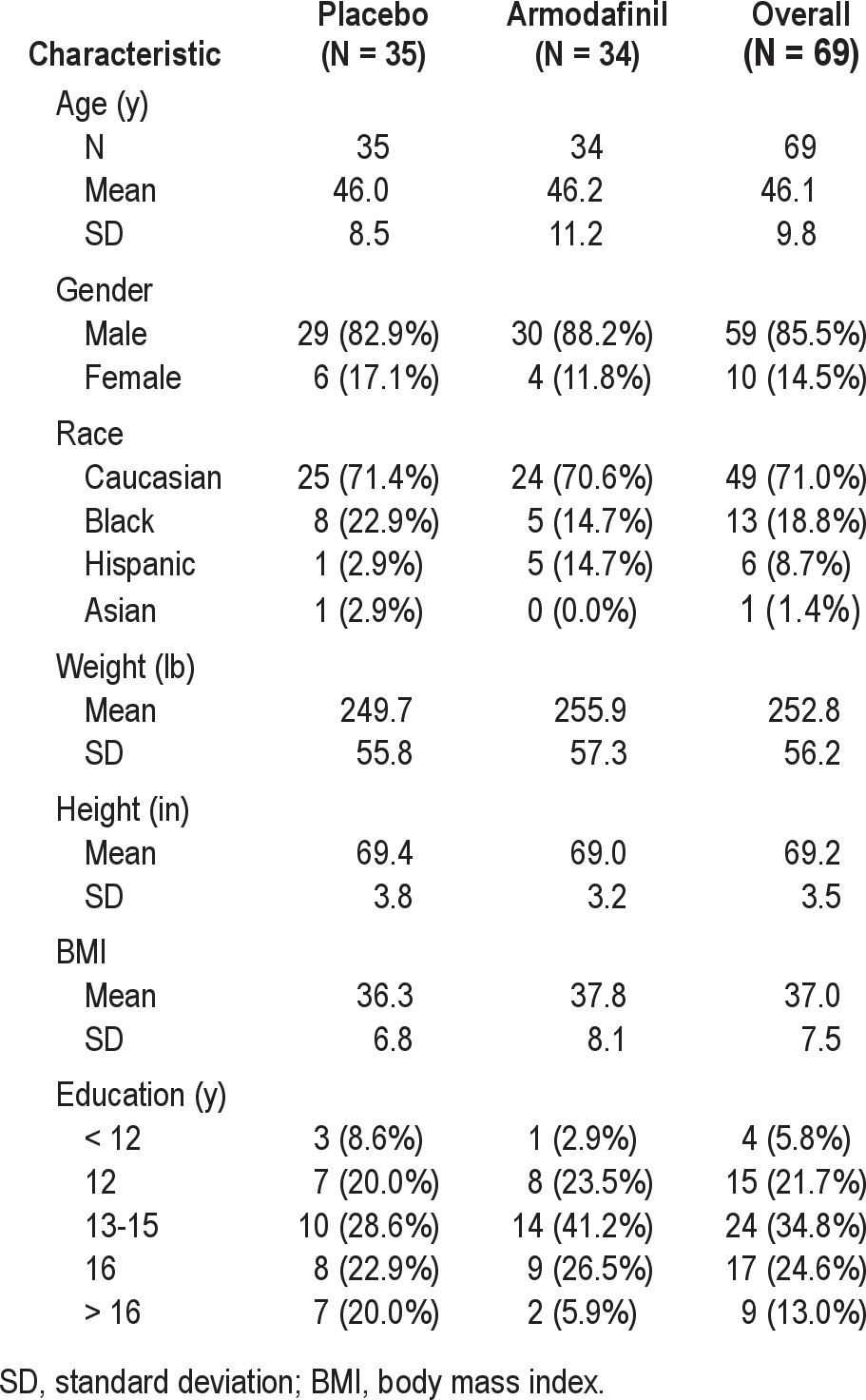
The subject demographics are summarized in Table 2. Sixty-nine (69) subjects were randomized to armodafinil or matching placebo treatment for 14 days. The mean age of participants was 46.1 ± 9.8 years (range 23 to 64). The majority of subjects were males (85.5%). The distribution by race shows that 71.0% were non-Hispanic Caucasian, 18.8% African American, 8.7% Hispanic, and 1.4% Asian. College education (13 or more years of school) was reported by 72.4% of participants. Of the remaining subjects, 21.7% had completed high school and 5.8% had not graduated from high school. The mean AHI at baseline was 43.12 ± 26.1 (range 15.07-114.6), and the mean body mass index (BMI) was 37.0 ± 7.5. The mean ESS score at baseline was 16.8 ± 3.0 (range 12 to 24). There were no significant differences between treatment groups on any of these variables.
Table 2.
Demography of the safety population
Simulated Driving Performance
Effect of Armodafinil
There was significant improvement in the DSS (p = 0.03) for subjects who received armodafinil compared to those treated with placebo (see Table 3). For the DSS, a lower value indicates safer driving. Subjects who received armodafinil had a significantly lower DSS following treatment. In contrast, for subjects who were treated with placebo, the DSS increased from Baseline to Visit 3.
Table 3.
DSS driving variables: change from Baseline to Visit 3 (mITT)
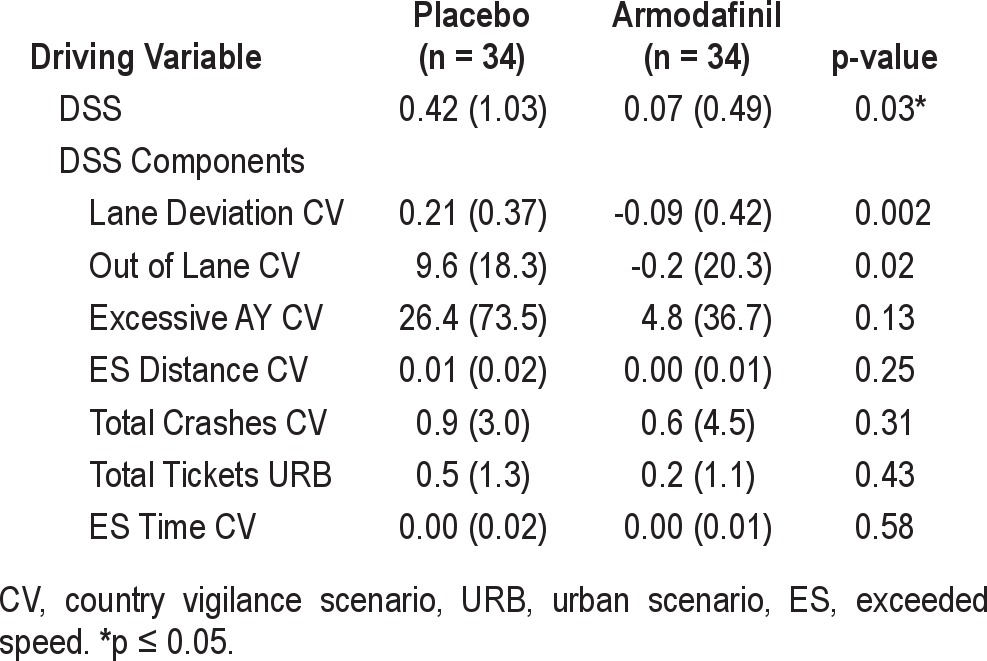
Of the 7 variables (see Table 3) that comprise the DSS, a significant difference between armodafinil and placebo was found for Out of Lane CV (p = 0.02) and Lane Deviation CV (p = 0.002). Except for the 2 speeding variables (ES Time CV and ES Distance CV), which showed no response to treatment, the 3 remaining DSS variables; Excessive Ay CV (cornering speed), Total Tickets URB, and Total Crashes CV demonstrate numerically better performance for the armodafinil group than the placebo group.
Additional driving variables demonstrating a significant difference of p ≤ 0.10 between armodafinil and placebo are found in Table 4. Of those comparisons, only Speed Deviation CV (p = 0.005) and Total Tickets V (p = 0.04) were found to be statistically significant (p ≤ 0.05). A trend towards significance was found for Time to First Crash CV (p = 0.09) and the Divided Attention Reaction Time VIG (p = 0.08) measure.
Table 4.
Additional driving variables (p-values < 0.10): change from baseline to Visit 3 (mITT)
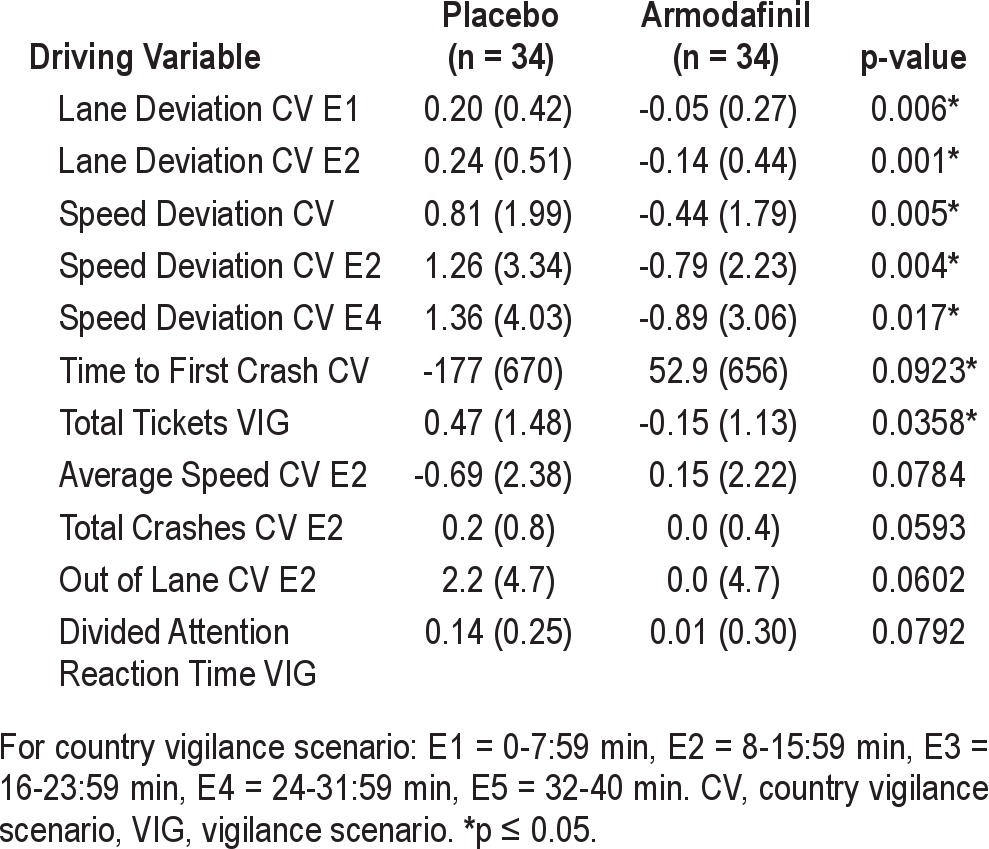
Analysis of time-on-task variables for the lengthy CV shows treatment group differences or trends favoring armodafinil for the second 8-min epoch (Lane Deviation E2, p = 0.001; Average Speed E2, p = 0.08; Speed Deviation E2, p = 0.004; Total Crashes E2, p = 0.06; and Out of Lane E2, p = 0.06). For the first 8-min epoch there was significantly better performance on the measure of Lane Deviation E1 (p = 0.006). In addition, for the measure of Speed Deviation, there was significantly better performance for the fourth 8-min epoch (Speed Deviation E4, p = 0.017).
Effect of CPAP
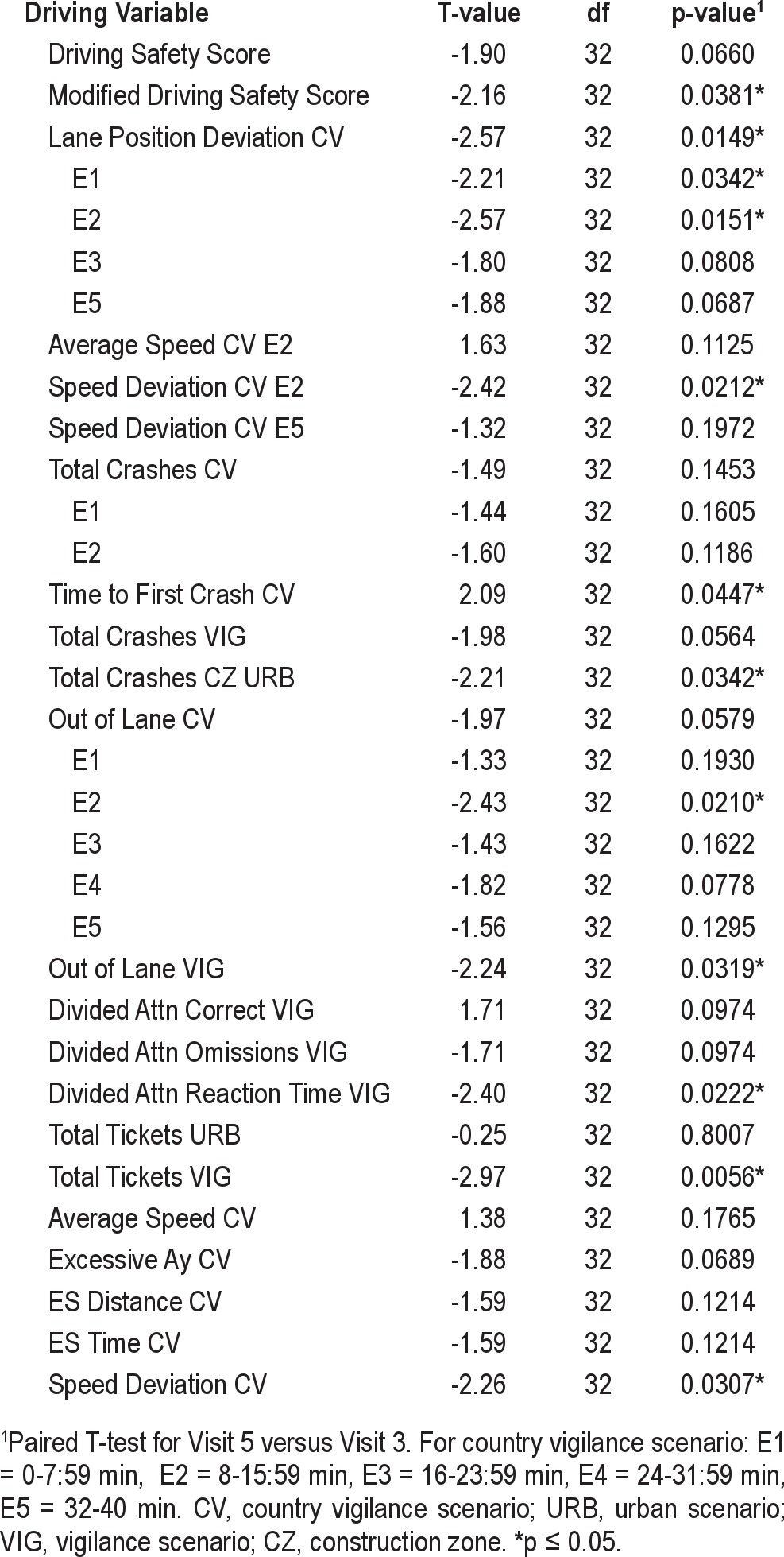
Evaluation of the impact of CPAP was complicated by the unanticipated brief interval between screening and baseline (i.e., < 24 h for 23 of 69 subjects). Experience in our laboratory has shown that the time between driving sessions significantly impacts simulated driving performance (i.e., very short intervals result in inflated retest scores). As a result of the unequal time interval between Visit 1 and Visit 2 (< 24 h), compared to the time between Visit 2 and Visit 3 (generally 2 weeks), the driving simulator results from the Baseline session (Visit 2) were not considered to be optimal for evaluating the effects of CPAP. Based on the interval between visits and the lack of a confounding treatment, assessment of the effect of CPAP on simulated driving performance was based on a comparison of the driving results from Visit 3 (end of drug treatment) and Visit 5 (after 4 weeks of CPAP treatment) for those subjects who were randomized to placebo.
The results for CPAP treatment on driving simulator performance are summarized in Table 5. The DSS showed a trend for improved performance with CPAP treatment (p = 0.07). Signifi-cant improvement was seen for Lane Deviation CV (p = 0.01). Only 1 of the DSS components, Total Tickets URB, failed to show a numerical benefit of CPAP treatment. All other DSS components showed a trend for improved performance (p = 0.06 to p = 0.15) with CPAP treatment. Many other driving simulator parameters showed significant improvement with CPAP, including; Time to First Crash CV (p = 0.045), Speed Deviation CV (p = 0.02), Crashes in the Construction Zone URB (p = 0.03), Out of Lane VIG (p = 0.03), Divided Attention Reaction Time VIG (p = 0.02), and Total Tickets VIG (p = 0.01). Analysis of time-on-task for the 40-min CV drive shows that for the five 8-min blocks (E1- E5), the most significant impact of CPAP was evident during the second 8-min block.
Table 5.
Driving variables: paired comparisons of CPAP effect, Visit 3 (pre-CPAP treatment) vs. Visit 5 (post-CPAP treatment)
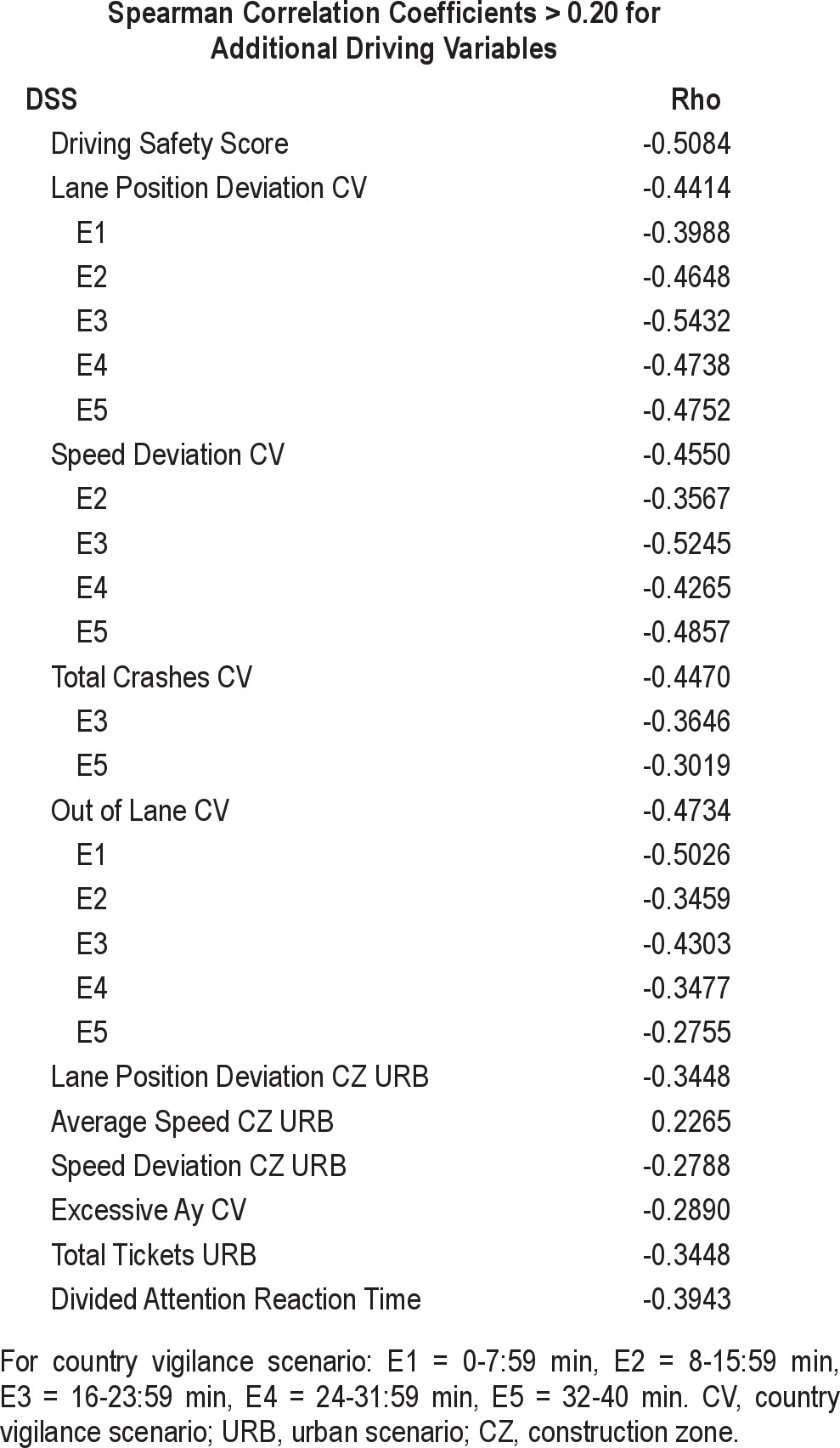
Analysis of the impact of CPAP compliance on simulated driving shows strong correlations between primary and secondary driving variables and hours of CPAP use. Correlations > 0.20 are shown in Table 6. CPAP compliance accounted for 26% of the variance (rho = 0.51) in the DSS.
Table 6.
Correlations between CPAP compliance and driving variables
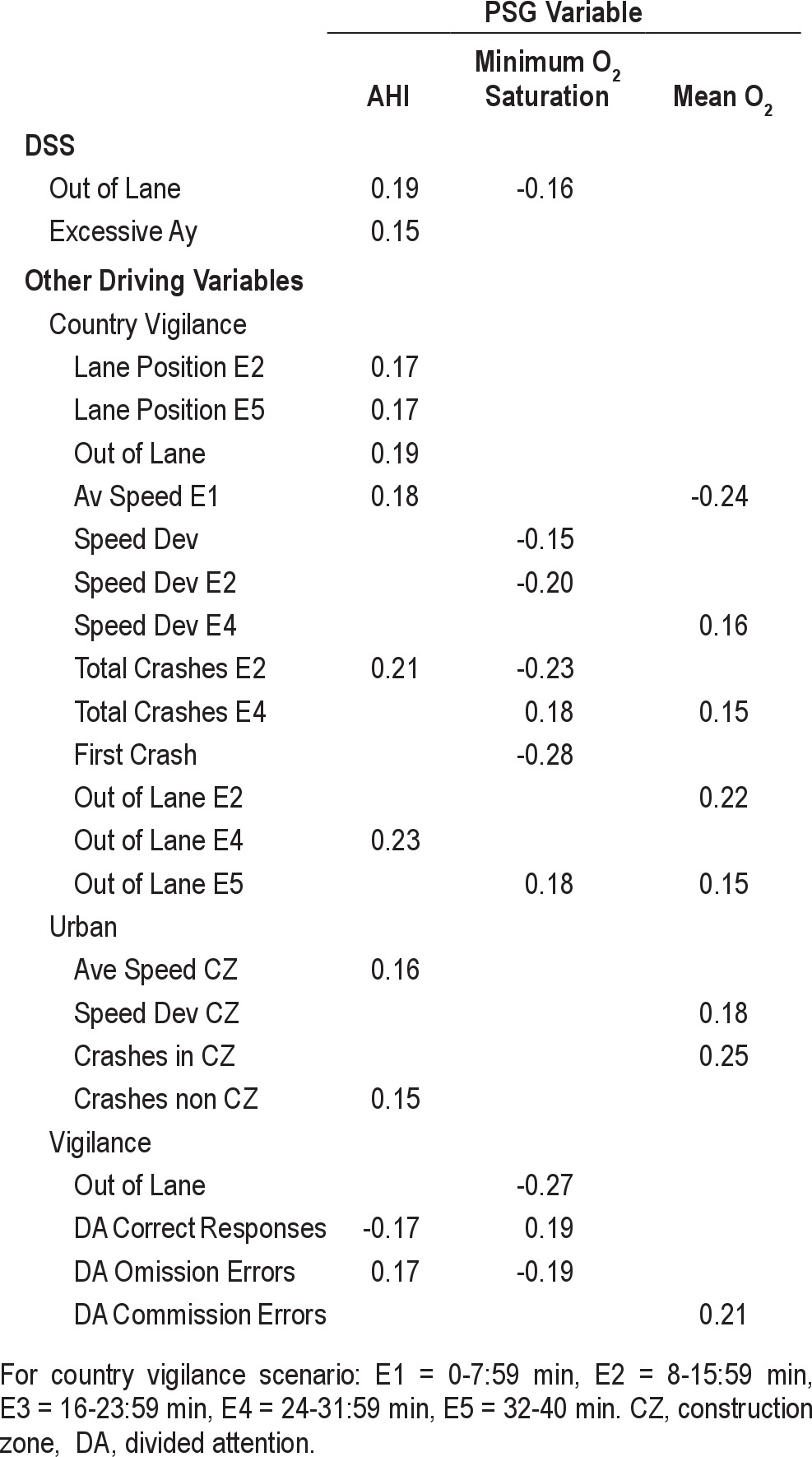
The relationship between baseline driving performance and PSG measures (AHI, mean O2, minimum O2 saturation, and arousal index) is shown in Table 7. This analysis shows that baseline AHI had the most impact on simulated driving performance, followed by minimum O2 saturation and mean O2.
Table 7.
Correlations between baseline PSG and baseline driving variables, Spearman correlation coefficients ≥ 0.15
Patient Reported Outcomes
Following treatment with armodafinil, there was a trend for improved self-reported sleepiness on the ESS (p = 0.066). Following CPAP, the improvement in ESS score was highly significant (p < 0.0001). ESS scores at Visit 5 were correlated with CPAP compliance (r = 0.33).
The FOSQ was administered to assess changes in quality of life. Following treatment with armodafinil, there was a significant improvement compared to placebo in 2 of the 5 FOSQ domains: General Productivity (p = 0.01) and Social Outcome (p = 0.005). Treatment with CPAP resulted in signifi-cant improvements in 3 FOSQ domains: General Productivity (p < 0.0001), Social Outcome (p < 0.0001), and Vigilance (p < 0.0001). FOSQ scores at Visit 5 for these 3 domains were correlated with CPAP compliance: General Productivity, r = 0.20; Social Outcome, r = 0.23; and Vigilance, r = 0.24.
The MOS-CF6 was administered at Baseline, Visit 3, and Visit 5. At Baseline, the placebo group reported more difficulty with cognitive functioning (65.4 ± 17.3) than subjects who were randomized to the armodafinil group (74.7 ± 13.6). Specifically, the placebo group reported more difficulty with problem solving, concentration, disorganization, and memory. At Visit 3, both groups reported less difficulty with cognitive functioning. However, the improvement for the placebo group (8.6 ± 18.1) was somewhat less (p = 0.06) than the improvement reported by the armodafinil group (9.3 ± 12.1). In contrast, CPAP treatment resulted in a highly significant reduction in self-reported cognitive function difficulty (p < 0.0001). The overall change from baseline in MOS-CF6 score for the mITT population following CPAP treatment was 17.4 ± 14.6.
After finishing the CV, subjects responded to 2 visual analog scales: (1) “How well do you think you drove for the last 60 minutes?” and (2) “How motivated did you feel to drive at your best during the last 60 minutes of driving?” From Baseline to Visit 3, subjects receiving placebo reported a decline (-10.9 mm) in their driving performance. In contrast, subjects receiving armodafinil reported an improvement (+1.9 mm) in their driving performance (p = 0.002). CPAP treatment resulted in a marked positive increase in “How well do you think you drove for the last 60 minutes?” (+12.5 mm, p = 0.0023). In response to the visual analog scale (VAS) measuring motivation, subjects receiving placebo reported a decline (-8.3 mm) in motivation. By comparison, those receiving armodafinil reported increased motivation (+4.3 mm, p = 0.0031). Following CPAP treatment, there was an improvement in motivation compared to baseline (+4.9 mm); however, the increase did not reach statistical significance (p = 0.107).
CPAP Compliance
The compliance results for the final 2 weeks of CPAP treatment were used to compare treatment compliance for the armodafinil and placebo groups. Results showed that hours of CPAP compliance did not differ for those subjects who had previously been treated with armodafinil compared to those who had received placebo (p = 0.80).
Clinician Assessment: CGI-s and CGI-c
The Principal Investigator, who was blind to both treatment group assignment and to CPAP compliance results, completed the Clinical Global Impression of Severity (CGI-s) rating at baseline and both the Clinical Global Impression of Change (CGI-c) and the CGI-s at subsequent visits. Mean CGI-s scores and score distributions were comparable at Baseline for the 2 treatment groups (placebo = 4.91; armodafinil = 5.06; 5 = markedly ill). Following treatment with armodafinil or placebo, the mean CGI-c score for the placebo group was 3.09 and for the armodafinil group 2.79. Although, numerically the change from baseline is more favorable for the armodafinil group, the difference between groups is statistically nonsignificant (p = 0.34). At the end of CPAP treatment, there was no difference in CGI-c scores between the 2 treatment groups (p = 0.82). However, for both groups there was a marked improvement in CGI-s (p < 0.0001) compared to Baseline. Of the 68 subjects in the mITT population, 41 (60%) were rated as normal, 14 (21%) were rated as borderline ill, and 4 (6%) were rated as mildly ill at the end of the study. At Baseline, the lowest rating was moderately ill (19%). Following CPAP, only 4 (6%) were rated as moderately ill, and 1 subject was rated as markedly ill (compared to 63% at baseline).
DISCUSSION
A primary objective of this study was to determine whether use of a new wake-promoting agent, armodafinil, prior to initiation of nasal continuous positive airway pressure (CPAP) therapy, would improve the simulated driving performance of patients with excessive daytime sleepiness (EDS) secondary to obstructive sleep apnea (OSA). Another objective of the study was to assess the impact of treatment with armodafinil prior to CPAP treatment on compliance with CPAP treatment.
The results demonstrate that armodafinil improved simulated driving performance prior to initiation of CPAP therapy. This improvement in driving performance was evident in the composite DSS, which was the primary study endpoint (p = 0.03). The DSS was shown to be sensitive to other stimulant agents in a prior study.26 In addition, according to the VAS, subjects appeared to be aware of their improved driving performance in response to treatment with armodafinil compared to placebo (p = 0.002).
The specific driving simulator measures which were most sensitive to the beneficial effects of armodafinil on driving performance were lane position deviation (p = 0.002), lane excursions (p = 0.02; Out of Lane), speed deviation (p = 0.005; Speed Variability), and total tickets (p = 0.036). Armodafinil also showed a trend to improvement (compared to placebo) on measures of reaction time on a divided attention task (p = 0.08) and time to first crash (p = 0.09).
Evaluation of the impact of CPAP on simulated driving was complicated by the unanticipated short interval (i.e., < 24 h) between the screening visit and the baseline visit. To eliminate this effect we assessed the impact of CPAP treatment by comparing the driving simulation results for Visit 3 (end of drug treatment) and Visit 5 (end of CPAP treatment) for the subjects randomized to placebo. Subjects who were randomized to armodafinil were excluded from this analysis to avoid the confounding effect of drug treatment.
In spite of the reduction in the number of subjects (n = 32), due to the exclusion of those treated with armodafinil, the results replicate the improvement in simulated driving performance previously shown following CPAP treatment.2,14
The DSS showed a trend for improved performance following CPAP (p = 0.07). The only DSS component significantly affected by CPAP treatment was Lane Deviation (p = 0.01). This is consistent with the literature demonstrating SDLP to be the most sensitive measure to treatment with CPAP treatment. The Out of Lane measure and the Excessive Ay (cornering speed) measures showed a trend for better performance following CPAP (p = 0.06, and p = 0.07, respectively).
The mDSS, which is based on recent simulation research with OSA patients, showed significant improvement following CPAP (p = 0.038). The four mDSS components which showed a significant effect of CPAP include: Time to First Crash (p = 0.045), Out of Lane (p = 0.06), Lane Position Deviation E5 (p = 0.07), and Total Crashes (p = 0.15).
In addition to the driving measures already mentioned, the beneficial effect of CPAP was also evident on measures of speed deviation (p = 0.03), speeding violations on the vigilance scenario (p = 0.006), divided attention reaction time (p = 0.02), lane excursions on the vigilance scenario (p = 0.03), and crashes in the urban scenario construction zone (p = 0.03). Furthermore, subjects reported awareness of their improved driving performance following treatment with CPAP (p = 0.0023).
As expected, the benefits of CPAP are dependent upon treatment compliance. More than 25% of the variance in the DSS (rho = 0.55) at Visit 5 can be accounted for by CPAP compliance (i.e., hours of CPAP use in the last 2 weeks of the study). The strongest correlations between CPAP compliance and driving variables were found for measures of lane position deviation, speed deviation, and lane excursions. The benefit of CPAP on simulated driving performance is most evident on measures that assess weaving (i.e., maintenance of lane position) and speed control.
The results of the current study also touched on the question of the underlying causes of the driving problems associated with OSA. Regression analyses revealed that simulated driving performance was most strongly associated with baseline AHI, followed by minimum O2 saturation and mean O2 level.
Patient reported outcome scores also demonstrate the value of treatment with armodafinil prior to initiation of CPAP. The improvement in self-reported sleepiness approached significance on the ESS (p = 0.066). Following two weeks of armodafinil, subjects reported significant improvement on sleep-related quality of life outcome scales (FOSQ Productivity, p < 0.001; and FOSQ Social Outcome, p < 0.001). On the other hand, the clinician-based ratings (CGI scales) showed a numerical advantage but not a statistically significant treatment difference in disease severity. Although subjects were less sleepy, they were still judged by the clinician as showing moderate disease severity.
Consistent with the benefits seen on measures of driving performance, the patient reported outcome measures and clinician ratings showed marked benefits of CPAP. The ESS dropped significantly following CPAP (p < 0.0001). ESS improvement was correlated with compliance (r = -0.33). Significant improvement was seen in three FOSQ domains (General Productivity, p < 0.0001), Social Outcome (p < 0.0001), and Vigilance (p < 0.0001). Similarly, CPAP treatment resulted in improved scores on the MOS-CF6. Subjects reported significantly less cognitive difficulties following CPAP (p < 0.0001). These benefits were also evident in the clinician's rating of the patient's condition following CPAP. Specifically, the clinician rating of disease severity (CGI-s) dropped significantly following CPAP (p < 0.0001).
Although treatment with armodafinil improved simulated driving performance in OSA patients, it is not our intention to encourage use of armodafinil as a substitute for treatment with CPAP. Our study was conducted to identify a safe alternative to CPAP during the waiting prior to the initiation of CPAP therapy (i.e., bridging therapy). However, it should be noted that treatment with armodafinil prior to initiation of CPAP did not have an impact on CPAP compliance (p = 0.80). This may allay the concerns that some practitioners may have as to whether treatment prior to initiation of CPAP would discourage subsequent use of CPAP treatment.
In summary, armodafinil was found to improve simulated driving performance in OSA patients with EDS prior to initiation of CPAP. The improvement in driving performance was most evident on measures of lane position control (including the number of lane excursions) and speed control. Subjects treated with armodafinil showed awareness of their improved driving performance. Treatment with armodafinil was not found to impact subsequent CPAP compliance. The improvement seen on driving simulator parameters in this study following CPAP is comparable to that reported in prior studies. Although the purpose of the current study was not to compare armodafinil to CPAP, a review of the study results shows a comparable effect size on DSS for treatment with armodafinil and treatment with CPAP (armodafinil vs. placebo, d = 0.42; Baseline vs. CPAP, d = 0.40).
DISCLOSURE STATEMENT
This was an investigator initiated research study supported by Cephalon, which provided no role in the conception and production of this study. Dr. Kay is President of Cognitive Research Corporation which provided the driving simulators and is an owner of CogScreen LLC which publishes the CogScreen test used to assess cognitive functioning in this trial. Dr. Kay has received research support from Merck, Schering-Plough, Novartis, Pfizer, Astellas, Watson, Shire, and Vivus. Dr. Feldman has received research support, consulting fees and speaker's bureau honoraria from Cephalon, Jazz Pharmaceuticals, Merck, Pfizer, Sanofi, Novartis, Sanofi, Lundbeck, Eli Lilly, Evotec, Bristol-Myers Squibb, Takeda, Sepracor, and Apnicure.
The use of armodafinil (Nuvigil) in this clinical trial is considered off-label, since the approved FDA labeling states that: “In OSA, Nuvigil is indicated as an adjunct to standard treatment(s) for the underlying obstruction. If continuous positive airway pressure (CPAP) is the treatment of choice for a patient, a maximal effort to treat with CPAP for an adequate period of time should be made prior to initiating Nuvigil.”
ACKNOWLEDGMENTS
The study was conducted at the St. Petersburg Sleep Disorder Center, St. Petersburg, FL. The authors thank Dr. Albert Azzaro, Mr. David McLaughlin, and Mr. Andrew Thurston for their critical review of the manuscript, and Dr. Julie Jones for performing the statistical analysis. We also thank Mary O'Brien, ARNP, study coordinator, for her dedication to this project, and Ms. Debbie Lees for organizing and finalizing the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANCOVA
analysis of covariance
- APPLES
Apnea Positive Pressure Long-term Efficacy Study
- BMI
body mass index
- CGI
clinical global impression
- CGI-c
clinical global impression of change
- CGI-s
clinical global impression of severity
- CMH
Cochran-Mantel-Haenszel
- CNS
central nervous system
- CPAP
continuous positive airway pressure
- CRCDS
Cognitive Research Corporation Driving Simulator
- CV
country vigilance driving scenario
- DSS
Driving Safety Score
- EDS
excessive daytime sleepiness
- EEG
electroencephalogram
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes Sleep Questionnaire
- mDSS
modified Driving Safety Score
- mITT
modified intent-to-treat
- MOS-CF6
Medical Outcomes Study 6-item Cognitive Functioning Scale
- MWT
maintenance wakefulness test
- nCPAP
nasal continuous positive airway pressure
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- PVT
psychomotor vigilance test
- SDLP
standard deviation of lateral position
- SSQ
simulator sickness questionnaire
- STI
Systems Technology, Inc.
- URB
urban driving scenario
- VAS
visual analog scale
- VIG
vigilance driving scenario
REFERENCES
- 1.National Commission on Sleep Disorders Research. Vol. 1. Bethesda, MD: National Institutes of Health; 1991. Executive summary and executive report. [Google Scholar]
- 2.Findley L, Smith C, Hooper J, Dineen M, Suratt PM. Treatment with nasal CPAP decreases automobile accidents in patients with sleep apnea. Am J Respir Crit Care Med. 2000;161:857–9. doi: 10.1164/ajrccm.161.3.9812154. [DOI] [PubMed] [Google Scholar]
- 3.George CF, Nickerson PW, Hanly PJ, Miller TW, Kryger MH. Sleep apnea patients have more automobile accidents. Lancet. 1987;ii:447. doi: 10.1016/s0140-6736(87)90974-3. [DOI] [PubMed] [Google Scholar]
- 4.Vaa T. Oslo: Institute of Transport Economics; 2003. Impairments, diseases, age and their relative risks of accident involvement: Results from a meta-analysis. [Google Scholar]
- 5.Vakulin A, Baulk SD, Catcheside PG, et al. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. Sleep. 2007;30:1327–33. doi: 10.1093/sleep/30.10.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George CF. Driving simulators in clinical practice. Sleep Med Rev. 2003;7:311–20. doi: 10.1053/smrv.2001.0233. [DOI] [PubMed] [Google Scholar]
- 7.Desai AP, Wilsmore B, Bartlett DJ, et al. The utility of the AusEd driving simulator in the clinical assessment of driver fatigue. Behav Res Methods. 2007;39:673–81. doi: 10.3758/bf03193039. [DOI] [PubMed] [Google Scholar]
- 8.Risser R, Risser MS, Catesby J, Ware C, Freeman FG. Driving simulation with EEG monitoring in normal and obstructive sleep apnea patients. Sleep. 2000;23:1–6. [PubMed] [Google Scholar]
- 9.Philip P, Sagaspe P, Taillard J, et al. Fatigue, sleepiness, and performance in simulated versus real driving conditions. Sleep. 2005;28:1511–6. doi: 10.1093/sleep/28.12.1511. [DOI] [PubMed] [Google Scholar]
- 10.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: Systematic review and meta-analysis. Sleep. 2010;33:1373–80. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George CFP. Reduction in motor vehicle collisions following treatment of sleep apnea with nasal CPAP. Thorax. 2001;56:508–12. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baulk SD, Biggs SN, Reid KJ, van den Heuvel CJ, Dawson D. Chasing the silver bullet: Measuring driver fatigue using simple and complex tasks. Accid Anal Prev. 2008;40:396–402. doi: 10.1016/j.aap.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Hack M, Davies RJO, Mullins R, et al. Randomized prospective parallel trial of therapeutic versus sub-therapeutic nasal continuous positive airway pressure on simulated steering performance in patients with obstructive sleep apnea. Thorax. 2000;55:224–31. doi: 10.1136/thorax.55.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orth M, Duchna HW, Leidag M, et al. Driving simulator and neuropsychological testing in OSAS before and under CPAP therapy. Eur Respir J. 2005;26:898–903. doi: 10.1183/09031936.05.00054704. [DOI] [PubMed] [Google Scholar]
- 15.Turkington PM, Sircar M, Saralaya D, Elliott MW. Time course of changes in driving simulator performance with and without treatment in patients with sleep apnea hypopnoea syndrome. Thorax. 2004;59:56–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 2005;28:464–71. doi: 10.1093/sleep/28.4.464. [DOI] [PubMed] [Google Scholar]
- 17.Dinges DF, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Med. 2003;4:393–402. doi: 10.1016/s1389-9457(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 18.Grunstein RR, Newcombe A, Desai A, Joffe D, Seale JP. Modafinil improves alertness and driving simulator performance in sleep-deprived mild obstructive sleep apnoea (OSA) patients. Road Safety Conference; 2001; Melbourne, Victoria. [Google Scholar]
- 19.Gurtman CG, Broadbear JH, Redman JR. Effects of modafinil on simulator driving and self-assessment of driving following sleep deprivation. Hum Psycho-pharmacol Clin Exp. 2008;23:681–92. doi: 10.1002/hup.983. [DOI] [PubMed] [Google Scholar]
- 20.Williams SC, Rogers NL, Marshall NS, Leung S, Starmer GA, Grunstein RR. The effect of modafinil following acute CPAP withdrawal: a preliminary study. Sleep Breath. 2008;12:359–64. doi: 10.1007/s11325-008-0175-9. [DOI] [PubMed] [Google Scholar]
- 21.Williams SC, Marshall NS, Kennerson M, Rogers NL, Liu PY, Grunstein RR. Modafinil effects during acute continuous positive airway pressure withdrawal: A randomized crossover double-blind placebo-controlled trial. Am J Respir Crit Med. 2010;181:825–31. doi: 10.1164/rccm.200908-1307OC. [DOI] [PubMed] [Google Scholar]
- 22.Hirshkowitz M, Black JE, Wesnes K, Niebler G, Arora S, Roth T. Adjunct armodafinil improves wakefulness and memory in obstructive sleep apnea/hypopnea syndrome. Respir Med. 2007;101:616–27. doi: 10.1016/j.rmed.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 23. Nuvigil [package insert]. Cephalon, Inc. Frazer, PA. 2010 [cited 2012 Oct 17]. Available from: http://www.nuvigil.com/media/Full_Prescribing_Information.pdf.
- 24.Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG. Simulator sickness questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol. 1993;3:203–20. [Google Scholar]
- 25.Kushida CA, Nichols DA, Quan SF, et al. The apnea positive pressure long-term efficacy study (APPLES): Rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2:288–300. [PubMed] [Google Scholar]
- 26.Kay GG, Michaels MA, Pakull B. Simulated driving changes in young adults with ADHD receiving mixed amphetamine salts extended release and atomoxetine. J Atten Disord. 2009;12:316–29. doi: 10.1177/1087054708322986. [DOI] [PubMed] [Google Scholar]
- 27.Findley LJ, Suratt PM, Dinges DF. Time-on-task decrements in “steer clear” performance of patients with sleep apnea and narcolepsy. Sleep. 1999;22:804–9. doi: 10.1093/sleep/22.6.804. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 30.Ware JE, Kosinski M, Dewey JE. Lincoln, RI: QualityMetric, Incorporated; 2000. How to score version two of the SF-36 health survey. [Google Scholar]


