Abstract
Study Objectives:
Obesity is a risk factor for sleep disordered breathing (SDB) in children. Plasma levels of high-sensitivity C-reactive protein (Hs-CRP) are predictive of cardiovascular morbidity in adults, and CRP levels are associated with over-weight. Increased carotid intima-media thickness (IMT) is associated with several cardiovascular risk factors. We evaluated the effect of SDB on CRP levels and IMT in lean and obese children not selected for snoring.
Methods:
101 children (age 5-15 years) attending a weight clinic or scheduled for routine visit. IMT was measured with quantitative B-mode ultrasound scans. The apnea-hypopnea index (AHI) was measured overnight: AHI < 1 defined controls, AHI ≥ 1 to < 5 = mild SDB, and AHI ≥ 5 = obstructive sleep apnea (OSA).
Results:
AHI was significantly associated with Hs-CRP concentration (r = 0.32, p = 0.002) in all 101 children irrespective of age and sex. Body mass index (BMI) was higher in OSA children than controls (25.5 ± 7.0 vs 22.1 ± 6.9, p = 0.05). Obese children had 3.3 times more probability of having OSA (HR 3.3, 95% CI 1.2-9.3, p = 0.02) than lean children.
Hs-CRP values were significantly higher in children with OSA than in children without (p = 0.011), but not when BMI z-score was added as covariate. IMT was not associated with AHI or SDB.
Conclusions:
The results of this study suggest an association between OSA and Hs-CRP concentrations (mainly mediated by overweight and obesity), but not between OSA and subclinical atherosclerosis. There is scope for prevention in childhood before OSA syndrome causes the irreversible damage to arteries observed in adult patients.
Citation:
Iannuzzi A; Licenziati MR; De Michele F; Verga MC; Santoriello C; Di Buono L; Renis M; Lembo L; D'Agostino B; Cappetta D; Polverino M; Polverino F. C-reactive protein and carotid intima-media thickness in children with sleep disordered breathing. J Clin Sleep Med 2013;9(5):493-498.
Keywords: C-reactive protein, intima-media thickness, obstructive sleep apnea
Sleep disordered breathing (SDB) is characterized by repeated events of partial or complete upper airway obstruction during sleep that result in disruption of normal ventilation, hypoxemia, and sleep fragmentation. SDB is frequent in obese individuals and is associated with cardiovascular morbidities.1,2
Over the last 30 years, the prevalence of overweight and obesity has increased substantially across all pediatric age groups. The “obesity pandemic” is expected to be deleterious to global health outcomes and life expectancy.3 Current evidence suggests that overweight is associated with SDB among children,4 and the rising prevalence of childhood overweight is likely to increase the prevalence of childhood obstructive sleep apnea syndrome (OSAS), as demonstrated in a recent Italian study.5 In adults, OSAS is associated with cardiovascular disease and obesity.6
A possible mechanism underlying an increased prevalence of atherogenesis among OSAS patients could be sympathetic activation,7 with consequent initiation and propagation of inflammatory responses within the microvasculature. Also, in non-obese children, OSAS may lead to comorbidities that are remarkably similar to those associated with obesity and may recruit inflammatory mechanisms like those activated by obesity, which suggests that the two disorders may amplify each other.8 One of the most relevant serum markers of inflammation, at least in adults, is the high-sensitivity C-reactive protein (Hs-CRP), which has recently emerged as one of the most powerful independent predictors of risk for future cardiovascular morbidity.9,10 However, a pediatric population would be a better cohort in which to evaluate a possible association between SDB and low-grade inflammation because in adulthood there is a strong confounding influence of other cardiovascular risk factors, complete elimination of which might be difficult if not impossible.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep apnea is often associated with cardiovascular disease and atherogenesis. This study was done to evaluate the association between sleep apnea and low-grade inflammation in a pediatric population without confounding influence of risk factors, as in adults.
Study Impact: The study demonstrated that obstructive sleep apnea in children is not associated with subclinical atherosclerosis. The major clinical implication of our study is that there is scope for prevention in childhood before sleep apnea syndrome causes the irreversible damage to arteries observed in adult patients.
The prevalence of OSAS in children differs among studies with estimates ranging between 0.7% and 10.3%.11 A possible limitation of most studies regarding SDB or OSAS in children is that they focus on children, obese or lean, who underwent polysomnography (PSG) for primary snoring. Snoring children are not representative of the general population of children. In the present study, we measured plasma Hs-CRP levels in a cohort of lean and obese children (not recruited for snoring) who were investigated for SDB. We also evaluated whether SDB severity is associated with subclinical carotid atherosclerosis.
METHODS
Patients and Methods
All the children from 5 to 15 years recruited from a subset of a community sample scheduled for a standard routine visit by their ambulatory pediatrician and all the children in the same age range evaluated for overweight or obesity in a specialistic setting care in September, October, November, and December 2009, were invited to participate in a study to evaluate their cardiovascular risk factors and to investigate sleep breathing disorders by full PSG. Carotid ultrasound was performed in all enrolled subjects and intima-media thickness (IMT) was used as a proxy of vascular health. The local Ethics Committee “ASL SA1” approved the study on April 2, 2007 (approval number n° 21/2007). A total of 150 children were invited to participate, and 130 (54% males) accepted. Written informed consent was provided by the children and their parents. No child had any acute or chronic disease, and none was on regular medication. There was no family history of premature cardiovascular disease. A complete medical history, including a standard symptom questionnaire and history of snoring or atopy, was obtained from the parents.
Twenty children with a history or tests positive for asthma or allergic rhinitis were excluded. An expert pediatrician assessed pubertal development, based on Tanner stage, by physical examination. All subjects underwent a physical examination, and tonsillar size was graded from 0 to 4.12 Nine children with a tonsillar size graded as 4 were excluded from the study, leaving a final cohort of 101 children.
Anthropometric Measurements
Anthropometric measurements were made with the children wearing only underclothes and no shoes. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body weight was measured to the nearest 0.1 kg with a digital scale. Waist circumference was measured at the level of the umbilicus and the superior iliac crest at the end of a normal expiration, while the child stood upright. The body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. BMI percentiles for age and standard deviation scores for BMI were based upon the Center for Disease Control normative curves.13 Obesity was defined as BMI > 95th percentile. The children had to rest for 10 min in a quiet, comfortable room before blood pressure measurements.
Pressure Measurement
Blood pressure was measured in the sitting position, using the right arm, with a standard mercury sphygmomanometer. After the appropriate-size cuff had been applied (covering approximately 80% of the circumference of the upper arm), the cuff was gradually inflated to about 20 mm Hg above the point at which the radial pulse disappeared. The pressure within the cuff was then deflated at a rate of 2-3 mm Hg/sec while the physician auscultated with a stethoscope over the brachial artery. The physician recorded systolic blood pressure (SBP) as the first Korotkoff sound and diastolic blood pressure (DBP) as the fifth. Pressures were measured 3 times at 1-min intervals, to the nearest 2 mm Hg. The measurements were then averaged for statistical analysis. Mean blood pressure was calculated as diastolic pressure plus one-third of pulse pressure.
Laboratory Analyses
Blood for high-sensitivity assessments of plasma CRP levels and for other biochemical parameters was drawn the morning after each child underwent a standard PSG evaluation in the sleep laboratory. C-reactive protein was measured by nephelometry (BNTnII, Dade Behring, Liederbach, Germany), and glycated hemoglobin was measured with high-performance liquid chromatography (Variant II, BioRad Laboratories, Hercules, CA). Plasma insulin was measured with an analyzer for heterogeneous immunoassays (Elecsys 2010, Roche Diagnostics, Mannheim, Germany). Total cholesterol, triglyceride, high-density lipoprotein cholesterol, and glucose concentrations were measured by enzymatic assays (Roche/ Hitachi 747, Roche Diagnostics GmbH, Mannheim, Germany). The low-density lipoprotein cholesterol concentration was calculated using Friedewald's formula. Insulin resistance was calculated with the homeostasis model assessment-insulin resistance (HOMA-IR) index (fasting insulin × fasting glucose / 22.5), as described by Matthews et al.14
Ultrasound B-mode Imaging
Carotid B-mode ultrasound examinations were performed with an Aloka, SSD 4000 and a 7 to 13 MHz linear array probe. The ultrasound protocol involved a detailed investigation of the distal 1.0 cm of the near and far walls of the right and left common carotid artery before the crest at the origin of the bifurcation. Different scanning angles (anterior, lateral, and posterior) were used to identify the greatest IMT in each wall. Quantitative measurements of the near and far wall IMT were performed at the end of each examination on digitally stored images using electronic calipers. The sonographers, unaware of the study aim, reviewed the images and selected the frame that contained the thickest IMT for each of the 4 carotid walls. The mean of these 4 maximum thicknesses was reported as carotid IMT. Common carotid IMT measurements were obtained in all children. To evaluate the repeatability and intraobserver variability of vascular measurements in children, we performed the carotid ultrasound scan in the same setting and with the same sonographer on 2 occasions (1 to 7 days apart) in 21 healthy children of a previous study. The coefficient of variation was 3.9% for the IMT measurements.15
Polysomnography
Children were evaluated in the sleep laboratories of the Respiratory Departments of the Cava de' Tirreni Hospital, in a quiet darkened room with an ambient temperature of 22-24°C, in the company, in a separate bed, of one of their parents. No sedation or sleep deprivation was used before the study. Subjects arrived at the laboratory at 21:00, and studies were terminated at 07:00. Children underwent a standard multichannel overnight PSG (Compumedics Sleep, Abbotsford, Australia). Electroencephalogram (C3/A2, 01/A2), right and left electroculograms, submental electromyogram, and tibial electromyogram were continuously monitored. Nasal/oral airflow was measured by thermistors (nasal pressure cannula with an oral thermistor bead). Chest and abdominal wall movements were assessed by inductance plethysmography. Heart rate was assessed by electrocardiography, and transcutaneous hemoglobin oxygen saturation by pulse oximetry (Pulsox, Minolta) with simultaneous monitoring of pulse waveforms. Snoring intensity was monitored with a microphone sensor placed at the level of the neck, and body position was continuously recorded. All polysomnograms were supervised and recorded by an experienced sleep technologist. All data were stored for off-line analysis and were subsequently reviewed by a single investigator, a physician with expertise in sleep medicine, to ensure consistency. The percentage of time spent in each sleep stage was expressed as percentage of the total sleep time (TST). Obstructive apneas were defined by a complete cessation of airflow with continued chest wall and abdominal movement of at least two breaths. Hypopneas were defined as a reduction in airflow ≥ 50% from the immediately preceding recordings, with a corresponding decrease in oxygen saturation of > 4% and/or arousal. Breathing disturbances in sleep were quantified as the apnea index (AI) and apnea-hypopnea index (AHI). The obstructive apnea index was defined as the number of apnea events per hour of TST, and AHI was defined as the number of obstructive apnea and hypopnea events per hour of TST. AHI values ≥ 5 episodes/h of TST or AI values > 1 indicated obstructive sleep apnea (OSA); AHI values ≥ 1 and < 5 indicated mild SDB; and AHI values < 1 indicated controls. These cutoff points are widely used in clinical practice in children and are associated with such meaningful clinical outcomes as hypertension. Clinically, the data support the threshold of AHI > 5 (or AI > 1) for the initiation of treatment for SDB in children.16,17 Arousals were identified by an abrupt shift in EEG frequency lasting > 3 seconds. Arousal index, defined as the number of arousals divided by the TST, and sleep efficiency, defined as the ratio of TST to nocturnal time in bed, were also measured.
Statistical Analysis
The sample size of the study has been calculated using the following assumptions:
0.055 mm as a relevant difference in carotid IMT between the groups18
standard deviation of 0.06 mm18
α error (2-sided): 0.05
β error: 0.20
Using these criteria, a minimum of 19 children per group was necessary to test the hypothesis of a difference in carotid IMT among children with OSA, SDB, and controls.
Because plasma Hs-CRP levels were not normally distributed, logarithmic transformation was applied (LnHs-CRP). Correlations of Hs-CRP levels with AHI were evaluated by linear regression followed by calculation of Pearson correlation coefficients. Comparisons of demographic, biochemical, ultra-sound, and PSG data among groups were made with independent t tests or analysis of variance, with p values adjusted for unequal variances when appropriate (Levene test for equality of variances). Because obesity would be expected to contribute to increased CRP levels, we developed a general linear model and performed an analysis of covariance with LnCRP as dependent variable, categorized SDB as fixed factor and age, sex, and BMI z-score as covariates. Logistic regression analyses were used to determine whether obese children were at increased odds of OSA. All p values reported were 2-tailed, with statistical significance set at > 0.05. All analyses were performed using the Statistical Package for the Social Sciences Software (version 17.0; SPSS, Inc., Chicago, IL).
RESULTS
In all 101 children enrolled in the study, AHI was significantly associated with LnHs-CRP concentrations (r = 0.32, p = 0.002), irrespective of age and sex. However, when BMI was added to the model, the association did not quite reach significance level (p = 0.075). Age was significantly correlated with DBP, also after correction for AHI and sex (r = 0.13, p = 0.041), but this association lost statistical significance after the addition of BMI to the model (p = 0.32). No statistical association was found between AHI and SBP.
Glucose concentrations were similar in obese and non-obese children, but obese children had significantly higher concentrations of serum insulin and a higher HOMA index, which indicates an early insulin-resistance status. Socioeconomic status, evaluated by the parents' level of education, was significantly lower in obese children. SBP, DBP, and carotid IMT values were significantly higher in obese children (Table 1). Moreover, obese children had higher plasma concentrations of transaminases, especially alanine-transaminase, which could be markers of early hepatic steatosis (Table 1).
Table 1.
Anthropometric, biochemical, subclinical atherosclerosis markers and sleep disordered breathing in obese and non-obese children (crude data)
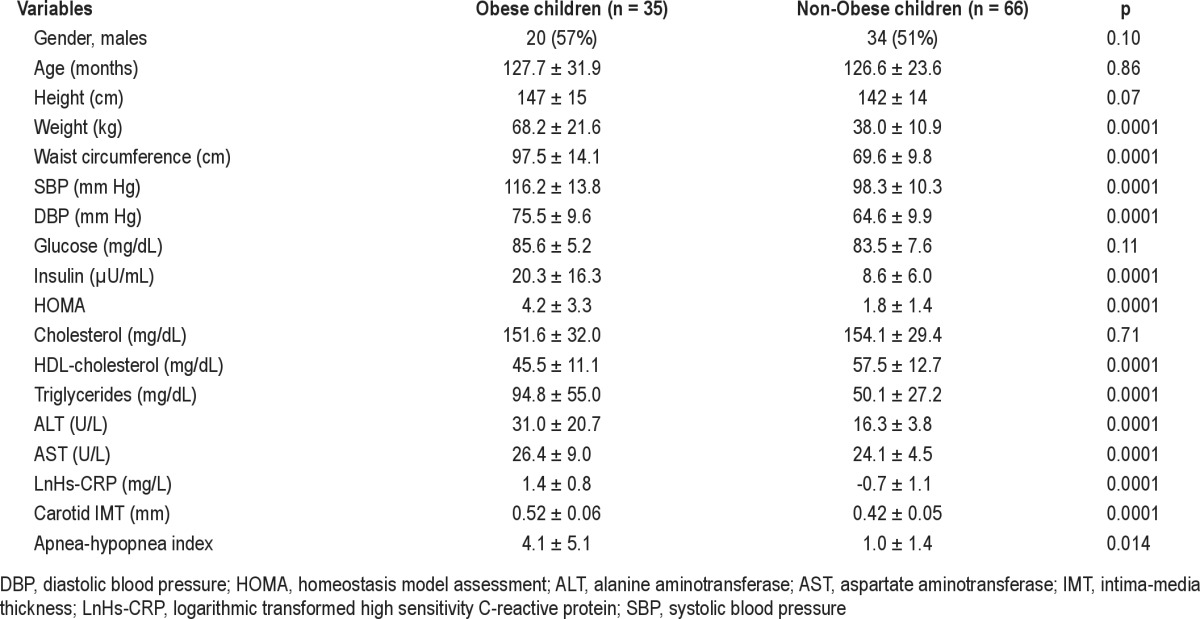
At logistic regression analysis, obese children had 3.3 times more probability of having OSA (hazard ratio 3.3, 95% CI 1.2-9.3, p = 0.02) than non-obese children. LnHs-CRP levels were significantly higher in OSA children than in children with mild SDB and controls (Table 2). Hs-CRP values were significantly higher in children with OSA than in children without OSA (p = 0.011; Figure 1), but statistical significance was lost after adjustment for BMI z-score.
Table 2.
Anthropometric, biochemical, and subclinical atherosclerosis markers in OSA (AHI > 5), mild sleep disordered breathing (AHI > 1 to < 5), and control children (AHI < 1) (crude data)
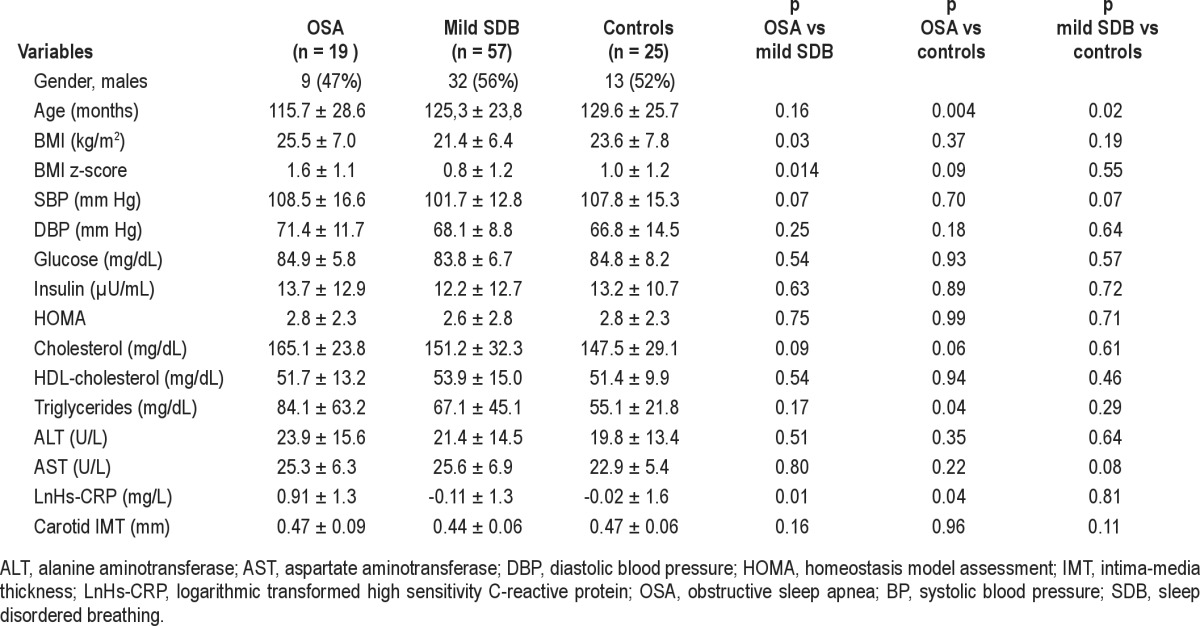
Figure 1. Ln CRP: High-sensitivity C-reactive protein (logarithmically transformed).
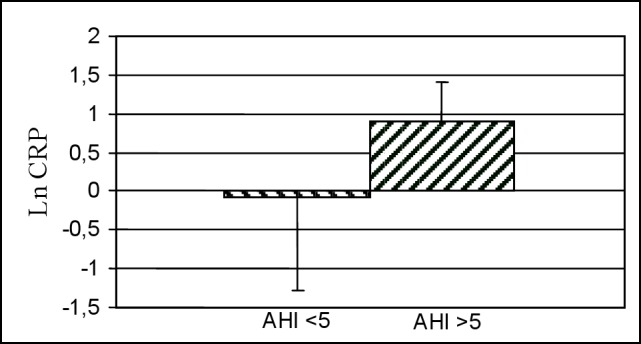
AHI, apnea-hypopnea index.
DISCUSSION
In an effort to identify pathophysiological links between obstructive sleep apnea-hypopnea and early markers of cardiovascular disease in the pediatric age group, we evaluated biochemical parameters and conducted carotid ultrasonography and PSG in a group of obese and lean children not selected for snoring. To our knowledge, this is the first study that puts together data regarding subclinical atherosclerotic markers, sleep-breathing disorder parameters, and low-grade inflammation biochemical markers in a pediatric population not affected by snoring. Here we demonstrate: (i) an association between AHI and CRP concentrations, mainly mediated by overweight and obesity; (ii) a significantly greater low-grade inflammation in children with OSA than in children without OSA; and (iii) that OSA is not associated with subclinical atherosclerosis.
Association between AHI and CRP Concentrations, Mainly Mediated by Overweight and Obesity
Linear regression analysis showed a significant association between AHI and LnHs-CRP in all children enrolled in the study. The novelty of this finding lies in the demonstration of this association in children not selected for snoring. In our cohort, logistic regression analysis showed that obese children had a three-fold higher risk of having OSA than non-obese children.
In adults, OSAS is associated with cardiovascular disease, and elevated CRP plasma concentration could be the link between the two disorders.19,20 In snoring children, Tauman et al. reported significantly higher CRP levels in children with OSA (AHI > 5) versus children with mild SDB (AHI > 1 and < 5) and control children (AHI < 1). Moreover, CRP levels were associated with SDB irrespective of relative BMI. The authors speculated that the strong epidemiologic links between CRP levels and athero-genesis could indicate that the coincidence of SDB with higher CRP levels could increase the risk of atheroma formation among children with OSA.21 In another study of an adolescent cohort free of known cardiovascular disease, SDB was associated with increasing levels of CRP.22 In agreement with our research, AHI was closely related to CRP only when AHI exceeded 5, whereas no significant association was found in children with an AHI < 5. Differently, in a survey of Greek children, mean CRP values did not differ between control subjects and snorers with an AHI < 1, snorers with mild SDB, and snorers with OSA.23
In our study, the association between AHI and LnHs-CRP maintained statistical significance when the covariates sex and age were added to the model, but was just below significance level when BMI was added. These data indicate that a high BMI is important in promoting the link between SDB and a pro-inflammatory state in children.
Low-Grade Inflammation Is Significantly Greater in Children with OSA than in Children without OSA
In our cohort, Hs-CRP values were significantly higher in children with OSA than in children without OSA. However, the addition of the covariate BMI z-score to the model abolished the significant difference between the two groups. This finding is in line with the well-known association between obesity and both SDB and a pro-inflammatory state also in children. In another study conducted in a clinical sample of obese children, SDB was associated with elevation of proinflammatory cytokines.16 In the latter study, Hs-CRP levels were elevated in obese children with SDB and the elevation was due to both SDB and obesity.
The increased CRP concentrations in children with OSA could indicate a link between the intermittent hypoxemia and sleep disturbance of SDB and inflammatory responses. The association in children of elevated CRP levels with OSA suggests that AHI > 5 facilitates the activation of low-grade inflammatory processes and that, together with overweight-obesity, may be responsible, at least in part, for the morbidity associated with SDB.
Association between SDB and Subclinical Atherosclerosis Markers
Primary snoring was associated with higher blood pressure and reduced arterial distensibility in a small cohort of Chinese children.24
In adults, carotid markers of subclinical atherosclerosis are significantly higher in OSAS.25 In our study, SDB was not associated with carotid IMT, a proxy of subclinical atherosclerosis. It is feasible that, in the pediatric age group, OSA, especially when associated with obesity, is able to elicit a low-grade inflammatory response, but, differently from adults,26,27 it does not cause clear signs of carotid atherosclerosis. However, SDB and obesity may be part of a negative profile of cardiovascular risk that continues into adulthood, thereby facilitating development of cardiovascular morbidities.
A limitation of the present study is the high prevalence of overweight/obesity and OSA in our cohort. However, the South of Italy has the greatest prevalence among western countries with 41.8% of children and teenagers being overweight or obese.28
In conclusion, our study demonstrates that SDB is associated with low-grade inflammation in children, but this association was mediated by BMI. Our study also reaffirms that overweight and obesity play a role in promoting low-grade inflammation and disturbances of sleep-breathing, and it provides the first demonstration that SDB and carotid atherosclerosis are not associated in children. Taken together, these findings confirm the importance of early intervention in childhood, before the onset of OSAS-induced ultrasound-detectable atherosclerosis and before OSAS causes the irreversible damage to arteries observed in adult patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Jean Ann Gilder (Scientific Communication srl) who edited the manuscript. The cost of this service was provided by private funds. Work for this study was performed at Cava de' Tirreni Hospital, ASL Salerno, Italy.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
apnea index
- BMI
body mass index
- DBP
diastolic blood pressure
- HOMA-IR
homeostasis model assessment-insulin resistance
- Hs-CRP
high-sensitivity C-reactive protein
- IMT
intima-media thickness
- LnHs-CRP
logarithmic transformed high sensitivity C-reactive protein
- OSA
obstructive sleep apnea
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
- SBP
systolic blood pressure
- SDB
sleep disordered breathing
- TST
total sleep time
REFERENCES
- 1.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Kheirandish-Gozal L. The obesity epidemic and disordered sleep during childhood and adolescence. Adolesc Med State Art Rev. 2010;21:480–4ix. [PubMed] [Google Scholar]
- 4.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–48. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunetti L, Tesse R, Miniello VL, et al. Sleep-disordered breathing in obese children: the southern Italy experience. Chest. 2010;137:1085–90. doi: 10.1378/chest.09-1529. [DOI] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 7.Phillips BG, Somers VK. Neural and humoral mechanisms mediating cardiovascular responses to obstructive sleep apnea. Respir Physiol. 2000;119:181–7. doi: 10.1016/s0034-5687(99)00113-9. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee R, Kim J, Kheirandish-Gozal L, Gozal D. Obesity and obstructive sleep apnea syndrome in children: A tale of inflammatory cascades. Pediatr Pulmonol. 2011;46:313–23. doi: 10.1002/ppul.21370. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–8. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin North Am. 1989;36:1551–69. doi: 10.1016/s0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts United States: methods and development. Vital and health statistics 2002. Data from the National Health Survey Series 11. 2000:1–190. [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Iannuzzi A, Licenziati MR, Acampora C, et al. Increased carotid intima-media thickness and stiffness in obese children. Diabetes Care. 2004;27:2506–8. doi: 10.2337/diacare.27.10.2506. [DOI] [PubMed] [Google Scholar]
- 16.Tsaoussoglou M, Bixler EO, Calhoun S, Chrousos GP, Sauder K, Vgontzas AN. Sleep-disordered breathing in obese children is associated with prevalent excessive daytime sleepiness, inflammation, and metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:143–50. doi: 10.1210/jc.2009-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bixler EO, Vgontzas AN, Lin HM, et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52:841–6. doi: 10.1161/HYPERTENSIONAHA.108.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iannuzzi A, Licenziati MR, Vacca M, et al. Comparison of two diets of varying glycemic index on carotid subclinical atherosclerosis in obese children. Heart Vessels. 2009;24:419–24. doi: 10.1007/s00380-008-1138-6. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30:29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lui MM, Lam JC, Mak HK, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135:950–6. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 21.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics. 2004;113:e564–9. doi: 10.1542/peds.113.6.e564. [DOI] [PubMed] [Google Scholar]
- 22.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 23.Kaditis AG, Alexopoulos EI, Kalampouka E, et al. Morning levels of C-reactive protein in children with obstructive sleep-disordered breathing. Am J Respir Crit Care Med. 2005;171:282–286. doi: 10.1164/rccm.200407-928OC. [DOI] [PubMed] [Google Scholar]
- 24.Kwok KL, Ng DK, Cheung YF. BP and arterial distensibility in children with primary snoring. Chest. 2003;123:1561–6. doi: 10.1378/chest.123.5.1561. [DOI] [PubMed] [Google Scholar]
- 25.Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension. 2009;53:64–9. doi: 10.1161/HYPERTENSIONAHA.108.119420. [DOI] [PubMed] [Google Scholar]
- 26.Silvestrini M, Rizzato B, Placidi F, Baruffaldi R, Bianconi A, Diomedi M. Carotid artery wall thickness in patients with obstructive sleep apnea syndrome. Stroke. 2002;33:1782–5. doi: 10.1161/01.str.0000019123.47840.2d. [DOI] [PubMed] [Google Scholar]
- 27.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 28.Archenti A, Pasqualinotto L. Childhood obesity: the epidemic of the third millenium. Acta Biomed. 2008;79:151–5. [PubMed] [Google Scholar]


