Abstract
Ultrasound has emerged to become a commonly used modality in the performance of chronic pain interventions. It allows direct visualization of tissue structure while allowing real time guidance of needle placement and medication administration. Ultrasound is a relatively affordable imaging tool and does not subject the practitioner or patient to radiation exposure. This review focuses on the anatomy and sonoanatomy of peripheral non-axial structures commonly involved in chronic pain conditions including the stellate ganglion, suprascapular, ilioinguinal, iliohypogastric, genitofemoral and lateral femoral cutaneous nerves. Additionally, the review discusses ultrasound guided intervention techniques applicable to these structures.
Keywords: genitofemoral nerve, ilioinguinal nerve, lateral femoral cutaneous nerve, stellate ganglion, suprascapular nerve, ultrasound
INTRODUCTION
Ultrasound has become a popular tool utilized in the performance of chronic pain interventions. Traditionally, procedures in pain medicine were performed using anatomic landmarks, fluoroscopy or CT scan. While these modalities continue to be utilized, there has been a tremendous growth in the use of ultrasound by pain practitioners. The first objective of this review article is to describe the anatomy and sonoanatomy of non-axial structures commonly involved in chronic pain conditions. The second objective is to describe the techniques utilized to perform ultrasound guided interventions for these structures.
METHODS
A MEDLINE database search was performed from January 1982 to Dec 2012 using the search terms ultrasound, ultrasound-guided, pain management and different structures relevant to this review including stellate ganglion, suprascapular, ilioinguinal, iliohypogastric, genitofemoral and lateral femoral cutaneous nerves. This article does not address perioperative nerve blocks, axial structures or trigger point injections.
ULTRASOUND VERSUS CONVENTIONAL IMAGING TECHNIQUES
Most pain management guidelines have moved towards recommending image guidance such as ultrasound, fluoroscopy or CT scan for interventional procedures. This is largely due to the increased accuracy, reliability (precision) and safety associated with structure visualization [1]. Fluoroscopy is effective for visualization of bony structures but not soft tissues. It thus has limitations when performing procedures for peripheral procedures. While CT scan is better than fluoroscopy for visualization of bony structures and soft tissue, it can be cost prohibitive and is associated with significant radiation exposure to the patient and practitioner [2]. Ultrasound has emerged as a popular modality in various disciplines because of its numerous advantages. It is generally more affordable and portable than other imaging modalities while avoiding any radiation exposure. Ultrasound provides direct visualization of various tissue structures including muscles, tendons, ligaments, nerves, vessels and bone surfaces. Ultrasound technology now allows visualization of small peripheral nerves and their associated branches [3]. Real time ultrasound guidance of needle placement and medication administration provides an advantage in ensuring accuracy. Furthermore, ultrasound is increasingly being utilized for the diagnosis of various conditions that may be associated with the patient's presentation such as nerve and joint pathology [4-6].
Although ultrasound is associated with significant advantages, it does have limitations. Visualization of certain structures including bone and deeper tissues can be limited. Bone has a high attenuation coefficient and casts an acoustic shadow; thus, structures hidden by bone are not well visualized. Visualization of deeper structures can also be challenging and requires use of a low frequency curve-linear probe. While this allows deeper visualization, the resolution is compromised. The technique required for ultrasound utilization is certainly user dependent. Obtaining an ideal image of the target structure while maintaining visualization of the needle requires practice and experience [7]. This is particularly true when the target structure is deep and the needle insertion angle is more acute. Like other interventional techniques, ultrasound in pain medicine requires a sound understanding of anatomy as well as sonoanatomy. The architecture of different tissues and organs are variable and interpretation of this is required when performing interventions. Despite these limitations, the advantages of ultrasound have resulted in its increasing popularity for various pain procedures.
STELLATE GANGLION (CERVICAL SYMPATHETIC) BLOCK
Stellate Ganglion Block (SGB) is commonly utilized for chronic pain conditions of the head, neck and upper extremity [8]. Increasingly this block is also being utilized for acute pain, as well as non-painful conditions [9-12].
1. Anatomy and sonoanatomy
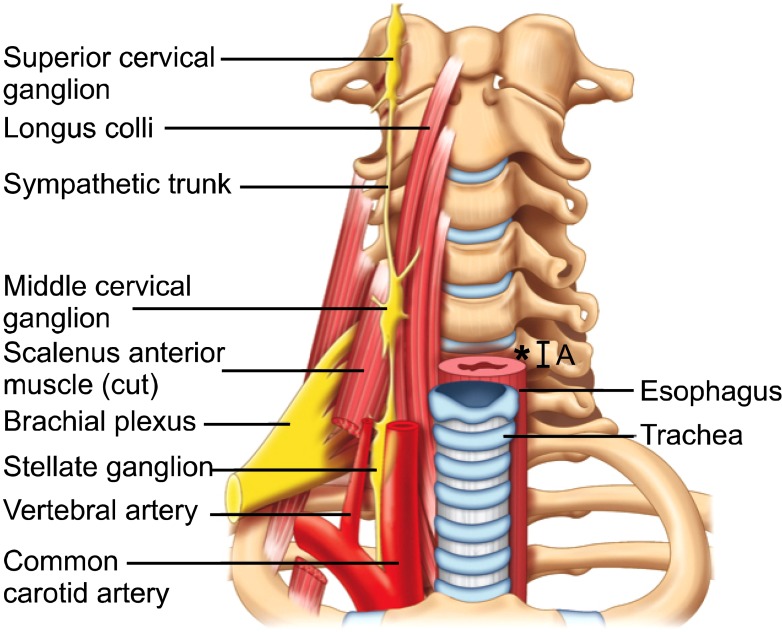
The stellate ganglion is part of the sympathetic chain and is formed by fusion of the inferior cervical and first thoracic ganglion. It is located at the C7.T1 level, posterior to the vertebral artery, lateral to the longus colli muscle and adjacent to the neck of the first rib (Fig. 1). Sympathetic innervation of the upper extremity arises from the stellate ganglion via post-ganglionic fibers that travel with C7, C8 and T1. The head and neck also receive sympathetic innervation from the cervical sympathetic chain. However, these fibers arise from the superior and middle cervical ganglion, which are more cephalad. The SGB is typically performed at the C6 level, with the goal of therapeutic medication spreading more caudally to reach the actual stellate ganglion. The C6 location is chosen to avoid the vertebral artery, which is classically described as having entered the foramen transversarium at this level. At C6 the cervical sympathetic chain is anterior to the longus colli muscle, embedded within the prevertebral fascia.
Fig. 1.
Prevertebral region of the neck. The target site for needle insertion in classical approach is marked as *. The breadth of the transverse process is marked as A. Reproduced with permission from USRA (www.usra.ca).
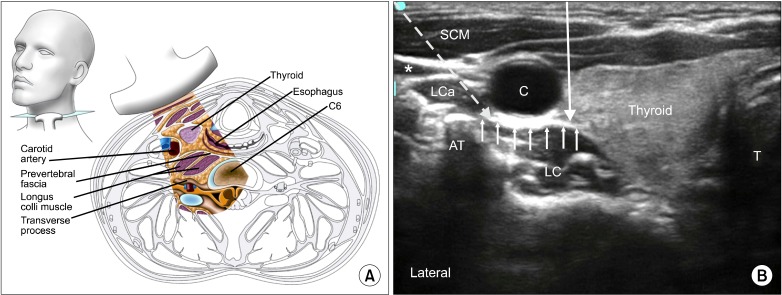
Ultrasound allows visualization of the thyroid gland, esophagus, carotid artery, internal jugular vein, C6 anterior and posterior tubercles, longus colli muscle, longus capitus muscle and prevertebral fascia (Fig. 2). The C6 transverse process is identified by its prominent anterior tubercle. The transverse process of C7 appears different from other cervical vertebrae due to its vestigial anterior and large posterior tubercles. The vertebral artery is identified by ultrasound and usually enters the foramen transversarium at C6; however, studies show that it can be extra-foraminal at this level in 7-10% of the population [13,14]. The esophagus is deviated to the left of the trachea in approximately 50-70% of the population, as revealed by studies utilizing various imaging modalities [15-17]. Mediastinitis can result from esophageal injury, particularly if the patient has an unrecognized diverticulum [18]. Another important identifiable structure is the inferior thyroidal artery, which can be seen passing in front of the prevertebral fascia. Injury to this artery can lead to a hematoma; this was a common complication found in the first published case series comparing "blind" injection with ultrasound for SGB [19].
Fig. 2.
(A) Cross section of the neck at the sixth cervical vertebral level correlating with the ultrasonographic image. (B) Ultrasonographic image of neck at C6. C: carotid artery, *: internal jugular vein (compressed), SCM: sternocleidomastoid muscle, LC: longus colli muscle, LCa: longus capitis muscle, T: airway, AT: anterior tubercle. The prevertebral fascia is marked by small solid arrows. The needle paths of anterior and lateral approach are marked by long solid and dotted arrow respectively. Reproduced with permission from USRA (www.usra.ca).
The target for injection at C6 is the plane between the longus colli muscle and the prevertebral fascia, which can be seen with ultrasound. Studies suggest that subfascial injections have better caudal spread and greater temperature change between arms than suprafascial injection [20,21]. Furthermore, suprafascial injections may be associated with more cephalad, medial and anterior spread of local anesthetic. This spread can result in an increased risk of hoarseness, likely secondary to contact with the recurrent laryngeal nerve.
2. Existing techniques
The classically described technique for SGB is performed at the C6 anterior tubercle (Chassaignac's tubercle) with anatomic or fluoroscopic guidance. The needle is placed onto Chassaignac's tubercle, medial to the carotid artery, and withdrawn 0.1-0.5 mm prior to injection of medication. Several disadvantages exist with this approach. Firstly, the landmark of Chassaignac's tubercle is quite small, with a cephalo-caudad dimension as narrow as 6 mm. If the needle tip slides off the tubercle it could penetrate the vertebral artery [22]. Fluoroscopy increases the accuracy of identifying Chassaignac's tubercle, however identifying the tissue plane between the longus colli muscle and the prevertebral fascia is not accomplished. Furthermore, vascular structures (carotid artery, vertebral artery, inferior thyroidal artery) and soft tissue structures (thyroid and esophagus) are not seen with fluoroscopy and are therefore at risk of puncture [18,23].
3. Ultrasound guided injection technique
The patient is placed in the supine position with the neck extended. A high frequency (6-13 MHz) linear ultrasound probe is placed at the C6 level to identify the longus colli muscle, longus capitus muscle, prevertebral fascia and surrounding structures (Fig. 2). The level should be confirmed by scanning caudally to visualize the C7 transverse process. There are two common approaches utilized; the medial and the lateral approach. The medial approach is similar to the classically described technique in which the needle is placed medial to the carotid artery, and advanced using an out of plane technique towards the preverbal fascia. Performing a pre-scan is important to plan needle trajectory. The path of the esophagus and inferior thyroidal artery in particular, may dissuade the operator from utilizing the approach medial to the carotid artery [24]. The author generally prefers the lateral in-plane approach, where the needle passes lateral to the carotid artery and anterior to Chassaignac's tubercle. Color Doppler determines whether there are any vessels in the needle trajectory. The tip of the needle is directed between the prevertebral fascia and the longus colli muscle (Fig. 2). A total of 5ml of local anesthetic is injected in the subfascial plane. Visualization of real time spread of local anesthetic is important to minimize risk of intravascular injection.
SUPRASCAPULAR NERVE BLOCK
Suprascapular nerve (SSN) block was originally described in 1941 [25]. Indications for this procedure include treatment of chronic shoulder pain (adhesive capsulitis, frozen shoulder and glenohumeral joint arthritis) [26-29], treatment of acute pain (shoulder trauma or surgery), [26,30-32] as well as diagnosis of suspected suprascapular neuropathy [33].
1. Anatomy and sonoanatomy
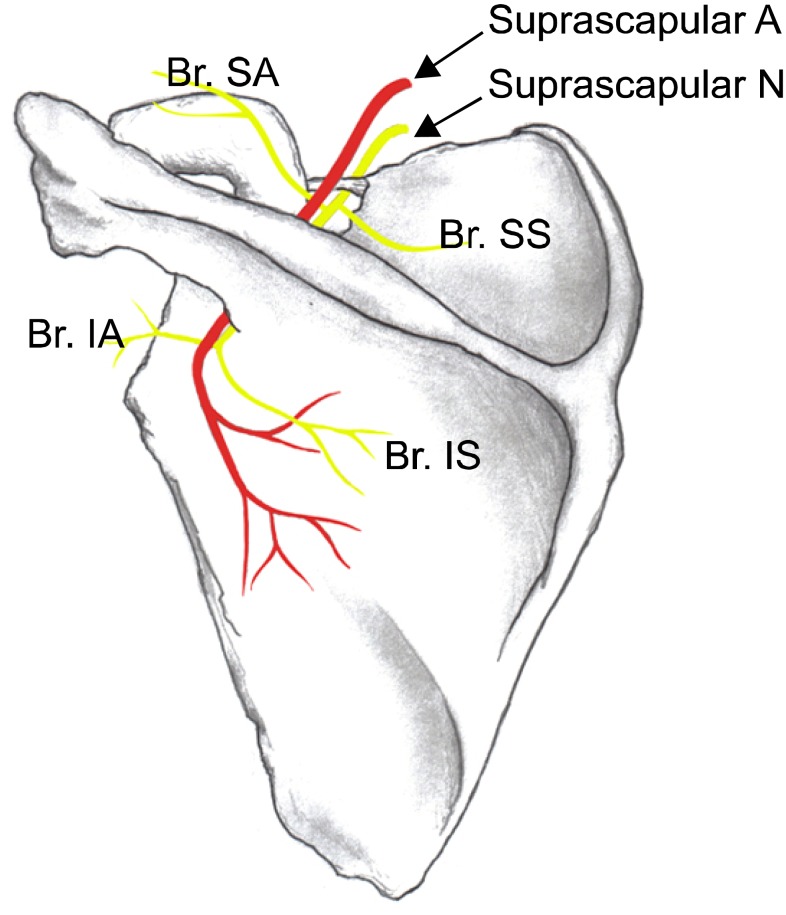
The SSN provides sensory innervation to approximately 70% of the shoulder joint. It has multiple branches including sensory fibers to the shoulder and shoulder joint, as well as motor fibers to the supraspinatus and infraspinatus muscles (Fig. 3).
Fig. 3.
Suprascapular nerve and its branches of the left shoulder. Superior articular branch (Br. SA) supplies the coracohumeral ligament, subacromial bursa and posterior aspect of the acromioclavicular joint capsule; Inferior articular branch (Br. IA) supplies the posterior joint capsule; Br. SS: branch to the supraspinatus muscle, Br. IS: branch to the infraspinatus muscle. Reproduced with permission from USRA (www.usra.ca).
The SSN arises from the superior trunk (union of C5 and C6) of the brachial plexus and runs adjacent to the omohyoid muscle. It continues under the trapezius muscle and then under the transverse scapular ligament at the level of the suprascapular notch (Fig. 3). The suprascapular notch is classically described as an u-shaped dip on the superior margin of the scapula, medial to the coracoid process. The size and shape of the notch are highly variable and can be absent in up to 8% of cadavers [34]. The suprascapular artery and vein can usually be identified crossing over top the transverse scapular ligament.
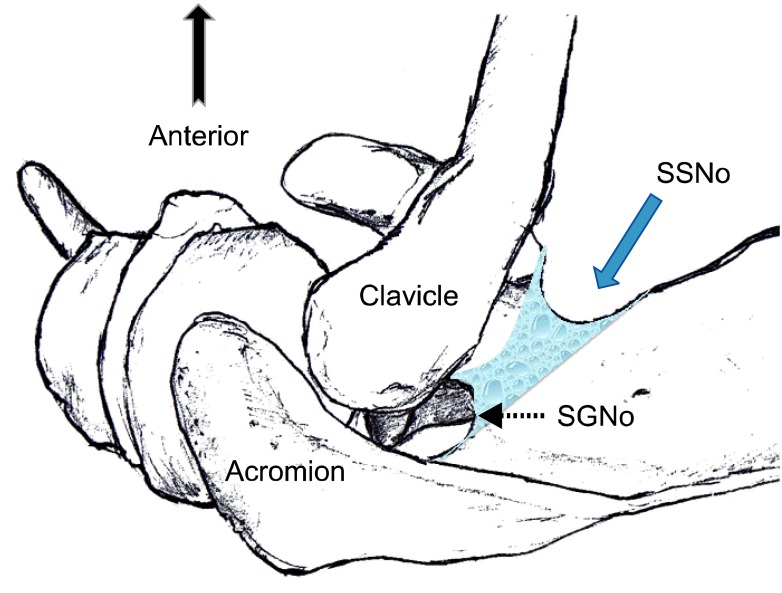
Once the SSN passes the suprascapular notch, it is within the suprascapular fossa, deep to the supraspinatus fascia superiorly. The SSN then leaves the suprascapular fossa by preceding infero-laterally under the supraspinatus muscle towards the spinoglenoid notch to enter the infraspinatus fossa. It curves around the lateral aspect of the scapular spine and then proceeds more medially on the posterior aspect of the scapula (Fig. 4).
Fig. 4.
Superior view of the left shoulder. The course of the suprascapular nerve enters the suprascapular fossa through the suprascapular notch (SSNo) and then enters the infrascapular fossa through the spinoglenoid notch (SGNo). Reproduced with permission from USRA (www.usra.ca).
The ideal location to perform the SSN block is in the suprascapular fossa on the floor of the scapular spine, between the suprascapular notch and the spinoglenoid notch [35]. The suprascapular fossa is a good target because it forms a compartment and retains the therapeutic medication with a small volume [36]. More importantly, this technique is advantageous because it avoids the more anterior suprascapular notch, which can be absent in some individuals. At the level of the suprascapular notch it can be difficult to visualize the full needle using the in-plane technique. Furthermore, if the needle advances too anteriorly there is a risk of pneumothorax. Finally, medication administered at the anterior level of the notch is more likely to spread to the brachial plexus [35].
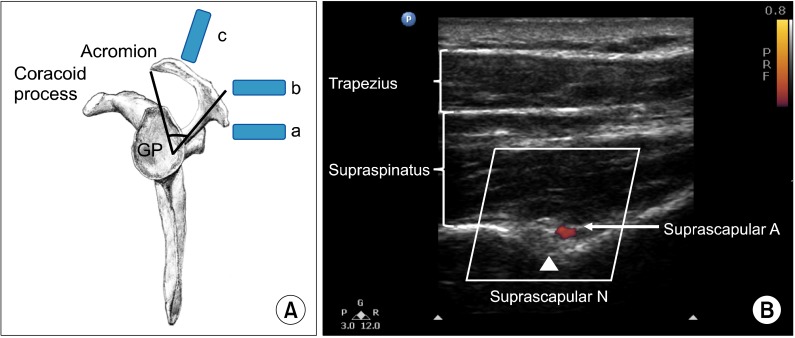
Ultrasound imaging of the suprascapular fossa allows visualization of the trapezius and supraspinatus muscles (Fig. 5). The SSN is often seen along with the suprascapular artery on the floor of the suprascapular fossa. Aligning the probe in the coronal plane with slight anterior angulation is required to better visualize the contents of suprascapular fossa, as the scapular spine forms an angle (39.5 degrees +/- 5.8 degrees) to the axis of the scapula blade [37].
Fig. 5.
(A) Lateral view of the scapula. The scapula spine forms an angle (39.5° ± 5.8°) to the axis of the scapula blade. The content of the suprascapular fossa cannot be revealed when scanning from the ultrasound probe (a), which is behind the dorsal border of the scapular spine, because of the obstruction of view from the scapular spine. By moving the ultrasound probe position to (b), the content of the suprascapular fossa cannot be revealed either. The optimal ultrasound probe position is at (c) when the probe is almost at the coronal plane with a slight anterior tilt. (B) Ultrasonographic image of the suprascapular nerve on the floor of the scapular spine between suprascapular notch and spinoglenoid notch. Both suprascapular nerve and artery run underneath the fascia of supraspinatus muscle. Reproduced with permission from USRA (www.usra.ca).
2. Existing techniques
Several different techniques have been described for SSN block, most of which target the SSN posteriorly at the level of the suprascapular notch or the suprascapular fossa. Modalities utilized include anatomic landmarks [25], peripheral nerve stimulator [30], electromyography [27], fluoroscopy [38] and CT guidance [39]. The primary disadvantages to anatomic landmarks include risk of pneumothorax, intravascular injection and nerve injury [40]. Studies evaluating the accuracy of using only anatomic landmarks demonstrated that needle placement could often end up within or above the supraspinatus muscle [36]. Fluoroscopy or CT guidance can be used to increase the accuracy of needle position, with the suprascapular fossa being a popular target. An adequate volume of therapeutic medication is required to ensure appropriate coverage of the SSN. However, a recent cadaveric CT scan study which targeted the suprascapular fossa showed that 10 ml of injectate spread to the brachial plexus within the axilla in 3 of 33 subjects [36].
Five articles describe the sonoanatomy of suprascapular nerve injection [35,41-44]. The majority of these describe a posterior approach. Some of the early articles inaccurately identified the floor of the suprascapular fossa as the suprascapular notch. More recently an anterior approach has also been described which visualizes the suprascapular nerve branching off the superior trunk of the brachial plexus, deep to the omohyoid muscle [44]. The major limitation of the anterior approach is the proximity to the brachial plexus (median 8 mm; range 4-15 mm), which potentially increases the risk of unintended spread of local anesthetic. Also, at this level the nerve is close to the pleura, potentially increasing the risk of pneumothorax.
3. Ultrasound guided injection technique
The patient is placed in either the sitting or prone position. A high frequency (7-13 MHz) linear ultrasound probe is used. The probe is placed over the suprascapular fossa in a coronal plane, using a slight anterior tilt (Fig. 5). The mid point of the probe in short axis should bisect a line between the medial aspect of the coracoid process and the posterior aspect of the acromion. This line follows the orientation of the suprascapular nerve towards the spinoglenoid notch. Targets visualized from superficial to deep include the trapezius muscle, supraspinatus muscle and floor of the suprascapular fossa (Fig. 5). The nerve is approximately 2.5 mm in size but can sometimes be difficult to visualize because of orientation. An in-plane technique from medial to lateral is utilized for needle insertion due to the position of the acromion on the lateral side. A 22 Gauge, 80-mm needle is utilized and an injectate volume of 5-8 ml is usually sufficient.
ILIOINGUINAL, ILIOHYPOGASTRIC AND GENITOFEMORAL NERVE BLOCK
The Ilioinguinal (IL), iliohypogastric (IH) and genitofemoral nerves (GF) supply the skin bordering the lower abdomen, groin and thigh [45]. These nerves have a superficial course and are at risk of injury during common surgeries such as cesarean section, inguinal hernia, and laparoscopic procedures [46-48]. Perioperative and traumatic nerve injury have been shown to be associated with chronic post surgical pain [49,50]. Patients may present with pain that is neuropathic in nature radiating to the low abdomen, groin, and medial thigh, as well as to the scrotum in males and labia majora in females. Blocking these can be of diagnostic and therapeutic value.
1. Anatomy and sonoanatomy
There is significant anatomic variability and overlap in sensory innervation of the lower abdomen, groin and medial thigh. This variability is due to the various communication and dominance patterns of the IH, IL and GF nerves. Classically, the Iliohypogastric (IH) nerve provides sensory innervation to the skin over the lower part of the rectus abdominus muscle [51]. The terminal branch of the ilioinguinal (IL) nerve is often accompanied by the genital branch of the GF nerve. Either collectively or individually, the terminal sensory branches of these nerves may innervate the skin of the mons pubis, upper medial thigh, inguinal crease, anterior surface of the scrotum and part of the labia majora.
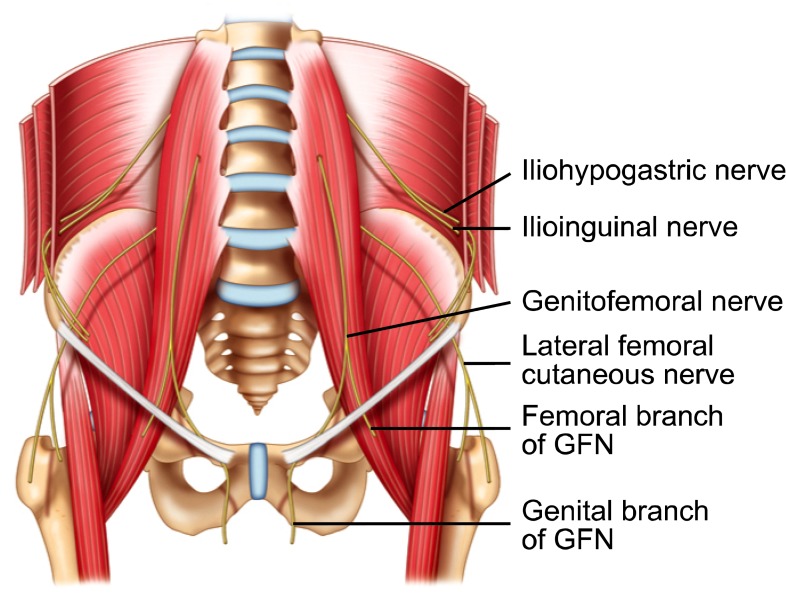
The ilioinguinal (IL) and iliohypogastric (IH) nerves arise from T12 and L1 (Fig. 6). They run along the lateral aspect of the psoas major muscle and course obliquely across the quadratus lumborum and iliacus muscles. Above the iliac crest these nerves travel from deep to superficial and pierce the transversus abdominus muscle (TA) to lie between TA and the internal oblique muscle (IO). This is the most common location for finding both nerves together and is thus a common target for injection.
Fig. 6.
Schematic diagram showing the pathway of ilioinguinal, iliohypogastric and genitofemoral nerve. GFN: genitofemoral nerve. Reproduced with permission from USRA (www.usra.ca).
The IL and IH nerves then continue on different paths. The IH nerve pierces the IO above the ASIS and travels between IO and external oblique muscles (EO) above the inguinal canal. Just above the superficial inguinal ring it pierces the EO to innervate the skin. The IL nerve runs parallel and below the IH nerve. After it pierces the IO, the IL nerve eventually travels within the inguinal canal. It exits the inguinal canal via the superficial inguinal ring along with the spermatic cord. Within the inguinal canal the IL nerve may join or travel with the GF nerve.
The genitofemoral nerve (GF) arises from L1 and L2. It pierces the psoas muscle at L3/L4 and divides into the genital and femoral branches at variable distances from the inguinal canal (Fig. 6). The femoral branch follows the course of the external iliac artery and continues with it under the inguinal ligament. It goes on to supply the skin over the femoral triangle. The genital branch travels through the inguinal canal, either within or outside the spermatic cord and terminates in its cutaneous branches. Within the inguinal canal the IL and GF nerve may travel together.
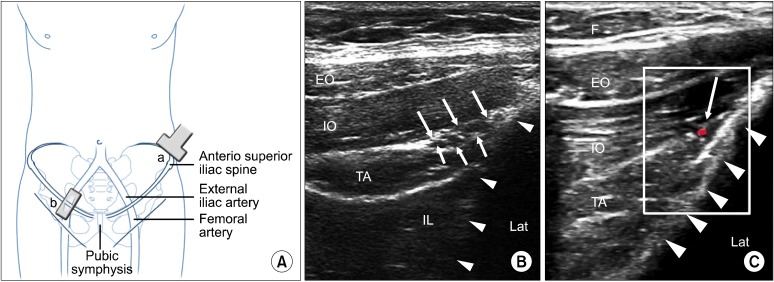
The ideal location to scan the IL and IH nerves together is cephalad and posterior to the ASIS, with the probe perpendicular to the inguinal canal. This position should allow visualization of the hyperechoic iliac crest, all three layers of abdominal muscles (EO, IO and TA) and the peritoneum (Fig. 7). The nerves are often visualized in the fascial plane between the IO and TA muscles as it slopes upwards towards the iliac crest. The IL and IH nerves are usually in close proximity to each other and often accompanied by the deep circumflex iliac artery (Fig. 7). Occasionally the nerves can be visualized piercing the IO muscle to lie between the IO and EO muscles.
Fig. 7.
(A) Schematic diagram to show the position of the ultrasound probe. The probe (a) is placed above and 3 finger breadth lateral to the anterior superior iliac spine and is in the short axis of the course of ilioinguinal nerve (i.e. at right angle to the iliac crest). The probe (b) is placed in the inguinal line in long axis of femoral and external iliac artery. (B) Figure showing the three layers of muscles and the fasica split (white line arrows) with the ilioinguinal and iliohypogastric nerves inside. Solid triangles outline the iliac crest. (C) Similar to figure 7b with Doppler showing the deep circumflex iliac artery. EO: external oblique muscle, IO: internal oblique muscle, TA: transverse abdominus muscle, IL: iliacus, F: adipose tissue, Lat: lateral. Reproduced with permission from USRA (www.usra.ca).
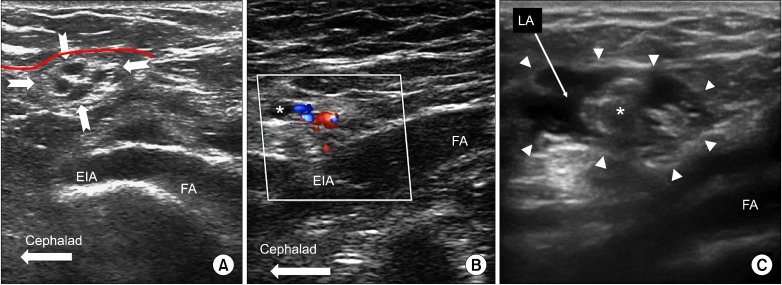
The genital branch of the GF nerve is a common target for injection, however this structure cannot be visualized routinely with US unless a high frequency probe (>18 MHz) is used. Scanning focuses on identifying surrounding structures including the inguinal canal because it contains the genital branch of the GF nerve (Fig. 8). Note that branches of the IL nerve may also travel within the inguinal canal at this location. The inguinal canal also contains the spermatic cord (round ligament of uterus in females), the testicular artery and the artery to vas deferens. The inguinal canal is better visualized in males than females.
Fig. 8.
(A) Long axis view of the femoral and external iliac artery showing the cross section of spermatic cord (outlined by solid arrows) in a male patient. The red dashed line outlines the deep abdominal fascia. (B) Similar view as 8a with color Doppler showing the vessels inside the spermatic cord. (C) Following injection, the inguinal canal can be well visualized (outlined by solid arrows) filled with local anesthetic (LA). The spermatic cord is indicated by *. Reproduced the with permission from USRA (www.usra.ca).
It is important to note that the descriptions provided above may only pertain to 42% of dissections [52]. The point at which the IL and IH nerves pierce the abdominal layers is highly variable [47]. In 29% of patients the IL nerve joins the IH nerve or one of the nerves may be entirely absent [52]. One cadaver study demonstrated that the IL nerve could be responsible for the expected innervation of the GF nerve in 28% of dissections [53]. This anatomic variability must be taken into consideration when performing diagnostic and therapeutic injections for these structures.
2. Existing techniques
Several anatomically guided techniques have been described for the IL and IH nerve blocks. Two of the suggested targets include 1 inch medial to the ASIS along a line joining the ASIS and the umbilicus or 3 cm medial and inferior to the ASIS [54,55]. These blind techniques are associated with high failure rates in the range of 10-40% [56,57]. These low success rates are likely because the IL and IH nerves can be located in various fascial planes between the IO, EO and TA muscles [56]. In addition, there are several nearby structures that can be damaged with blind injection as demonstrated by reported cases of femoral nerve palsy, bowel perforation and vascular injury [58-61].
The described anatomic techniques for the GF nerve block actually only target the genital branch, as the femoral branch has already separated by this point. One such technique involves identifying the pubic tubercle and infiltrating medication 1cm superior and lateral to it [62]. This is an infiltration-based approach that likely targets spread of medication towards the inguinal canal. Nearby structures that are at risk of damage include the spermatic cord, testicular artery and bowel.
3. Ultrasound guided injection technique
The use of US guidance for the IL and IH nerve block has been evaluated in clinical practice and has been validated in a cadaver study [63-66]. The patient is placed in a supine position and a high frequency (6-13 MHz) linear ultrasound probe is utilized. In the author's experience, the ideal probe location is perpendicular to the inguinal ligament with the probe cephalad and 3 fingerbreadths lateral to the ASIS. The lateral end of the probe should be in contact with the iliac crest. All three muscle layers of the abdomen (EO, IO, TA) should be well visualized with the nerves most commonly lying in the fascial plane between the IO and TA at this location. The nerves should be found within 1.5 cm of the iliac crest with the IL nerve being more lateral [67]. This technique has been validated by a cadaver study with an associated 95% likelihood of identifying both nerves [63]. Color Doppler can be utilized to identify the deep circumflex iliac artery, often identified in the same fascial plane as the nerves. Caution should be taken if another nerve is found further away from the iliac crest. This is most likely the twelfth intercostal nerve or subcostal nerve. An out of plane technique is used to approach the IL and IH nerves. A total of 6-8 ml of injectate is usually adequate for coverage. If the nerves cannot be visualized, consideration can be given to injecting the fascial plane between the IO and TA muscles as this is the most reliable nerve location [64].
Ultrasound guidance for blockade of the genital branch of the GF nerve has been described in a prior review article [68]. It involves scanning the inguinal canal, which may also include the IL nerve. For the GF nerve block the patient is placed supine and a high frequency (6-13 MHz) linear ultrasound probe is utilized. The final probe position is perpendicular to the inguinal canal, 2 finger breadths lateral to the pubic tubercle [68]. The inguinal canal and its contents can be visualized. If difficulty is encountered identifying the inguinal canal, the femoral artery can be used as a reference landmark. The probe is used to obtain a long axis view of the femoral artery. The artery is traced cephalad until it dives deep where it transitions to the external iliac artery. At this point the inguinal canal and its contents should be seen superficially as a round structure (Fig. 8). The canal can be traced medially by maintaining the probe perpendicular to the inguinal ligament and shifting the probe towards the pubic tubercle. Color doppler can be utilized to identify the testicular artery and artery to vas deferens, as well as the pampiniform venous plexus. Asking the patient to perform a gentle valsalva maneuver may accentuate the vascular structures within the canal. An out of plane technique from lateral to medial is commonly utilized for needle placement, however an in-plane technique can also be used. As the nerve can be either within or outside the spermatic cord in males, 4 ml of medication is deposited within the spermatic cord and 4 ml within the inguinal canal outside of the spermatic cord. Care should be taken to avoid epinephrine-containing solution in males due to the potential vasoconstrictive effects on the testicular artery. In females a total of 5 ml of medication is deposited within the inguinal canal around the round ligament.
LATERAL FEMORAL CUTANEOUS NERVE BLOCK
Meralgia Paresthetica was first described in 1885 and is characterized by pain, numbness and paresthesia along the antero-lateral aspect of the thigh [69]. It is a rare condition and is defined as a mono-neuropathy of the lateral femoral cutaneous nerve (LFCN). Injection of the LFCN can be of diagnostic and therapeutic value [70].
1. Anatomy and sonoanatomy
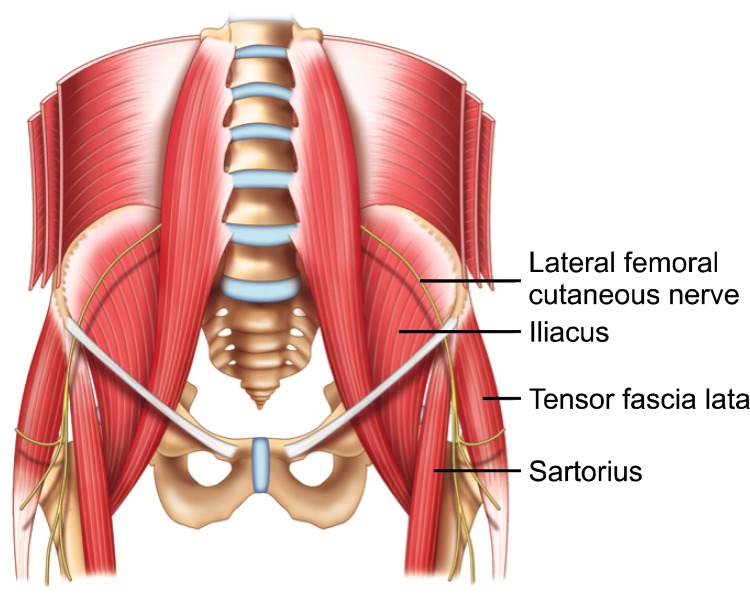
The LFCN is a part of the lumbar plexus and arises from L2 and L3 (Fig. 9). It continues as a purely sensory nerve and provides cutaneous supply to the antero-lateral thigh as high as the greater trochanter [71]. It emerges on the lateral border of the psoas muscle and continues caudally and laterally across the iliacus muscle towards the ASIS. Medial to the ASIS, the LFCN usually continues towards the inguinal ligament and travels through, above or below it [72-74]. The nerve location is quite variable at the inguinal ligament. Classically, it is found medial to the ASIS at a distance anywhere between 6 mm and 7.3 cm away [75]. The diameter of the nerve at this location is 3.2 +/- 0.7 mm [72]. Once at, or beyond the inguinal ligament the nerve divides into an anterior and posterior branch. Below the inguinal ligament the nerve, or one of its branches may lie superficial to the sartorius muscle or may course laterally between the sartorius and tensor fascia lata muscles. While the above descriptions often hold true, there are several anatomic variations along the course of the LFCN, particularly around the inguinal ligament. Cadaver studies demonstrate the LFCN passing over or posterior to the ASIS in 4-29% of dissections [73,76,77]. It may divide into branches (0-5 branches) before crossing the inguinal ligament in up to 28% of individuals [75]. Although the nerve usually travels into the thigh above the sartorius muscle, it may travel within the muscle in 22% of cases [72].
Fig. 9.
Schematic diagram showing the pathway of a typical course of lateral femoral cutaneous nerve. Note that the nerve course beneath the inguinal ligament and runs superficially to the sartorius muscle and then in between this muscle and tensor fascia lata muscle. Reproduced with permission from USRA (www.usra.ca).
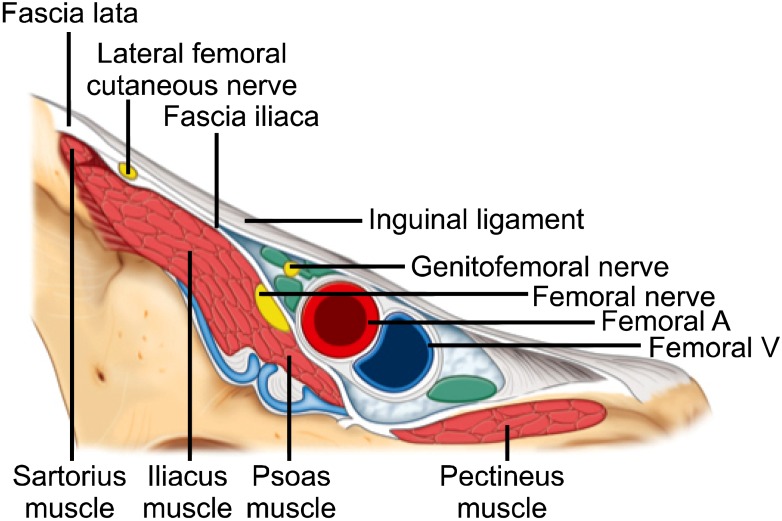
The LFCN is a small peripheral nerve with significant anatomic variability, thus a systematic approach is required to identify it. Dynamic scanning from cephalad to caudal along the course of the nerve may provide additional benefit. The nerve can be visualized with ultrasound at, above or below the inguinal ligament. Above the inguinal ligament the nerve can be visualized lying over the iliacus muscle, particularly in patients' with a low body mass index. At the inguinal ligament the nerve is most likely identified medial to the ASIS, between the fascia lata and fascia iliaca, superficial to the sartorius and iliacus muscles (Fig. 10). In the infrainguinal region the nerve is most likely identified superficial to the sartorius muscle or more laterally between the sartorius and tensor fascia lata muscles (Fig. 10).
Fig. 10.
Nerves at the inguinal area. Reproduced with permission from USRA (www.usra.ca).
2. Existing techniques
The anatomic landmark based technique for injection places the needle 2.5 cm medial to the ASIS, just caudal to the inguinal ligament. Depth of needle insertion is guided by feeling the needle pop through the first fascial layer or by loss of resistance [71,78,79]. Anatomically guided blind injections have been reported to be associated with a failure rate as high as 60% [71]. The use of a nerve stimulator can reduce the failure rate to 15%, however, this can be associated with patient discomfort [71]. Adjacent structures at risk of injury include the femoral nerve, femoral artery and bowel. One study found that unintentional femoral nerve block occurred in 35% of patients using the blind approach [71]. Given the variable course of LFCN course, an anatomic approach alone is often inadequate. One cadaver study comparing ultrasound and anatomic landmark based techniques found success rates of 84% and 5% respectively [80].
3. Ultrasound guided injection technique
The technique for ultrasound guided LFCN blockade has been well published [80-84]. The patient is placed in the supine position and a high frequency (6-13 MHz) linear ultrasound probe is used. The block is performed as close to the inguinal ligament as possible as the level of entrapment is usually around this level. However, the nerve can be difficult to locate proximally. The author recommends initiating the scan at the level of the inguinal ligament. The lateral end of the probe is placed on the ASIS and oriented in the longitudinal plane of the inguinal ligament. The LFCN is often found between the fascia lata and fascia iliaca at this location keeping in mind that the nerve can lie at variable distances medial to the ASIS. Adjacent structures can be visualized including the triangular shaped sartorius muscle deep to the inguinal ligament. Hydrodissection between the fascia lata and fascia iliaca with dextrose 5% can be utilized to better visualize the nerve. If the nerve is not identified at the level of the inguinal ligament, an attempt to visualize it in the infrainguinal location can be attempted. Here the nerve may be found superficial to the sartorius muscle or between the fat filled space between sartorius and fascia lata muscles, which is more lateral. Once identified, the nerve can be followed proximally and distally with the injection performed in the most proximal location possible. The LFCN may appear hyper or hypoechoic depending on the tissue architecture of the surrounding structures (Fig. 11). In cases of advanced meralgia paresthetica the nerve may appear larger (pseudoneuorma). An in-plane technique can be utilized for the injection. A total injectate volume of 5 ml is usually adequate. If the nerve is not identifiable despite scanning these locations, consideration can be given to utilizing nerve stimulation for additional guidance.
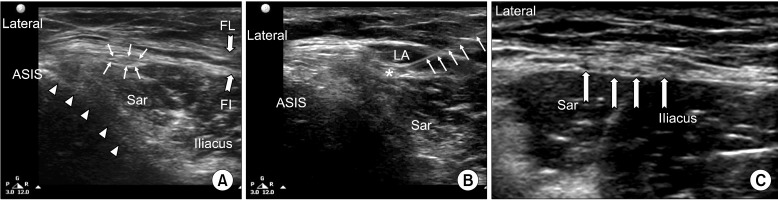
Fig. 11.
(A) Ultrasonographic picture showing the lateral femoral cutaneous nerve (LFCN). The LFCN is indicated by line arrows. The fascia is indicated by bold arrows (FL: Fascia Lata, FI: Fascia Iliaca); the ilium is indicated by solid arrows; Sar: Sartorius muscle, ASIS: Anterior Superior Iliac Spine. (B) Post-injection ultrasonographic picture; the needle is indicated by line arrows, LA: local anesthetic, *LFCN. (C) The LFCN has already branched into smaller nerves and appears as hypoechoic structures (solid line arrows). Reproduced with permission from USRA (www.usra.ca).
CONCLUSIONS
Ultrasound has emerged to become one of the principle tools used in the performance of chronic pain interventions. It allows identification of target structures, visualization of needle placement and observation of therapeutic medication in real time. Furthermore, utilization of ultrasound instead of fluoroscopy spares healthcare providers and patients the risk of radiation exposure. Ultrasound utilization for chronic pain interventions is still in its relatively early stages and additional studies are required to further evaluate the efficacy and limitations of employing this modality.
References
- 1.Manchikanti L, Boswell MV, Singh V, Benyamin RM, Fellows B, Abdi S, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12:699–802. [PubMed] [Google Scholar]
- 2.Davros WJ. Fluoroscopy: basic science, optimal use, and patient/operator protection. Tech Reg Anesth Pain Manag. 2007;11:44–54. [Google Scholar]
- 3.Suk JI, Walker FO, Cartwright MS. Ultrasonography of peripheral nerves. Curr Neurol Neurosci Rep. 2013;13:328. doi: 10.1007/s11910-012-0328-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouffard JA, Lee SM, Dhanju J. Ultrasonography of the shoulder. Semin Ultrasound CT MR. 2000;21:164–191. doi: 10.1016/s0887-2171(00)90041-6. [DOI] [PubMed] [Google Scholar]
- 5.Martinoli C, Bianchi S, Gandolfo N, Valle M, Simonetti S, Derchi LE. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20 Spec No:S199–S213. doi: 10.1148/radiographics.20.suppl_1.g00oc08s199. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi S, Martinoli C, Bianchi-Zamorani M, Valle M. Ultrasound of the joints. Eur Radiol. 2002;12:56–61. doi: 10.1007/s00330-001-1162-8. [DOI] [PubMed] [Google Scholar]
- 7.Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound-guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg Anesth Pain Med. 2012;37:334–339. doi: 10.1097/AAP.0b013e3182475fba. [DOI] [PubMed] [Google Scholar]
- 8.Aeschbach A, Mekhail NA. Common nerve blocks in chronic pain management. Anesthesiol Clin North America. 2000;18:429–459. doi: 10.1016/s0889-8537(05)70171-1. [DOI] [PubMed] [Google Scholar]
- 9.Lipov EG, Joshi JR, Sanders S, Wilcox K, Lipov S, Xie H, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008;9:523–532. doi: 10.1016/S1470-2045(08)70131-1. [DOI] [PubMed] [Google Scholar]
- 10.Mulvaney SW, McLean B, de Leeuw J. The use of stellate ganglion block in the treatment of panic/anxiety symptoms with combat-related post-traumatic stress disorder; preliminary results of long-term follow-up: a case series. Pain Pract. 2010;10:359–365. doi: 10.1111/j.1533-2500.2010.00373.x. [DOI] [PubMed] [Google Scholar]
- 11.Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician. 2000;3:294–304. [PubMed] [Google Scholar]
- 12.Kakazu CZ, Julka I. Stellate ganglion blockade for acute postoperative upper extremity pain. Anesthesiology. 2005;102:1288–1289. doi: 10.1097/00000542-200506000-00039. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia A, Flamer D, Peng PW. Evaluation of sonoanatomy relevant to performing stellate ganglion blocks using anterior and lateral simulated approaches: an observational study. Can J Anaesth. 2012;59:1040–1047. doi: 10.1007/s12630-012-9779-4. [DOI] [PubMed] [Google Scholar]
- 14.Matula C, Trattnig S, Tschabitscher M, Day JD, Koos WT. The course of the prevertebral segment of the vertebral artery: anatomy and clinical significance. Surg Neurol. 1997;48:125–131. doi: 10.1016/s0090-3019(97)90105-1. [DOI] [PubMed] [Google Scholar]
- 15.Siegenthaler A, Mlekusch S, Schliessbach J, Curatolo M, Eichenberger U. Ultrasound imaging to estimate risk of esophageal and vascular puncture after conventional stellate ganglion block. Reg Anesth Pain Med. 2012;37:224–227. doi: 10.1097/AAP.0b013e31823d40fe. [DOI] [PubMed] [Google Scholar]
- 16.Smith KJ, Ladak S, Choi PT, Dobranowski J. The cricoid cartilage and the esophagus are not aligned in close to half of adult patients. Can J Anaesth. 2002;49:503–507. doi: 10.1007/BF03017931. [DOI] [PubMed] [Google Scholar]
- 17.Smith KJ, Dobranowski J, Yip G, Dauphin A, Choi PT. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology. 2003;99:60–64. doi: 10.1097/00000542-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Narouze S, Vydyanathan A, Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician. 2007;10:747–752. [PubMed] [Google Scholar]
- 19.Kapral S, Krafft P, Gosch M, Fleischmann D, Weinstabl C. Ultrasound imaging for stellate ganglion block: direct visualization of puncture site and local anesthetic spread. A pilot study. Reg Anesth. 1995;20:323–328. [PubMed] [Google Scholar]
- 20.Shibata Y, Fujiwara Y, Komatsu T. A new approach of ultrasound-guided stellate ganglion block. Anesth Analg. 2007;105:550–551. doi: 10.1213/01.ane.0000265691.02963.a4. [DOI] [PubMed] [Google Scholar]
- 21.Christie JM, Martinez CR. Computerized axial tomography to define the distribution of solution after stellate ganglion nerve block. J Clin Anesth. 1995;7:306–311. doi: 10.1016/0952-8180(95)00037-i. [DOI] [PubMed] [Google Scholar]
- 22.Janik JE, Hoeft MA, Ajar AH, Alsofrom GF, Borrello MT, Rathmell JP. Variable osteology of the sixth cervical vertebra in relation to stellate ganglion block. Reg Anesth Pain Med. 2008;33:102–108. doi: 10.1016/j.rapm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Narouze S. Beware of the "serpentine" inferior thyroid artery while performing stellate ganglion block. Anesth Analg. 2009;109:289–290. doi: 10.1213/ane.0b013e3181a20197. [DOI] [PubMed] [Google Scholar]
- 24.Peng PW, Narouze S. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures: part I: nonaxial structures. Reg Anesth Pain Med. 2009;34:458–474. doi: 10.1097/AAP.0b013e3181aea16f. [DOI] [PubMed] [Google Scholar]
- 25.Wertheim HM, Rovenstine EA. Suprascapular nerve block. Anesthesiology. 1941;2:541–545. [Google Scholar]
- 26.Emery P, Bowman S, Wedderburn L, Grahame R. Suprascapular nerve block for chronic shoulder pain in rheumatoid arthritis. BMJ. 1989;299:1079–1080. doi: 10.1136/bmj.299.6707.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones DS, Chattopadhyay C. Suprascapular nerve block for the treatment of frozen shoulder in primary care: a randomized trial. Br J Gen Pract. 1999;49:39–41. [PMC free article] [PubMed] [Google Scholar]
- 28.Karataş GK, Meray J. Suprascapular nerve block for pain relief in adhesive capsulitis: comparison of 2 different techniques. Arch Phys Med Rehabil. 2002;83:593–597. doi: 10.1053/apmr.2002.32472. [DOI] [PubMed] [Google Scholar]
- 29.Shanahan EM, Ahern M, Smith M, Wetherall M, Bresnihan B, FitzGerald O. Suprascapular nerve block (using bupivacaine and methylprednisolone acetate) in chronic shoulder pain. Ann Rheum Dis. 2003;62:400–406. doi: 10.1136/ard.62.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleeson AP, Graham CA, Jones I, Beggs I, Nutton RW. Comparison of intra-articular lignocaine and a suprascapular nerve block for acute anterior shoulder dislocation. Injury. 1997;28:141–142. doi: 10.1016/s0020-1383(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 31.Ritchie ED, Tong D, Chung F, Norris AM, Miniaci A, Vairavanathan SD. Suprascapular nerve block for postoperative pain relief in arthroscopic shoulder surgery: a new modality? Anesth Analg. 1997;84:1306–1312. doi: 10.1097/00000539-199706000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Neal JM, McDonald SB, Larkin KL, Polissar NL. Suprascapular nerve block prolongs analgesia after nonarthroscopic shoulder surgery but does not improve outcome. Anesth Analg. 2003;96:982–986. doi: 10.1213/01.ANE.0000052380.69541.D4. [DOI] [PubMed] [Google Scholar]
- 33.Romeo AA, Rotenberg DD, Bach BR., Jr Suprascapular neuropathy. J Am Acad Orthop Surg. 1999;7:358–367. doi: 10.5435/00124635-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Natsis K, Totlis T, Tsikaras P, Appell HJ, Skandalakis P, Koebke J. Proposal for classification of the suprascapular notch: a study on 423 dried scapulas. Clin Anat. 2007;20:135–139. doi: 10.1002/ca.20318. [DOI] [PubMed] [Google Scholar]
- 35.Peng PW, Wiley MJ, Liang J, Bellingham GA. Ultrasound-guided suprascapular nerve block: a correlation with fluoroscopic and cadaveric findings. Can J Anaesth. 2010;57:143–148. doi: 10.1007/s12630-009-9234-3. [DOI] [PubMed] [Google Scholar]
- 36.Feigl GC, Anderhuber F, Dorn C, Pipam W, Rosmarin W, Likar R. Modified lateral block of the suprascapular nerve: a safe approach and how much to inject? A morphological study. Reg Anesth Pain Med. 2007;32:488–494. doi: 10.1016/j.rapm.2007.06.394. [DOI] [PubMed] [Google Scholar]
- 37.Mallon WJ, Brown HR, Vogler JB, 3rd, Martinez S. Radiographic and geometric anatomy of the scapula. Clin Orthop Relat Res. 1992;(277):142–154. [PubMed] [Google Scholar]
- 38.Shah RV, Racz GB. Pulsed mode radiofrequency lesioning of the suprascapular nerve for the treatment of chronic shoulder pain. Pain Physician. 2003;6:503–506. [PubMed] [Google Scholar]
- 39.Schneider-Kolsky ME, Pike J, Connell DA. CT-guided suprascapular nerve blocks: a pilot study. Skeletal Radiol. 2004;33:277–282. doi: 10.1007/s00256-003-0733-y. [DOI] [PubMed] [Google Scholar]
- 40.Moore DC. Block of the suprascapular nerve. In: Thomas CC, editor. Regional nerve block. 4th ed. Springfield, IL: Charles C Thomas Publisher LTD; 1979. pp. 300–303. [Google Scholar]
- 41.Gofeld M. Ultrasonography in pain medicine: a critical review. Pain Pract. 2008;8:226–240. doi: 10.1111/j.1533-2500.2008.00215.x. [DOI] [PubMed] [Google Scholar]
- 42.Harmon D, Hearty C. Ultrasound-guided suprascapular nerve block technique. Pain Physician. 2007;10:743–746. [PubMed] [Google Scholar]
- 43.Yücesoy C, Akkaya T, Ozel O, Cömert A, Tüccar E, Bedirli N, et al. Ultrasonographic evaluation and morphometric measurements of the suprascapular notch. Surg Radiol Anat. 2009;31:409–414. doi: 10.1007/s00276-008-0458-7. [DOI] [PubMed] [Google Scholar]
- 44.Siegenthaler A, Moriggl B, Mlekusch S, Schliessbach J, Haug M, Curatolo M, et al. Ultrasound-guided suprascapular nerve block, description of a novel supraclavicular approach. Reg Anesth Pain Med. 2012;37:325–328. doi: 10.1097/AAP.0b013e3182409168. [DOI] [PubMed] [Google Scholar]
- 45.Rab M, Ebmer J, Dellon AL. Anatomic variability of the ilioinguinal and genitofemoral nerve: implications for the treatment of groin pain. Plast Reconstr Surg. 2001;108:1618–1623. doi: 10.1097/00006534-200111000-00029. [DOI] [PubMed] [Google Scholar]
- 46.Alfieri S, Rotondi F, Di Giorgio A, Fumagalli U, Salzano A, Di Miceli D, et al. Influence of preservation versus division of ilioinguinal, iliohypogastric, and genital nerves during open mesh herniorrhaphy: prospective multicentric study of chronic pain. Ann Surg. 2006;243:553–558. doi: 10.1097/01.sla.0000208435.40970.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whiteside JL, Barber MD, Walters MD, Falcone T. Anatomy of ilioinguinal and iliohypogastric nerves in relation to trocar placement and low transverse incisions. Am J Obstet Gynecol. 2003;189:1574–1578. doi: 10.1016/s0002-9378(03)00934-7. [DOI] [PubMed] [Google Scholar]
- 48.Seid AS, Amos E. Entrapment neuropathy in laparoscopic herniorrhaphy. Surg Endosc. 1994;8:1050–1053. doi: 10.1007/BF00705717. [DOI] [PubMed] [Google Scholar]
- 49.Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS. Chronic pain after hysterectomy. Acta Anaesthesiol Scand. 2008;52:327–331. doi: 10.1111/j.1399-6576.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 50.Aasvang E, Kehlet H. Chronic postoperative pain: the case of inguinal herniorrhaphy. Br J Anaesth. 2005;95:69–76. doi: 10.1093/bja/aei019. [DOI] [PubMed] [Google Scholar]
- 51.Mandelkow H, Loeweneck H. The iliohypogastric and ilioinguinal nerves. Distribution in the abdominal wall, danger areas in surgical incisions in the inguinal and pubic regions and reflected visceral pain in their dermatomes. Surg Radiol Anat. 1988;10:145–149. doi: 10.1007/BF02307823. [DOI] [PubMed] [Google Scholar]
- 52.al-dabbagh AK. Anatomical variations of the inguinal nerves and risks of injury in 110 hernia repairs. Surg Radiol Anat. 2002;24:102–107. doi: 10.1007/s00276-002-0006-9. [DOI] [PubMed] [Google Scholar]
- 53.Liu WC, Chen TH, Shyu JF, Chen CH, Shih C, Wang JJ, et al. Applied anatomy of the genital branch of the genitofemoral nerve in open inguinal herniorrhaphy. Eur J Surg. 2002;168:145–149. doi: 10.1080/110241502320127748. [DOI] [PubMed] [Google Scholar]
- 54.Katz J. Atlas of regional anesthesia. Norwalk, CT: Appleton-Century-Crofts; 1985. [Google Scholar]
- 55.Brown D. Atlas of regional anesthesia. Philadelphia, PA: WB Saunders; 1999. [Google Scholar]
- 56.van Schoor AN, Boon JM, Bosenberg AT, Abrahams PH, Meiring JH. Anatomical considerations of the pediatric ilioinguinal/iliohypogastric nerve block. Paediatr Anaesth. 2005;15:371–377. doi: 10.1111/j.1460-9592.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- 57.Weintraud M, Marhofer P, Bösenberg A, Kapral S, Willschke H, Felfernig M, et al. Ilioinguinal/iliohypogastric blocks in children: where do we administer the local anesthetic without direct visualization? Anesth Analg. 2008;106:89–93. doi: 10.1213/01.ane.0000287679.48530.5f. [DOI] [PubMed] [Google Scholar]
- 58.Ghani KR, McMillan R, Paterson-Brown S. Transient femoral nerve palsy following ilio-inguinal nerve blockade for day case inguinal hernia repair. J R Coll Surg Edinb. 2002;47:626–629. [PubMed] [Google Scholar]
- 59.Jöhr M, Sossai R. Colonic puncture during ilioinguinal nerve block in a child. Anesth Analg. 1999;88:1051–1052. doi: 10.1097/00000539-199905000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Amory C, Mariscal A, Guyot E, Chauvet P, Leon A, Poli-Merol ML. Is ilioinguinal/iliohypogastric nerve block always totally safe in children? Paediatr Anaesth. 2003;13:164–166. doi: 10.1046/j.1460-9592.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- 61.Vaisman J. Pelvic hematoma after an ilioinguinal nerve block for orchialgia. Anesth Analg. 2001;92:1048–1049. doi: 10.1097/00000539-200104000-00045. [DOI] [PubMed] [Google Scholar]
- 62.Waldman SD. Atlas of interventional pain management. 2nd ed. Philadelphia, PA: Saunders; 2004. [Google Scholar]
- 63.Eichenberger U, Greher M, Kirchmair L, Curatolo M, Moriggl B. Ultrasound-guided blocks of the ilioinguinal and iliohypogastric nerve: accuracy of a selective new technique confirmed by anatomical dissection. Br J Anaesth. 2006;97:238–243. doi: 10.1093/bja/ael103. [DOI] [PubMed] [Google Scholar]
- 64.Hu P, Harmon D, Frizelle H. Ultrasound guidance for ilioinguinal/iliohypogastric nerve block: a pilot study. Ir J Med Sci. 2007;176:111–115. doi: 10.1007/s11845-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 65.Gofeld M, Christakis M. Sonographically guided ilioinguinal nerve block. J Ultrasound Med. 2006;25:1571–1575. doi: 10.7863/jum.2006.25.12.1571. [DOI] [PubMed] [Google Scholar]
- 66.Gucev G, Yasui GM, Chang TY, Lee J. Bilateral ultrasound-guided continuous ilioinguinal-iliohypogastric block for pain relief after cesarean delivery. Anesth Analg. 2008;106:1220–1222. doi: 10.1213/ane.0b013e3181683821. [DOI] [PubMed] [Google Scholar]
- 67.Jamieson RW, Swigart LL, Anson BJ. Points of parietal perforation of the ilioinguinal and iliohypogastric nerves in relation to optimal sites for local anaesthesia. Q Bull Northwest Univ Med Sch. 1952;26:22–26. [PMC free article] [PubMed] [Google Scholar]
- 68.Peng PW, Tumber PS. Ultrasound-guided interventional procedures for patients with chronic pelvic pain - a description of techniques and review of literature. Pain Physician. 2008;11:215–224. [PubMed] [Google Scholar]
- 69.Grossman MG, Ducey SA, Nadler SS, Levy AS. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9:336–344. doi: 10.5435/00124635-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 70.Harney D, Patijn J. Meralgia paresthetica: diagnosis and management strategies. Pain Med. 2007;8:669–677. doi: 10.1111/j.1526-4637.2006.00227.x. [DOI] [PubMed] [Google Scholar]
- 71.Shannon J, Lang SA, Yip RW, Gerard M. Lateral femoral cutaneous nerve block revisited. A nerve stimulator technique. Reg Anesth. 1995;20:100–104. [PubMed] [Google Scholar]
- 72.Dias Filho LC, Valença MM, Guimarães Filho FA, Medeiros RC, Silva RA, Morais MG, et al. Lateral femoral cutaneous neuralgia: an anatomical insight. Clin Anat. 2003;16:309–316. doi: 10.1002/ca.10106. [DOI] [PubMed] [Google Scholar]
- 73.de Ridder VA, de Lange S, Popta JV. Anatomical variations of the lateral femoral cutaneous nerve and the consequences for surgery. J Orthop Trauma. 1999;13:207–211. doi: 10.1097/00005131-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 74.Ropars M, Morandi X, Huten D, Thomazeau H, Berton E, Darnault P. Anatomical study of the lateral femoral cutaneous nerve with special reference to minimally invasive anterior approach for total hip replacement. Surg Radiol Anat. 2009;31:199–204. doi: 10.1007/s00276-008-0433-3. [DOI] [PubMed] [Google Scholar]
- 75.Grothaus MC, Holt M, Mekhail AO, Ebraheim NA, Yeasting RA. Lateral femoral cutaneous nerve: an anatomic study. Clin Orthop Relat Res. 2005;(437):164–168. doi: 10.1097/01.blo.0000164526.08610.97. [DOI] [PubMed] [Google Scholar]
- 76.Aszmann OC, Dellon ES, Dellon AL. Anatomical course of the lateral femoral cutaneous nerve and its susceptibility to compression and injury. Plast Reconstr Surg. 1997;100:600–604. doi: 10.1097/00006534-199709000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Murata Y, Takahashi K, Yamagata M, Shimada Y, Moriya H. The anatomy of the lateral femoral cutaneous nerve, with special reference to the harvesting of iliac bone graft. J Bone Joint Surg Am. 2000;82:746–747. doi: 10.2106/00004623-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Patel NJ, Flashburg MH, Paskin S, Grossman R. A regional anesthetic technique compared to general anesthesia for outpatient knee arthroscopy. Anesth Analg. 1986;65:185–187. [PubMed] [Google Scholar]
- 79.Hopkins PM, Ellis FR, Halsall PJ. Evaluation of local anaesthetic blockade of the lateral femoral cutaneous nerve. Anaesthesia. 1991;46:95–96. doi: 10.1111/j.1365-2044.1991.tb09347.x. [DOI] [PubMed] [Google Scholar]
- 80.Ng I, Vaghadia H, Choi PT, Helmy N. Ultrasound imaging accurately identifies the lateral femoral cutaneous nerve. Anesth Analg. 2008;107:1070–1074. doi: 10.1213/ane.0b013e31817ef1e5. [DOI] [PubMed] [Google Scholar]
- 81.Bodner G, Bernathova M, Galiano K, Putz D, Martinoli C, Felfernig M. Ultrasound of the lateral femoral cutaneous nerve: normal findings in a cadaver and in volunteers. Reg Anesth Pain Med. 2009;34:265–268. doi: 10.1097/AAP.0b013e31819a4fc6. [DOI] [PubMed] [Google Scholar]
- 82.Hurdle MF, Weingarten TN, Crisostomo RA, Psimos C, Smith J. Ultrasound-guided blockade of the lateral femoral cutaneous nerve: technical description and review of 10 cases. Arch Phys Med Rehabil. 2007;88:1362–1364. doi: 10.1016/j.apmr.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 83.Tumber PS, Bhatia A, Chan VW. Ultrasound-guided lateral femoral cutaneous nerve block for meralgia paresthetica. Anesth Analg. 2008;106:1021–1022. doi: 10.1213/ANE.0b013e3181632487. [DOI] [PubMed] [Google Scholar]
- 84.Damarey B, Demondion X, Boutry N, Kim HJ, Wavreille G, Cotten A. Sonographic assessment of the lateral femoral cutaneous nerve. J Clin Ultrasound. 2009;37:89–95. doi: 10.1002/jcu.20521. [DOI] [PubMed] [Google Scholar]