Abstract
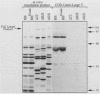
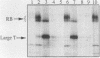
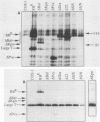
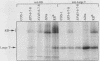
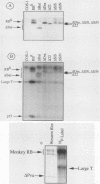
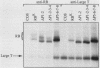
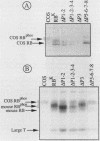
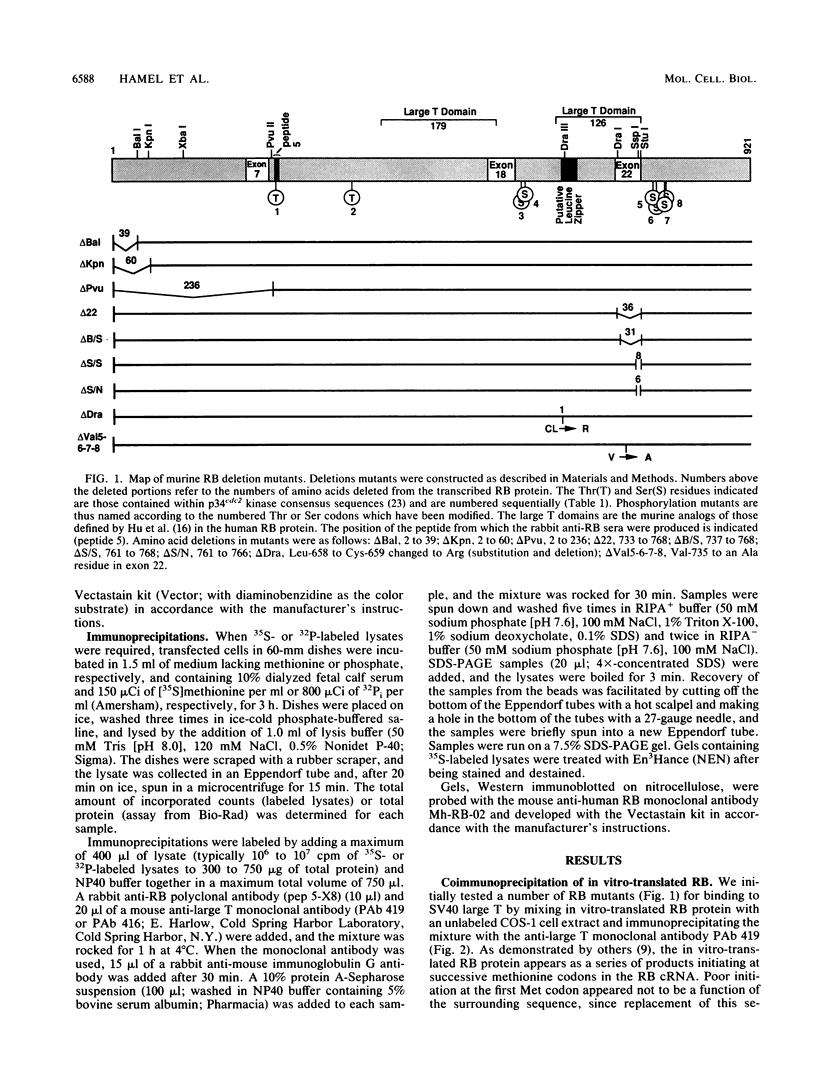
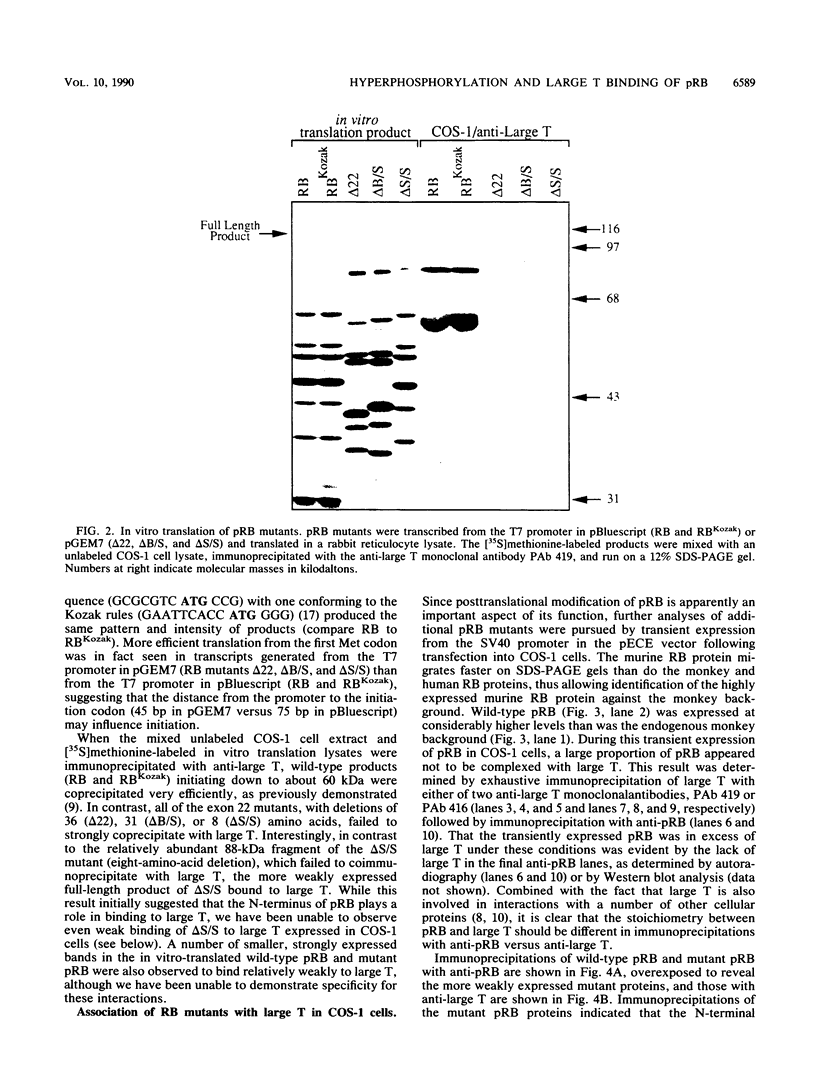
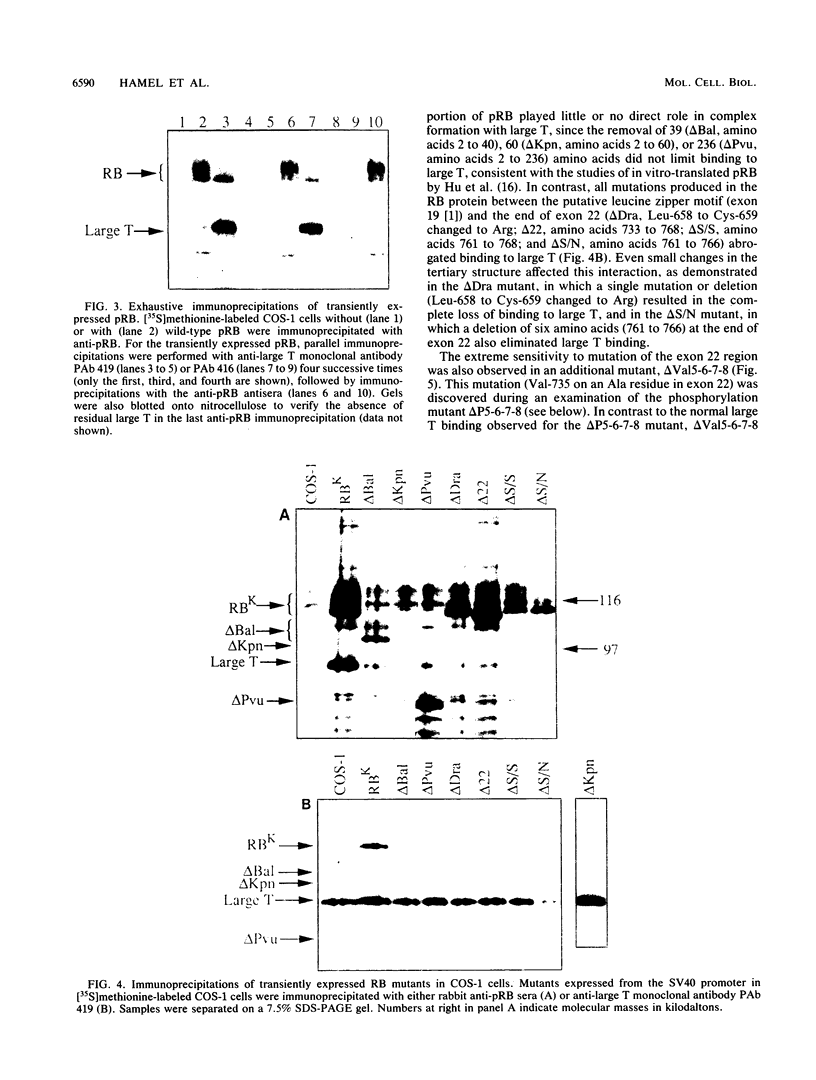
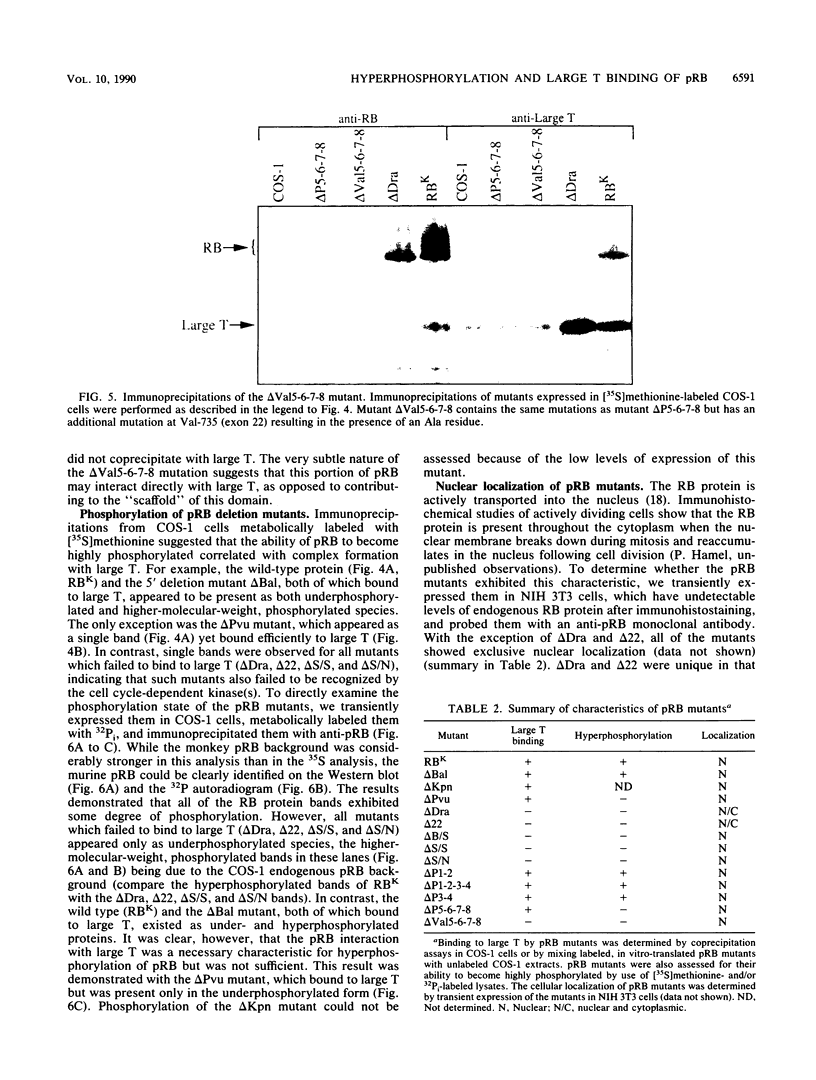
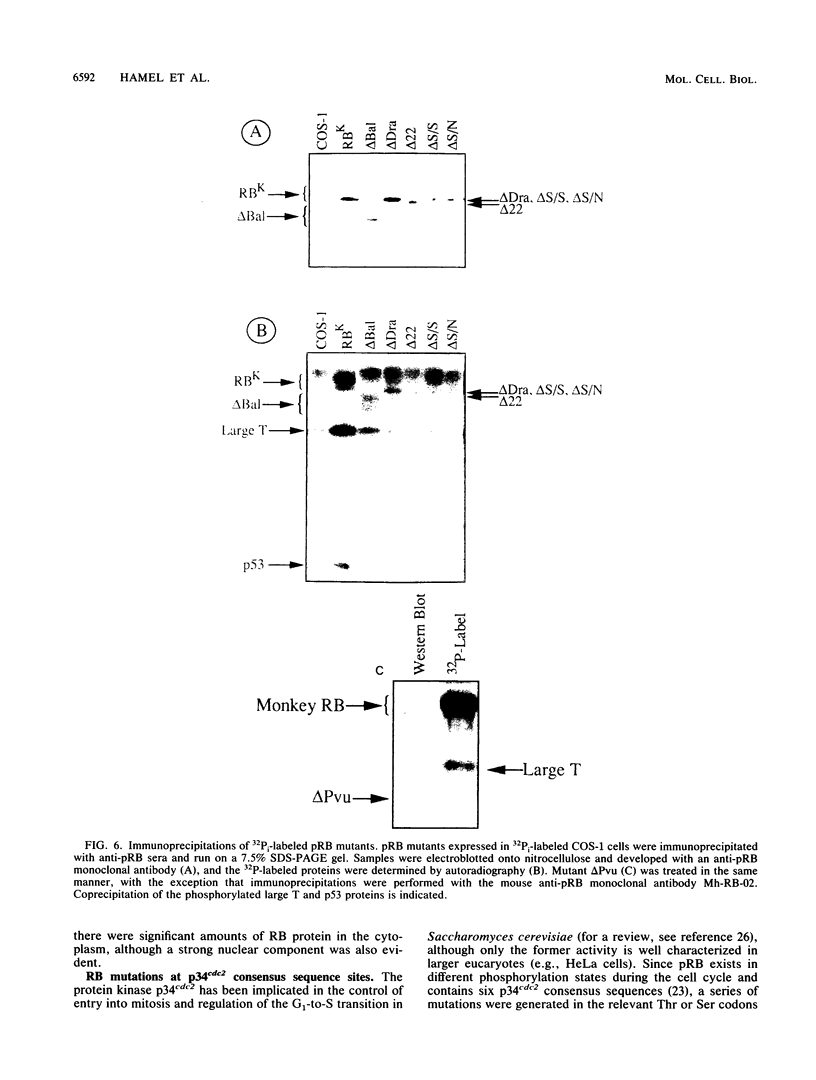
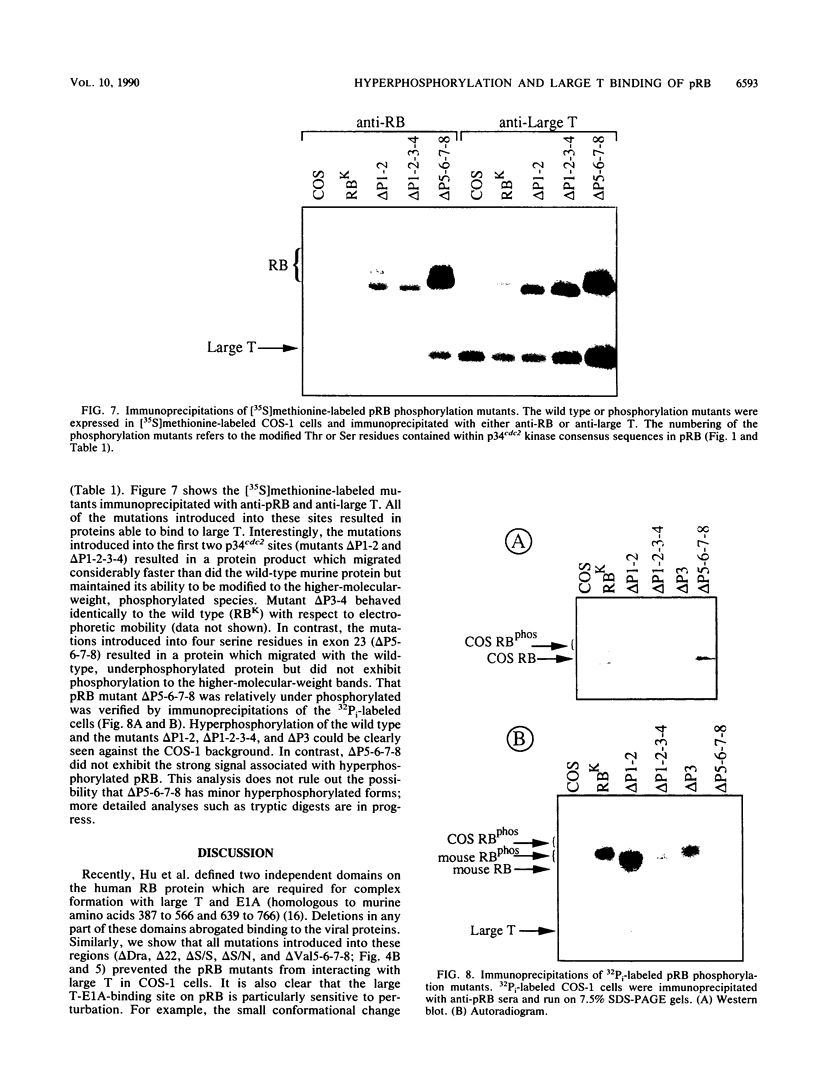
With the murine retinoblastoma (RB) cDNA, a series of RB mutants were expressed in COS-1 cells and the pRB products were assessed for their ability (i) to bind to large T antigen (large T), (ii) to become modified by phosphorylation, and (iii) to localize in the nucleus. All point mutations and deletions introduced into regions previously defined as contributing to binding to large T abolished pRB-large T complex formation and prevented hyperphosphorylation of the RB protein. In contrast, a series of deletions 5' to these sites did not interfere with binding to large T. While some of the 5' deletion mutants were clearly phosphorylated in a cell cycle-dependent manner, one, delta Pvu, failed to be phosphorylated depsite binding to large T. pRB with mutations created at three putative p34cdc2 phosphorylation sites in the N-terminal region behaved similarly to wild-type pRB, whereas the construct delta P5-6-7-8, mutated at four serine residues C terminal to the large T-binding site, failed to become hyperphosphorylated despite retaining the ability to bind large T. All of the mutants described were also found to localize in the nucleus. These results demonstrate that the domains in pRB responsible for binding to large T are distinct from those recognized by the relevant pRB-specific kinase(s) and/or those which contain cell cycle-dependent phosphorylation sites. Furthermore, these data are consistent with a model in which cell cycle-dependent phosphorylation of pRB requires complex formation with other cellular proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards R., Shackleford G. M., Schackleford G. M., Gerber M. R., Horowitz J. M., Friend S. H., Schartl M., Bogenmann E., Rapaport J. M., McGee T. Structure and expression of the murine retinoblastoma gene and characterization of its encoded protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6474–6478. doi: 10.1073/pnas.86.17.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Becker A. J., Gallie B. L. Identification of germline and somatic mutations affecting the retinoblastoma gene. Science. 1988 Sep 30;241(4874):1797–1800. doi: 10.1126/science.3175621. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge J., Webster T., Smith T. F., Paucha E. Prediction of similar transforming regions in simian virus 40 large T, adenovirus E1A, and myc oncoproteins. J Virol. 1988 May;62(5):1814–1818. doi: 10.1128/jvi.62.5.1814-1818.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Griep A. E., Westphal H. Antisense Myc sequences induce differentiation of F9 cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6806–6810. doi: 10.1073/pnas.85.18.6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., DeCaprio J. A., Huang C. M., Lee W. H., Paucha E., Livingston D. M. SV40 large T antigen binds preferentially to an underphosphorylated member of the retinoblastoma susceptibility gene product family. Cell. 1989 Jan 13;56(1):57–65. doi: 10.1016/0092-8674(89)90983-5. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Kaplan J. M., Bishop J. M., Varmus H. E. Mitosis-specific phosphorylation of p60c-src by p34cdc2-associated protein kinase. Cell. 1989 Jun 2;57(5):775–786. doi: 10.1016/0092-8674(89)90792-7. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts P. H., Watson J. V., Lamond A., Forster A., Stinson M. A., Evan G., Fischer W., Atherton E., Sheppard R., Rabbitts T. H. Metabolism of c-myc gene products: c-myc mRNA and protein expression in the cell cycle. EMBO J. 1985 Aug;4(8):2009–2015. doi: 10.1002/j.1460-2075.1985.tb03885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew J. Y., Chen P. L., Bookstein R., Lee E. Y., Lee W. H. Deletion of a splice donor site ablates expression of the following exon and produces an unphosphorylated RB protein unable to bind SV40 T antigen. Cell Growth Differ. 1990 Jan;1(1):17–25. [PubMed] [Google Scholar]
- Shew J. Y., Lin B. T., Chen P. L., Tseng B. Y., Yang-Feng T. L., Lee W. H. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):6–10. doi: 10.1073/pnas.87.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. J., Hu S. X., Hashimoto T., Takahashi R., Benedict W. F. The retinoblastoma susceptibility gene product: a characteristic pattern in normal cells and abnormal expression in malignant cells. Oncogene. 1989 Jun;4(6):807–812. [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Imamoto F. Transcriptional control of the endogenous MYC protooncogene by antisense RNA. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7363–7367. doi: 10.1073/pnas.84.21.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]