Abstract
Purpose
Parameters of positron emission tomography-computed tomography (PET-CT) were compared with the results of histopathologic examination in order to determine which can provide an objective indication of response after neoadjuvant chemoradiation for treatment of thoracic esophageal squamous cell carcinoma (SCC).
Materials and Methods
Between August 2003 and January 2010, data on 25 patients who underwent neoadjuvant chemoradiation and subsequent resection for treatment of esophageal SCC were retrospectively reviewed. Changes in maximum standardized uptake value (ΔSUVmax), metabolic tumor volume (ΔMTV), and total lesion glycolysis (ΔTLG) were analyzed by comparison with the histopathologic findings.
Results
Pathologic complete remission (CR) for the main tumor was achieved in 11 patients. Postradiation esophagitis was observed in 10 patients. ΔSUVmax of the main tumor was significantly greater in the CR group than in the partial response (PR) group (p=0.039), while ΔMTV and ΔTLG of the main tumor were not (p=0.141 and p=0.349, respectively). The cut-off ΔSUVmax value for CR was estimated as 72.1%, indicating significantly better accuracy than visual interpretation (p=0.045). Of the 48 involved lymph nodes, ΔSUVmax and ΔMTV of lymph nodes were significantly greater in the CR group than in the PR group (p=0.045 and p=0.014, respectively), while ΔTLG was not (p=0.063). The cut-off value of ΔSUVmax for prediction of CR in lymph nodes was calculated as 50.67%.
Conclusion
PET-CT could be used for prediction of response to neoadjuvant treatment in thoracic esophageal SCC. ΔSUVmax may be a more significant predictor for CR after neoadjuvant chemoradiation than ΔTLG and ΔMTV.
Keywords: Esophageal squamous cell carcinoma, Neoadjuvant therapy, Positron-emission tomography and computed tomography
Introduction
Despite the increase of routine health exam, many patients with esophageal cancer have advanced disease at initial diagnosis. Surgery is often combined with other modalities in order to improve outcomes, and neoadjuvant treatment was introduced in order to increase the rate of complete resection by decreasing tumor extent and preventing possible distant metastasis. Some recent studies have reported an encouraging rate of complete pathologic response (pCR) using neoadjuvant therapy [1-6].
Evaluation of response, during or after neoadjuvant treatment, is important for prediction of resectability in subsequent surgeries, as well as in balancing the benefits and possible harmful side effects. However, previous examinations, such as endoscopy, endoscopic ultrasonography (EUS), computed tomography (CT), and magnetic resonance imaging, were not satisfactorily useful because these modalities cannot effectively distinguish a viable tumor from post-treatment inflammation or fibrosis [7,8].
By reflecting metabolic tumor activity, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has emerged as an important noninvasive imaging modality for staging, response monitoring, and detection of recurrence in various types of malignancies [9-11]. Among the various PET parameters, maximum standardized uptake value (SUVmax) is most frequently used in clinical situations. However, SUVmax represents only a single voxel value of PET and hence can be easily confounded. Recent studies have reported that volumetric parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) were useful in predicting tumor response after neoadjuvant treatment for esophageal adenocarcinoma [11-13]. However, discussion of the utility of these PET parameters in esophageal squamous cell carcinoma (SCC) has been limited. In addition, despite the fact that lymph node metastasis is an important determinant of resectability in esophageal cancer, little has been studied about its response after neoadjuvant treatment.
The purpose of this study was to identify the most useful metabolic PET parameter for prediction of complete remission (CR) and resectability after neoadjuvant treatment for thoracic esophageal SCC by comparison of changes in PET parameters of both main tumors and suspected metastatic lymph nodes with postoperative pathologic results.
Materials and Methods
1. Patient enrollment
Between August 2003 and January 2010, 135 patients in our institution underwent neoadjuvant chemoradiation and subsequent resection with curative intent for treatment of advanced esophageal SCC. The eligibility criteria for this study were as follows: 1) candidates for neoadjuvant chemoradiation for advanced esophageal cancer, in whom local invasion of mediastinal structures or vertebra, or massive or extrathoracic lymph node metastasis were suspected by preoperative evaluations, 2) patients without distant metastasis, 3) completion of a planned schedule of chemotherapy and radiation, and 4) both pretreatment and post-treatment PET-CTs were performed in our institution in order to ensure consistency of data. Complete information regarding both prechemoradiation and postchemoradiation PET-CT was available for 25 patients, who were included as the final subjects. Our institutional review board approved this retrospective study protocol.
2. PET-CT imaging
Pretreatment PET-CT was performed as a part of routine preoperative evaluation. Post-treatment PET-CT was evaluated two or three days before surgery. The mean interval between pretreatment and post-treatment PET-CT was 75 days (range, 52 to 105 days).
After six hours of fasting before examination, blood glucose levels before injection of 18F-FDG were lower than 200 mg/dL in all patients. PET-CT imaging was performed using one of two dedicated PET-CT scanners (Discovery LS or Discovery STe, GE Healthcare, Milwaukee, WI) without intravenous or oral contrast material. A follow-up scan was performed after administration of neoadjuvant therapy using the same scanner in each patient.
First, whole-body CT was performed using a continuous spiral technique with a helical CT at 45-60 minutes after injection of 18F-FDG (5.5 MBq/kg). After the CT scan, an emission scan was obtained from the thigh to the head for 2.5-4 minutes per frame. Attenuation-corrected PET images were reconstructed from the CT data using an orderedsubset expectation maximization algorithm (28 or 20 subsets, two iterations).
3. Neoadjuvant chemoradiation therapy and operative procedures
Patients were scheduled to receive concomitant chemoradiotherapy before surgery. Patients received two cycles of cisplatin/fluorouracil on weeks 1 and 3 during radiotherapy. The chemotherapy regimen included cisplatin 60 mg/m2 on day 1, followed by fluorouracil 1,000 mg/m2/day by continuous infusion from day 1 to 4 of each cycle. Radiation therapy was administered with a target dose of 40 Gy (range, 38 to 44 Gy), delivered over five weeks by a daily dose of 1.8 or 2 Gy per fraction using a linear accelerator with 6 or 10 MV photons.
Surgery was generally performed three or four weeks after completion of neoadjuvant treatment. The type of surgery was selected according to the location of the tumor and suspected involved lymph nodes by initial PET-CT. Patients with upper thoracic esophageal cancer or suspected cervical lymph node involvement underwent 3-field resection, which included transthoracic esophagectomy, cervical esophagogastrostomy, and radical dissection of lymph nodes in the neck, chest, and abdomen. Patients with middle to lower thoracic tumors without cervical node involvement underwent Ivor Lewis operations with radical lymphadenectomy in the chest and abdomen. Cervical lymph node dissection routinely included level II-V stations and supraclavicular lymph nodes. In the thorax, exploration and dissection of both recurrent laryngeal, aortopulmonary, paraesophageal, subcarinal and nearby peribronchial, and both pulmonary ligaments and diaphragmatic lymph nodes were performed. Abdominal lymph node dissections were also performed in all patients for perigastric, celiac, left gastric, and common hepatic artery lymph nodes.
4. Data collection and statistical analysis
Medical records, including patient demographics, operation, information regarding neoadjuvant treatment, results of preoperative evaluation, including chest CT and PET-CT, histopathologic results, and follow-up were retrospectively reviewed. Post-treatment PET-CT was performed within one week prior to surgery if the patient completed the scheduled treatment.
Two experienced nuclear medicine physicians reviewed all 18F-FDG PET-CT images for initial staging and response evaluation on a dedicated workstation (GE Advantage Workstation 4.4). Initial visual interpretations regarding response after chemoradiation had been determined considering changes in PET parameters, radiologic changes in CT images, and other clinical situations. Metabolic and volumetric parameters were measured using Volume Viewer software (Kai Uwe Barthel, Berlin, Germany), which provides an automatically delineated volume of interest (VOI) using an isocontour threshold method based on the SUV. SUVmax was defined as the SUV on the highest image pixel in the tumor region. MTV was defined as the total tumor volume segmented by the threshold SUV [14]. Mediastinal blood pool activity of the aortic arch was used as a threshold for determining the VOI boundary [15,16]. SUVmean plus two standard deviations of the VOI in the aortic arch was adopted as the threshold SUV for the primary tumor and metastatic lymph nodes. Using the threshold SUV, VOIs of the primary tumor and metastatic lymph nodes were generated automatically. The software calculated the SUVmax, SUVmean, and MTV of each relevant lesion. TLG was obtained by multiplying the SUVmean by the number of voxels. Changes in metabolic parameters, including SUVmax, MTV, and TLG for both main tumors and individual involved lymph nodes were measured and compared with the histopathologic findings of surgical specimens. Surgical specimens were inspected and re-examined by a single pathologist. Determination of CR according to PET-CT results and pathologic results followed the revised Response Evaluation Criteria in Solid Tumors (RECIST ver. 1.1), and European Organization for Research and Treatment of Cancer (EORTC) recommendations [17,18]. In detail, pCR was defined as absence of histological evidence of neoplasia, gross tumor or individual cells in the resected esophageal specimen. Partial pathologic response was defined as a change in stage from preoperative evaluation or greater than 50% reduction in size of the tumor postoperatively. Nonresponders were defined as those with no change in tumor stage when comparing preoperative and postoperative pathologic stage.
Correlations of parameters with the prediction of CR were analyzed by maximum likelihood estimates using logistic regression analysis. Prediction accuracy was assessed using area under the receiver-operating characteristic curve (AUC). The cut-off values for best predictive value were calculated using Fisher's exact test. SAS ver. 9.1 (SAS Institute Inc., Cary, NC) was used in performance of statistical analysis. A p<0.05 was considered statistically significant.
Results
Patient demographics, information relevant to surgery, and recurrence are shown in Table 1. All 25 patients finished their scheduled chemoradiation therapy. Twenty one patients received neoadjuvant treatment due to extrathoracic (mostly cervical) or extensive intrathoracic lymph node involvement. In the remaining four patients, direct mediastinal or vertebra invasion was suspected. None of the patients in this series experienced significant side effects during neoadjuvant treatment, except two cases of nausea, which required additional anti-emetics administration.
Table 1.
Patient demographics and information at surgery and follow-up
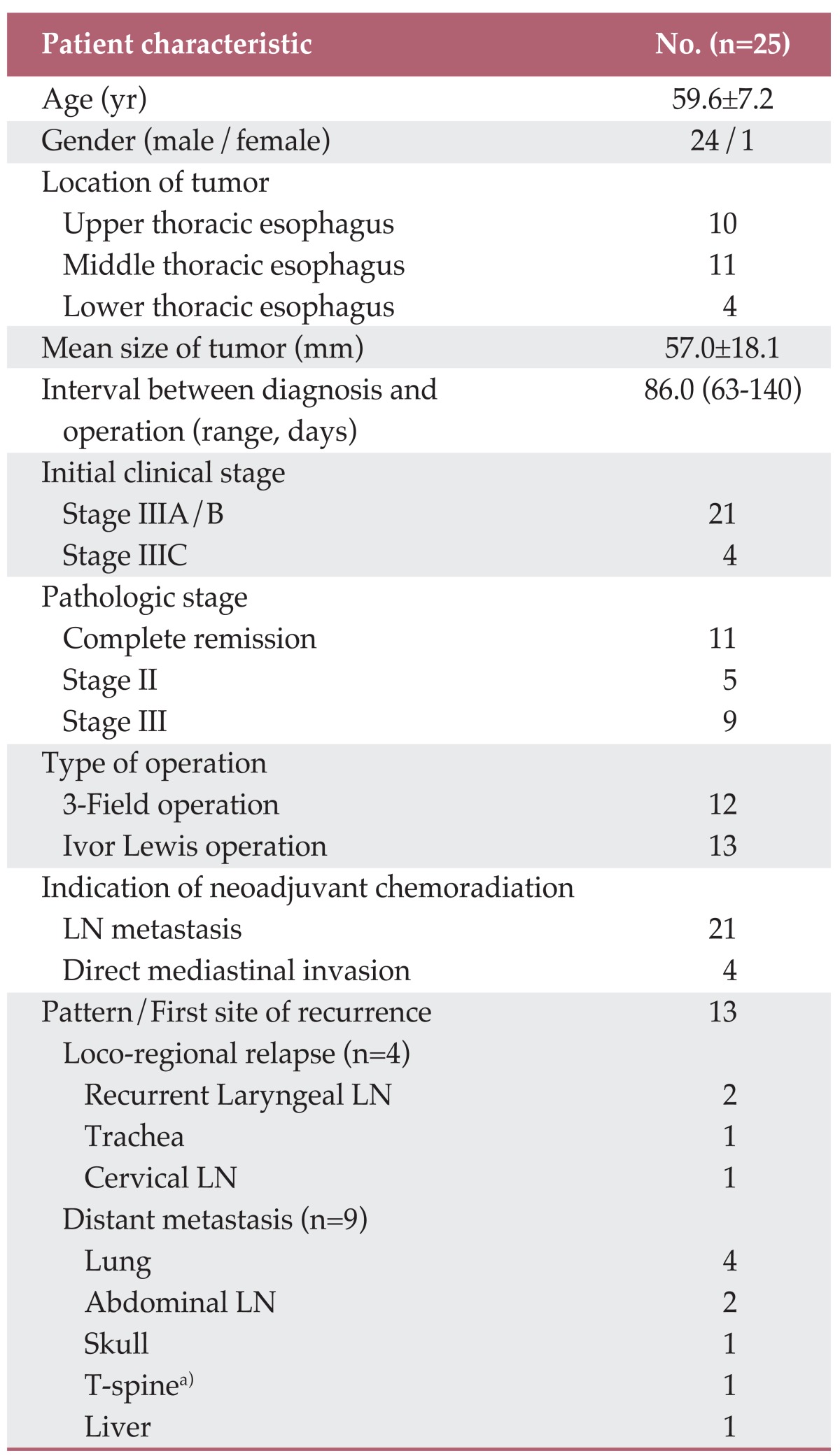
LN, lymph node. a)Found with abdominal LN metastasis.
Ten patients with upper thoracic esophageal cancer and another two patients with middle thoracic tumors with suspicious cervical nodal metastasis underwent 3-field resections. The other 13 patients underwent Ivor Lewis operations. Complete resection was achieved in 22 patients. The three remaining patients were considered to have residual microscopic tumors after removal of gross tumors invading the vertebra or aorta. The mean number of harvested lymph nodes was 43.2±16.8. Operative mortality occurred in one patient due to postoperative respiratory failure. Another five patients had pulmonary complications such as pneumonia or acute lung injury. Anastomosis leakage was suspected in three patients, based on esophagography performed on the seventh postoperative day, but showed improvement without surgical management.
All of the PET-CT parameters of the individual tumor and lymph nodes could be verified by histopathologic examination. For the main tumor, pathologic CR was achieved in 11 patients, and partial response (PR), including stable disease was found in 14 patients. Combined hypermetabolic postradiation esophagitis was observed in 10 of 25 patients (40%).
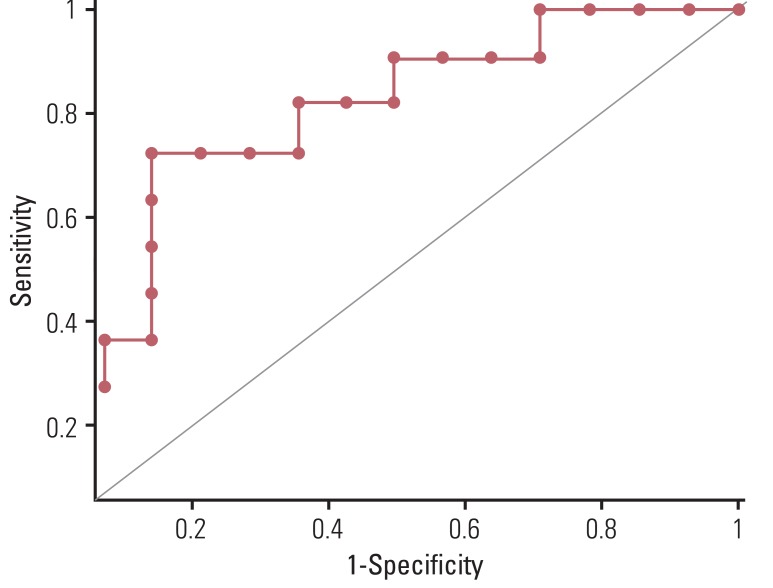
Reduction of SUVmax (ΔSUVmax) of the main tumor was significantly greater in the CR group than in the PR group (83.0%±17.0% vs. 55.6%±28.4%, p=0.039). The AUC was calculated as 0.789 (95% confidence interval, 0.581 to 0.925) for prediction of CR by ΔSUVmax of the main tumor. (Fig. 1) However, ΔMTV and ΔTLG of the main tumor did not differ significantly between the two groups (p=0.141 and p=0.349, respectively). Multivariable analysis identified an independent and positive association of ΔSUVmax with pathologic CR of the main tumor (Table 2). The optimum cut-off value of ΔSUVmax showing the best accuracy was 72.1% (p=0.02), which resulted in a sensitivity of 72.73, specificity of 85.7, and accuracy of 80.0%. These values were compared with those of visual interpretation results, which were calculated as follows: sensitivity, 36.4%; specificity, 85.7%; and accuracy, 64.0%. Both sensitivity and accuracy were significantly higher for ΔSUVmax than for visual interpretation (p=0.045, both). Visual interpretation assumed PR in all of the 10 cases with radiation-induced esophagitis, while seven of them were confirmed as having CR by pathologic examination (Figs. 2 and 3).
Fig. 1.
ROC curve of ΔSUVmax. Area under ROC was calculated as 0.789 (95% confidence interval, 0.581 to 0.925) for prediction of complete remission. ROC, receiveroperating characteristic; ΔSUVmax, changes in maximum standardized uptake value.
Table 2.
Multivariable analysis by logistic regression test for factors related to pathologic complete remission of the main tumor after neoadjuvant chemoradiation for thoracic esophageal squamous cell carcinoma
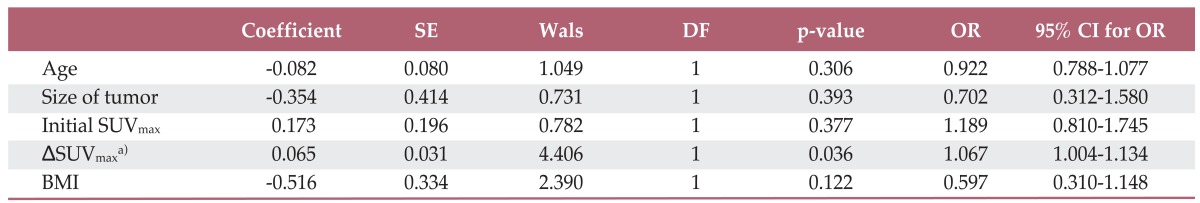
SE, standard error; DF, degree of freedom; OR, odd ratio; CI, confidence interval; SUVmax, maximum standardized uptake value; BMI, body mass index. a)Refers to the changes of SUVmax after neoadjuvant chemoradiation.
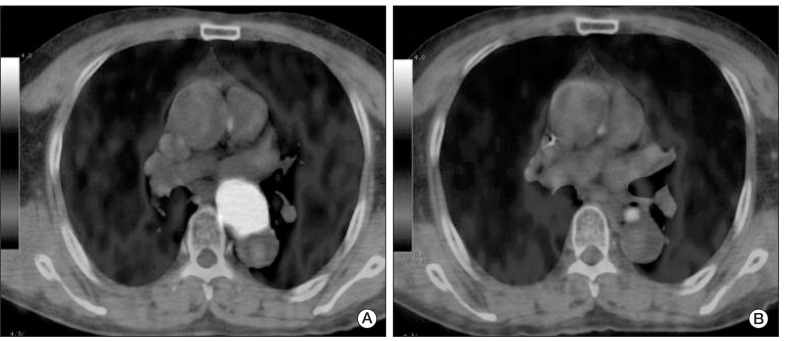
Fig. 2.
(A) Pretreatment fused positron emission tomography-computed tomography image shows a large hypermetabolic esophageal cancer (SUVmax=24.8). (B) Post-treatment image shows markedly decreased tumor uptake (SUVmax=4.4). Although there was residual hypermetabolic viable tumor by visual interpretation, reduction of SUVmax (ΔSUVmax) was 82.3%, by which complete remission could have been predicted according to the cut-off ΔSUVmax of 72%. Permanent pathologic examination revealed no remaining viable tumor. SUVmax, maximum standardized uptake value.
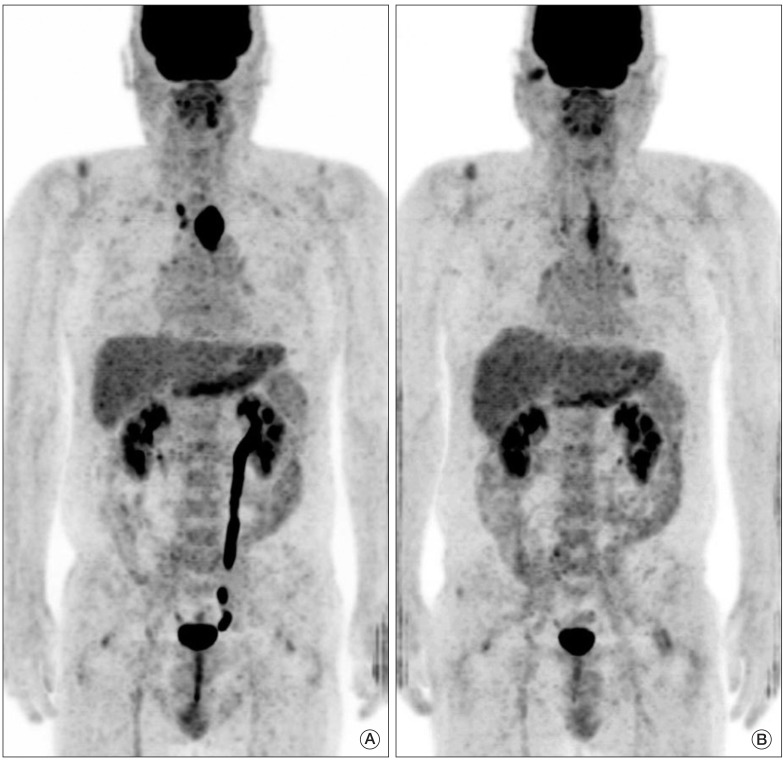
Fig. 3.
(A) Pretreatment fused positron emission tomography-computed tomography image shows a large hypermetabolic esophageal cancer (SUVmax=26.6). (B) post-treatment image shows linearly increase uptake in the upper thoracic esophagus beyond the boundary of the primary tumor (SUVmax=7.1), which suggests active radiation esophagitis. Due to esophagitis, diagnosis of complete remission by visual interpretation is difficult, while reduction of SUVmax (ΔSUVmax) was 73.3%, which implies complete remission according to the cut-off ΔSUVmax of 72%. Permanent pathologic examination revealed no remaining viable tumor. SUVmax, maximum standardized uptake value.
The number of lymph node stations with suspected metastasis according to prechemoradiation PET-CT was 48, and the mean number of lymph node stations with suspected metastasis per patient was 1.92 (range, 0 to 4). For these 48 involved lymph nodes, CR was achieved in 31 lymph nodes and PR in 17. Unexpected lymph node metastasis was found in four patients, which included three perigastric lymph nodes and two right recurrent laryngeal nerve lymph nodes. The ΔSUVmax and ΔMTV of lymph nodes was significantly greater in the CR group than in the PR group (p=0.045 and p=0.014, respectively). However, ΔTLG of lymph nodes did not differ significantly between the two groups (p=0.063). The optimum cut-off value of ΔSUVmax for lymph nodes showing the best accuracy was 50.7% (p=0.008), which resulted in a sensitivity of 90.3%, specificity of 64.7%, and accuracy of 81.3%.
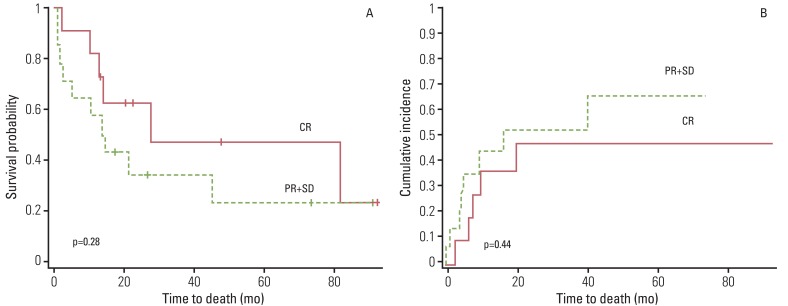
During follow-up, 13 patients experienced tumor recurrence, including nine distant metastases to lung, bone, or liver and four loco-regional relapses. All loco-regional relapses were found in the area of lymph nodes that were suspected as having residual disease by post-treatment PET-CT (Table 1). Patients showing pathological CR appear to have better survival than those with stable disease or PR, although statistical significance was not reached (p=0.286 and p=0.444, respectively) (Fig. 4A and B). No pretreatment and post-treatment PET parameters showed significant correlation with survival or recurrence of these patients.
Fig. 4.
Kaplan-Meier survival curve for overall survival (A) and cumulative incidence of recurrence (B). Patients showing pathological CR appear to have better survival than those with PR, although statistical significance was not reached, respectively. CR, complete remission; PR+SD, partial response and stable disease.
Discussion
Evaluation of response after neoadjuvant chemoradiation for treatment of esophageal cancer is very important not only to determination of the continuation of current treatment strategies but also to reducing the adverse effects of unnecessary chemoradiotherapy [19]. Some authors have reported increased survival in patients with CR after neoadjuvant treatment, while others have reported that neoadjuvant treatment did not lead to improvements in survival or recurrence [2,3,20,21]. In some cases, comparable outcomes were observed only by neoadjuvant chemotherapy without radiation, which could expedite resection, and decrease operative mortality and postoperative complications by radiation [22]. Recent meta-analysis provided strong evidence for a survival benefit of neoadjuvant treatment [6]. Evaluation of response for suspicious lymph node metastasis also has important clinical implications for determining the extent of resection and predicting prognosis [23]. However, lymph node metastasis has not been extensively explored [24].
Previous morphologic exams cannot identify a true residual tumor from post-treatment fibrosis. PET can provide information regarding changes in metabolic activity of tumors, and integrated PET-CT can give both anatomical extent and metabolic activity of the main tumor as well as involved lymph nodes. It is also helpful in distinguishing metastatic lesions from benign inflammation such as reactive lymph nodes. Various PET parameters are used for interpretation of results, and some recent reports have shown that volumetric parameters such as MTV or TLG were more effective in evaluation of response to neoadjuvant treatment than SUVmax. However, most of these studies were conducted on thoracic esophageal adenocarcinomas, and discussion regarding SCC has been limited, although correlations of changes in metabolic activity with response and survival were reported [25]. SCC, the most prevalent esophageal cancer in Asians, comprises more than 99% of esophageal cancers at our institution. Besides, there has been a lack of objective index for interpreting PET parameters, while interpretation of PET-CT results has been highly subjective and empirical according to persons who interpret the results.
Since 2003, integrated PET-CT has been used at our institution for both initial metastatic surveillance and for response evaluation of various tumors [14]. In the current study, we attempted to find a potential objective yardstick for judgment of complete resection and resectability in patients who underwent neoadjuvant chemoradiation for treatment of thoracic esophageal SCC. In this series, tumors were located at the upper or middle esophagus in 21 patients, and only four had distal esophageal tumors. The mean tumor size was 5.7 cm. Unlike the findings reported by Wieder et al. [25], in ten patients with radiation esophagitis after neoadjuvant treatment, the region of radiation esophagitis showed substantial PET uptake due to active inflammation, which made differentiation of true tumor-uptake from inflammation very difficult. In addition, in large tumors, it was also difficult to define the precise extent of viable tumors from post-treatment fibrosis radiologically. Therefore, in many cases, a significant difference was observed between the calculated volume of the viable tumor and actual viable tumor volume assessed by pathologic examination of the resected specimen. As a result, determination of response by TLG or MTV was more difficult, especially in patients with radiation esophagitis. Consequently, the correlation of ΔTLG or ΔMTV with pathologic findings was less consistent than that of ΔSUVmax. When we compared the effectiveness of ΔSUVmax with that of visual interpretation, the sensitivity and accuracy of predicting CR by ΔSUVmax at its optimum cut-off value were superior to those of visual interpretation.
Unlike adenocarcinoma, which occurs primarily in the confined space of the distal esophagus and esophagogastric junction, SCC occurs evenly throughout the whole thoracic esophagus, and therefore, tends to be extensive in size at initial diagnosis. In addition, some authors have reported that adenocarcinoma showed a more rapid response to increasing doses of chemoradiation than SCC [24]. For these reasons, development of inflammation and post-treatment fibrosis in response to chemoradiation appears more likely with SCC, and these could be confused with viable tumors. We also observed that PET results showed better correlation with pathologic findings for lymph nodes than for the main tumor due to these PET artifacts.
Our study had some limitations. First, it was pretrospective in design. Only 18.5% (25 out of 135) of patients who received neoadjuvant chemoradiation underwent both pretreatment and post-treatment PET-CT at our institution because post-treatment PET-CT is not routinely performed in the early period of disease and because many patients had undergone PET-CT in other hospitals with different equipment and protocols before transfer to our institution. We retrospectively reviewed pathologically proven specimens and compared the PET-CT findings with the pathologic reports. Conduct of a prospectively designed study might be required in order to more accurately include false positive or false negative results of PET-CT scans. Second, our study failed to show statistically significant correlation between CR and survival benefit (Fig. 4), although all of the loco-regional recurrence was observed in the areas of residual lymph node metastasis. This might result from a small number of subjects. Third, measurement of PET parameters was performed using two different PET scanner models with different protocols. Future studies with a larger population might be able to provide exact correlation of the changes of PET-CT parameter with prognosis in thoracic esophageal SCC. In addition, guidelines for adjuvant treatment according to reduction of SUVmax could be provided.
Conclusion
PET-CT can be used for prediction of treatment response after neoadjuvant chemoradiation for both the main tumor and involved lymph nodes in thoracic esophageal SCC. Reduction of SUVmax may be a more useful PET parameter for prediction of CR than that of visual interpretation or volume-adjusted metabolic parameters, such as TLG or MTV. Reduction of SUVmax greater than 70% for the main tumor and 50% for metastatic lymph nodes were associated with an increased chance of curative resection.
Acknowledgments
We would like to thank Hwan Joo Lee in the Department of Nuclear Medicine, Samsung Medical Center, and Sook Young Woo on the Biostatistics team, Samsung Biomedical Research Institute, for assistance with data analysis. This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (no. 1120150).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Geh JI, Crellin AM, Glynne-Jones R. Preoperative (neoadjuvant) chemoradiotherapy in oesophageal cancer. Br J Surg. 2001;88:338–356. doi: 10.1046/j.1365-2168.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Poplin E, Fleming T, Leichman L, Seydel HG, Steiger Z, Taylor S, et al. Combined therapies for squamous-cell carcinoma of the esophagus, a Southwest Oncology Group Study (SWOG-8037) J Clin Oncol. 1987;5:622–628. doi: 10.1200/JCO.1987.5.4.622. [DOI] [PubMed] [Google Scholar]
- 3.Bates BA, Detterbeck FC, Bernard SA, Qaqish BF, Tepper JE. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996;14:156–163. doi: 10.1200/JCO.1996.14.1.156. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med. 1996;335:462–467. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 5.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–313. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 6.Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 7.Zuccaro G, Jr, Rice TW, Goldblum J, Medendorp SV, Becker M, Pimentel R, et al. Endoscopic ultrasound cannot determine suitability for esophagectomy after aggressive chemoradiotherapy for esophageal cancer. Am J Gastroenterol. 1999;94:906–912. doi: 10.1111/j.1572-0241.1999.985_h.x. [DOI] [PubMed] [Google Scholar]
- 8.Jones DR, Parker LA, Jr, Detterbeck FC, Egan TM. Inadequacy of computed tomography in assessing patients with esophageal carcinoma after induction chemoradiotherapy. Cancer. 1999;85:1026–1032. doi: 10.1002/(sici)1097-0142(19990301)85:5<1026::aid-cncr3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 10.Brucher BL, Weber W, Bauer M, Fink U, Avril N, Stein HJ, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamede M, Abreu-E-Lima P, Oliva MR, Nose V, Mamon H, Gerbaudo VH. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: correlation with histopathology results. Am J Clin Oncol. 2007;30:377–388. doi: 10.1097/COC.0b013e31803993f8. [DOI] [PubMed] [Google Scholar]
- 12.Sendler A. Metabolic response evaluation by PET during neoadjuvant treatment for adenocarcinoma of the esophagus and esophagogastric junction. Recent Results Cancer Res. 2010;182:167–177. doi: 10.1007/978-3-540-70579-6_14. [DOI] [PubMed] [Google Scholar]
- 13.Roedl JB, Colen RR, Holalkere NS, Fischman AJ, Choi NC, Blake MA. Adenocarcinomas of the esophagus: response to chemoradiotherapy is associated with decrease of metabolic tumor volume as measured on PET-CT: comparison to histopathologic and clinical response evaluation. Radiother Oncol. 2008;89:278–286. doi: 10.1016/j.radonc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17:115–122. doi: 10.1245/s10434-009-0719-7. [DOI] [PubMed] [Google Scholar]
- 15.Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–578. doi: 10.1200/JCO.2006.08.2305. [DOI] [PubMed] [Google Scholar]
- 16.Le Roux PY, Gastinne T, Le Gouill S, Nowak E, Bodet-Milin C, Querellou S, et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011;38:1064–1071. doi: 10.1007/s00259-011-1741-0. [DOI] [PubMed] [Google Scholar]
- 17.van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010;20:1456–1467. doi: 10.1007/s00330-009-1685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 19.Thurau K, Palmes D, Franzius C, Minin E, Senninger N, Juergens KU, et al. Impact of PET-CT on primary staging and response control on multimodal treatment of esophageal cancer. World J Surg. 2011;35:608–616. doi: 10.1007/s00268-010-0946-x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JH, Choi EK, Kim SB, Park SI, Kim DK, Song HY, et al. Preoperative hyperfractionated radiotherapy with concurrent chemotherapy in resectable esophageal cancer. Int J Radiat Oncol Biol Phys. 2001;50:1–12. doi: 10.1016/s0360-3016(01)01459-6. [DOI] [PubMed] [Google Scholar]
- 21.van Heijl M, Omloo JM, van Berge Henegouwen MI, Hoekstra OS, Boellaard R, Bossuyt PM, et al. Fluorodeoxyglucose positron emission tomography for evaluating early response during neoadjuvant chemoradiotherapy in patients with potentially curable esophageal cancer. Ann Surg. 2011;253:56–63. doi: 10.1097/SLA.0b013e3181f66596. [DOI] [PubMed] [Google Scholar]
- 22.Luu TD, Gaur P, Force SD, Staley CA, Mansour KA, Miller JI, Jr, et al. Neoadjuvant chemoradiation versus chemotherapy for patients undergoing esophagectomy for esophageal cancer. Ann Thorac Surg. 2008;85:1217–1223. doi: 10.1016/j.athoracsur.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 23.Aiko S, Yoshizumi Y, Ishizuka T, Sakano T, Kumano I, Sugiura Y, et al. Reduction rate of lymph node metastasis as a significant prognostic factor in esophageal cancer patients treated with neoadjuvant chemoradiation therapy. Dis Esophagus. 2007;20:94–101. doi: 10.1111/j.1442-2050.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 24.Bollschweiler E, Holscher AH, Metzger R. Histologic tumor type and the rate of complete response after neoadjuvant therapy for esophageal cancer. Future Oncol. 2010;6:25–35. doi: 10.2217/fon.09.133. [DOI] [PubMed] [Google Scholar]
- 25.Wieder HA, Brucher BL, Zimmermann F, Becker K, Lordick F, Beer A, et al. Time course of tumor metabolic activity during chemoradiotherapy of esophageal squamous cell carcinoma and response to treatment. J Clin Oncol. 2004;22:900–908. doi: 10.1200/JCO.2004.07.122. [DOI] [PubMed] [Google Scholar]