Abstract
Purpose
In some unusual cases, in patients with cervical cancer, an elevation of squamous cell carcinoma antigen (SCC-Ag) was not observed at diagnosis but was observed on recurrence, or vice versa. The objective of this study was to identify patient-, disease-, and treatment-related factors associated with this unusual level of SCC-Ag, and to determine whether SCC-Ag is a useful tumor marker in such patients.
Materials and Methods
Among 129 patients with recurrence, 14 who showed a normal SCC-Ag level at diagnosis but an elevated level at recurrence were classified as group I; 22 patients with an elevated SCC-Ag level at diagnosis but not at recurrence were classified as group II; and 76 patients with an elevated SCC-Ag level at both diagnosis and recurrence were classified as group III.
Results
In univariate analysis, unusual SCC-Ag showed statistically significant relationships with pathology and biochemical response to treatment. However, in the multivariate analysis, none of the clinicopathologic factors showed a statistical relationship with unusual levels of SCC-Ag. The 5-year disease-free survival rates for groups I, II, and III were 7.1%, 9.1%, and 0% (p=0.418), and the 5-year overall survival rates were 34.3%, 58.4%, and 33.3% (p=0.142), respectively.
Conclusion
The value of SCC-Ag has been confirmed in all patients; thus, check of SCC-Ag level at follow-up should be considered. Although no statistically significant differences were observed among the groups, we conclude that patients with a high initial SCC-Ag and elevated SCC-Ag at relapse have poor prognosis due to high SCC-Ag level.
Keywords: Uterine cervical neoplasms, Squamous cell carcinoma-related antigen, Biological tumor markers
Introduction
Cervical cancer is the third most commonly diagnosed cancer and the fourth leading cause of cancer death in females worldwide, despite improvements in screening programs, which have allowed the diagnosis of preinvasive lesions and reduced the incidence of advanced stage cervical cancer [1].
Several features of cervical cancer are prognostically important, including the extent of tumor, lymph node involvement, tumor size, lymph vascular space invasion, and depth of stromal invasion by tumor cells [2,3]. Optimal management consists of precise staging and implementation of appropriate treatment followed by deliberate post-treatment surveillance, early detection of recurrence, and appropriate salvage therapy. Early detection of recurrence has been found to improve survival [4-6].
Several methods are available for detection of relapse, which serve as a significant prognostic factor for patients with cervical cancer. However, the disease is usually far advanced when the accompanying symptoms are observed. Screening Pap smear is insufficient for detection of early disease, diagnosis of relapse by physical examination is difficult because of anatomical changes after the initial treatment, and frequent performance of computed tomography (CT) or magnetic resonance imaging (MRI) is too expensive. To overcome these drawbacks, tumor marker analysis is widely used for early detection of recurrent disease.
Several tumor markers have been investigated in the search for prognostic parameters that could be used in monitoring of treatment response and in detection of recurrence in patients with cervical cancer. The squamous cell carcinoma antigen (SCC-Ag) is the most commonly used tumor marker for cervical cancer. Serum SCC-Ag level has shown correlation with the extent of the disease [7-9] and response to treatment and provides a valid tool for early detection of disease recurrence [10-13]. A very large proportion of patients (74-88%) present with an elevated SCC-Ag serum level in association with, or preceding, the disease [10-13], and 70-86% of cervical cancer patients with recurrent disease were found to have elevated SCC-Ag levels during follow-up [7,10,14,15]. However, in some unusual cases, elevation of SCC-Ag was not observed at diagnosis but was observed at diagnosis but not at recurrence. Scambia et al. [10] reported on two patients with a normal initial SCC-Ag level but an elevated level at recurrence, and Rose et al. [16] reported on two patients whose pretreatment SCC-Ag level was abnormally high but a normal level was observed at recurrence. With regard to this phenomenon, Choi et al. [17] explained that the reason for an unusual level of SCC-Ag from normal to overexpression is that the number of tumor cells with antigen expression at diagnosis is insufficient. However, invasiveness and tumor size increase upon recurrence, resulting in an elevated level of SCC-Ag. For the opposite case, which is more difficult to explain, they proposed that most tumor cells with high antigen expression are removed after treatment, and the subsequent recurrence involves tumor cells with low levels of antigen expression.
The first objective of this study was to identify patient-, disease-, and treatment-related factors associated with an unusual SCC-Ag response, and the second objective was to determine whether SCC-Ag is a useful tumor marker in patients with unusual SCC-Ag patterns or whether an alternative approach is needed.
Materials and Methods
We conducted a retrospective review of clinical information from 1,610 patients with cervical cancer between January 1994 and December 2010. Among these patients, 895 were excluded due to the absence of pretreatment or post-treatment SCC-Ag value, incomplete treatment, or missing some clinical information. We therefore analyzed 715 patients with histologically proven cervical cancer who were treated with radiotherapy and/or chemotherapy or surgery with adjuvant radiotherapy and/or chemotherapy at Samsung Medical Center (SMC) in Seoul, Korea. We analyzed information regarding age, menopausal status, International Federation of Gynecology and Obstetrics (FIGO) stage, pathology, tumor size, lymph node involvement, pretreatment and post-treatment SCC-Ag levels, treatment modality, response to treatment, and recurrence.
Inclusion criteria were as follows: 1) biopsy-confirmed cervical cancer of clinical stage I-IV according to the FIGO Staging System; 2) definitive or postoperative radiotherapy combined with chemotherapy in any sequence; and 3) measurement of serum SCC-Ag levels before and after treatment.
All of the patients underwent pretreatment work-ups and routine clinical staging. The largest diameter of lymph nodes greater than 1 cm was interpreted as involved. We collected serum samples from all patients in order to determine serum SCC-Ag levels before and after treatment and performed a retrospective review of the patients' records. Serum SCC-Ag was measured using a SCC immunoradiometric assay kit (Imx, Abbott Diagnostics, Abbott Park, IL) with a lower level of sensitivity of 0.1 ng/mL. The cut-off level of serum SCC used at SMC was 1.5 ng/mL. For assessment of biochemical response, SCC-Ag levels measured before and 1-2 months after the treatment were compared. When SCC-Ag showed a decrease after treatment, it was defined as a "Positive biochemical response," while, in the reverse case, defined as a "Negative biochemical response." Biochemical failure was defined as two consecutive increases in the SCC-Ag value of more than 1 ng/mL or elevation of greater than 1.5 ng/mL.
The median length of follow-up for all patients was 38.3 months (range, 1.1 to 161.4 months). Clinical examination, Pap smear, serum SCC-Ag measurement, and imaging work-up, including CT or MRI, were performed before and after treatment, followed by further work-up, depending on the clinical finding or serum SCC-Ag level. If a patient showed biochemical failure without evidence of local recurrence during the clinical follow-up examinations, all possible sites of recurrence were checked systemically. The initial site of recurrence was categorized as locoregional recurrence (LRR) or distant metastasis (DM) based on the location of the true pelvis. Among 129 patients with recurrence, 14 patients who showed a normal SCC-Ag level at diagnosis but an elevated level at recurrence were classified as group I; 22 patients with an elevated SCC-Ag level at diagnosis but not at recurrence were classified as group II; and 76 patients with an elevated SCC-Ag level at both diagnosis and recurrence were classified as group III. Patients with a normal SCC-Ag level at both diagnosis and recurrence were excluded in this study. We decided on an adequate treatment option for salvage therapy for recurred patients depending on their recurrence patterns. For patients with LRR, if operable, we performed salvage surgery; otherwise, reirradiation was performed. For patients showing distant metastasis, we chose chemotherapy prior to other treatment options for salvage treatment. It is notable that no significant difference in selection of the treatment option for recurred patients was observed among the three patient groups.
Statistical analyses were performed using chi-square test or Fisher's exact test, and one-way ANOVA was used to examine the relationships between unusual SCC-Ag levels and other clinicopathologic factors. Logistic regression analysis was used for multivariate analysis. Disease-free survival (DFS) and overall survival (OS) were calculated at the start of treatment using the Kaplan-Meier method. Log-rank test was used to determine the p-value, and p<0.05 was considered significant. Statistical software SPSS ver. 18 (SPSS Inc., Chicago, IL) was used in performance of all statistical analyses.
Results
The characteristics of the patient groups are shown in Table 1. The median ages of patients in groups I, II, and III were 45.5 years (range, 30 to 74 years), 56.0 years (range, 29 to 82 years), and 54.0 years (range, 30 to 79 years), respectively. Eight (57.1%), eight (36.4%), and 33 (43.4%) patients had a premenopausal status. With respect to clinical staging, five (35.7%), eight (36.4%), and 14 (18.4%) patients were stage I; eight (57.1%), 11 (50.0%), and 43 (56.6%) patients were stage II; 0 (0%), two (9.1%), and 13 (17.1%) patients were stage III; and one (7.1%), one (4.5%), and six (7.9%) patients were stage IV for groups I, II, and III, respectively. Regarding pathological characteristics, eight (57.10%), 19 (86.4%), and 72 (94.7%) patients in groups I, II, and III, respectively, had squamous cell carcinoma; five (35.7%), one (4.5%), and two (2.6%) patients had adenocarcinoma; and one (7.1%), one (4.5%), and two (2.6%) patients had adenosquamous cell carcinoma. Tumor size ranged between 1.6-8.0 cm (median, 4.2 cm) in group I, 2.1-14.0 cm (median, 4.4 cm) in group II, and 1.3-8.4 cm (median, 4.6 cm) in group III, and the patients were classified based on a size of 4 cm, which is the basis of size-based classification of FIGO stage. Five (35.7%), 12 (54.5%), and 50 (65.8%) patients in groups I, II, and III, respectively, had lymph node involvement. The initial levels of SCC-Ag in groups I, II, and III showed respective ranges of 0.2-1.4 ng/mL (median, 1.0 ng/mL), 1.5-21.0 ng/mL (median, 2.8 ng/mL), and 1.5-362.0 ng/mL (median, 8.4 ng/mL), and patients were classified based on a cut-off of 1.5 ng/mL, the value used in our hospital. Regarding response to treatment, nine (64.3%) patients in group I, 15 (68.2%) patients in group II, and 51 (67.1%) patients in group III showed complete response; two (14.3%), three (13.6%), and 19 patients (25.0%) showed partial response; two (14.3%), two (9.1%), and one (1.3%) patients showed progression of the disease; the result of treatment could not be assessed due to lack of records for one (7.1%), two (9.1%), and five patients (6.6%), respectively. Seven (50.0%), 16 (72.7%), and 62 (81.6%) patients in groups I, II, and III, respectively, showed a positive biochemical response. The site of recurrence was LRR in seven (50.0%), 12 (54.5%), and 37 patients (48.7%); DM in five (35.7%), nine (40.9%), and 33 patients (43.4%); and simultaneous LRR and DM in two (14.3%), one (4.5%), and six patients (7.9%) for groups I, II, and III, respectively.
Table 1.
Characteristics of patient groups
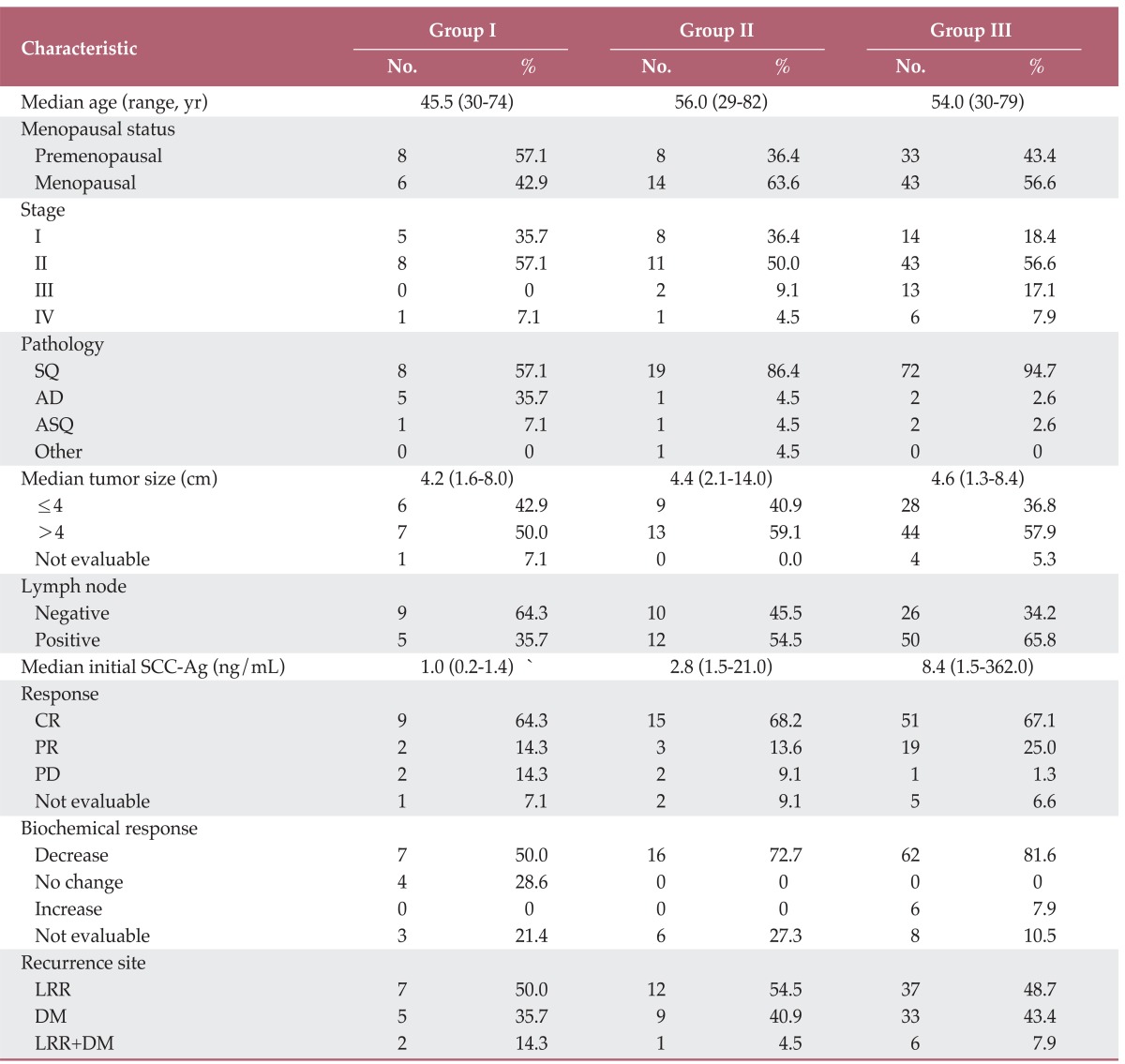
SQ, squamous cell carcinoma; AD, adenocarcinoma; ASQ, adenosquamous cell carcinoma; SCC-Ag, squamous cell carcinoma antigen; CR, complete response; PR, partial response; PD, progressive disease; LRR, locoregional recurrence; DM, distant metastasis.
A summary of the results of univariate and multivariate analyses for unusual SCC-Ag levels at initial diagnosis and recurrence is shown in Table 2. In univariate analysis, unusual SCC-Ag levels showed a statistically significant correlation with pathology and biochemical response. However, in the multivariate analysis, none of the clinicopathologic factors showed a statistically significant relationship with unusual SCC-Ag levels.
Table 2.
Univariate and multivariate analyses of clinicopathologic factors between patient groups
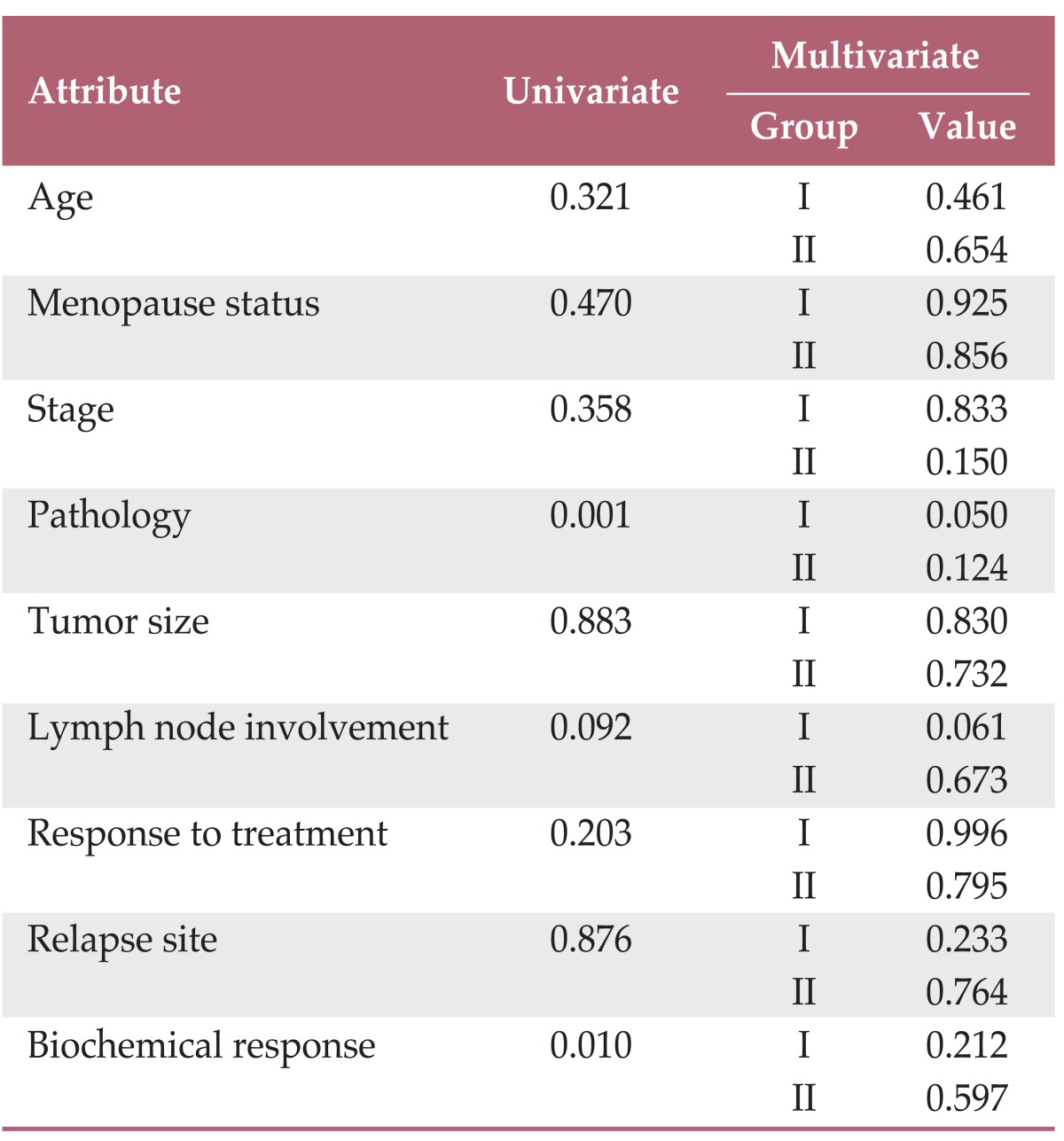
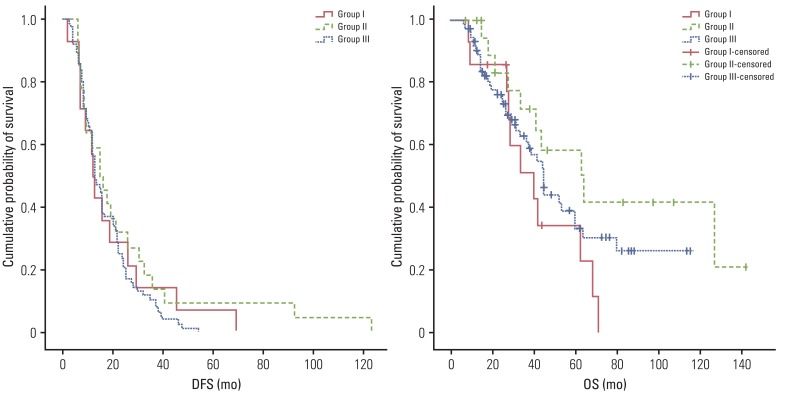
Table 3 and Fig. 1 show DFS and OS of the patient groups. The 5-year DFS rates of groups I, II, and III were 7.1%, 9.1%, and 0% (p=0.418), and the 5-year OS rates were 34.3%, 58.4%, and 33.3% (p=0.142), respectively.
Table 3.
Disease-free survival (DFS) and overall survival (OS) of patient groups
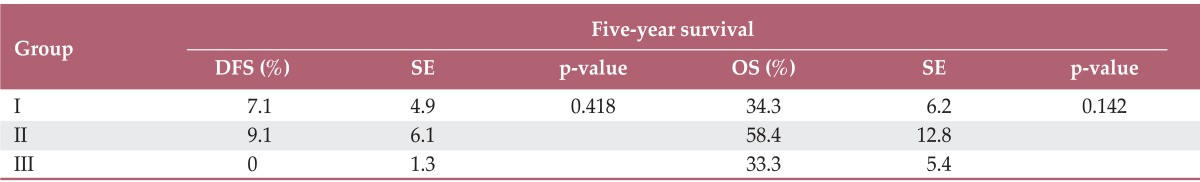
Fig. 1.
Disease-free survival (DFS) and overall survival (OS) of patient groups.
Discussion
Consistent with previous reports [18,19], SCC-Ag functioned as a tumor marker for most of the patients in our study. In classification of patients based on the standard cut-off level of 1.5 ng/mL SCC-Ag used at SMC, 66.3% of patients with cervical cancer showed an elevated SCC-Ag level on diagnosis, and 75.3% of patients showed an elevated SCC-Ag level at recurrence. In the current study, we assume that lower SCC-Ag levels at diagnosis, compared with previous studies, was due to a high proportion of patients with low stage and similar to that reported in other previously published studies [12,13,20]; we have shown that post-treatment SCC-Ag levels are helpful in detection of cervical cancer recurrence. In addition, the percentage of patients whose SCC-Ag level exceeded 1.5 ng/mL at diagnosis increased with increasing stage: 54.1%, 78.2%, 87.5%, and 95.5% for stages I, II, III and IV, respectively. Similar results were reported in a previous study [13]. Thus, the value of SCC-Ag as a tumor marker for the initial and relapse diagnoses of cervical cancer and its role as a prognostic factor have been confirmed by many previous studies, including the current study. However, a number of studies have reported unusual cases in which elevation of SCC-Ag level was observed at diagnosis but not at recurrence, or, conversely, was not observed at diagnosis but was observed at recurrence. We also observed 14 patients with the former pattern and 22 with the latter pattern among the patients in our recent study on the function of SCC-Ag [21]. Based on these findings, we became interested in the common characteristics of such patients and the role of SCC-Ag as a marker in these patients.
As shown in Tables 1 and 2, differences in stage, pathologic type, lymph node involvement, median value of initial SCC-Ag, and biochemical response were observed between the normal (group III) and unusual (groups I and II) SCC-Ag groups. Although only pathologic type showed statistical significance in multivariate analysis, pathology and biochemical response were statistically significant in univariate analysis. Regarding pathologic type, the proportion of squamous cell carcinoma, which was expected to show an association with higher SCC-Ag, compared with other pathologic types, was lower in groups I and II than in group III. The initial median SCC-Ag levels in groups I, II, and III increased by 1.0 ng/mL, 2.8 ng/mL, and 8.4 ng/mL, respectively. From these results, we conclude that the lack of elevation of SCC-Ag at diagnosis or relapse was due to low production of SCC-Ag. In group I, where absence of SCC-Ag elevation was observed at diagnosis, but elevation was observed at relapse, the initial production of SCC-Ag was low because there were few cells producing SCC-Ag. In contrast, the relapsed lesion contained many SCC-Ag-producing cells; therefore, elevated SCC-Ag was detected at recurrence. For group II, where elevation of SCC-Ag was observed at diagnosis, but normal levels of SCC-Ag were observed at relapse, we conclude that the primary lesion consisted of many cells producing SCC-Ag, however, these cells were no longer present after treatment. Thus, relapse involved cells that were not capable of producing SCC-Ag, indicating no elevation of SCC-Ag at recurrence.
Serum SCC-Ag has already been known to be a prognostic factor with respect to the aggressiveness of cervical cancer [7-9]. DFS and OS among the three groups are shown in Fig. 1 and Table 3. Although the current result lacked statistical significance, systematic relationship of SCC-Ag level with clinical outcome can be found. The 5-year DFS and OS of group III were lowest at 0% and 33.3%, and those of group II were highest at 9.1% and 58.4%, respectively. The lower DFS and OS of group III can be easily understood by the fact that it showed the highest level of SCC-Ag at initial diagnosis among the groups, which adversely affects the prognosis, as reported in the literature [7,9,10]. However, this simple explanation based only on the initial SCC-Ag level did not fully elucidate the differences in survival rates among the current patient groups. For example, group II showed a better survival rate than group I, even though the initial SCC-Ag level was higher in group II than in group I, implying that something other than the initial SCC-Ag level was necessary to explain the survival pattern. By cross checking the survival patterns in the three groups, we suggested that not only the initial SCC-Ag level but also the biochemical response according to SCC-Ag level may be related to the prognosis of cervical cancer. The better survival observed in group II, compared with that in groups I and III, can be explained by the fact that a larger portion of patients in group II showed a positive biochemical response after treatments and reduction of the productive capacity for SCC-Ag; hence, the expression of SCC-Ag can decline to the point that it cannot be detected at relapse.
The major finding in the current study is that the treatment response of patients with unusual change in SCC-Ag was not significantly different from that of the usual patient case with a typical SCC-Ag level. This implies that continuous check of SCC-Ag levels might be necessary for early detection of relapse, even in the case of an unusual patient. In particular, paying extra attention to group II patients with an elevated SCC-Ag level at initial diagnosis but with a normal SCC-Ag level at recurrence, including careful physical examination and imaging work-up, is strongly recommended. This result is consistent with those of previous studies [10-13], however, compared with previous studies, the reliability of the result may be further improved with the current analysis because a larger number of patients were enrolled in this study.
Nevertheless, this study has a number of limitations. Although this study included the largest number of patients with unusual SCC-Ag patterns analyzed to date, the sample size was not sufficient to validate statistical significance. In addition, although we identified some common characteristics of patients with unusual SCC-Ag patterns and found a similar response to treatment and a higher survival rate in these groups, we explained these findings only in terms of clinicopathologic factors without study of the underlying molecular biology. In order to overcome these limitations, further research involving biochemical analysis of a larger number of patients with unusual SCC-Ag patterns may be necessary in order to explain the clinical characteristics of these patients.
Conclusion
The effectiveness of SCC-Ag level as a tumor marker and its role as a prognostic factor have been validated in the present study as consistent with previous studies. Biochemical response as well as SCC-Ag could be considered as prognostic factor in both patients with usual and unusual SCC-Ag level. This result strongly suggest that continuous follow-up of SCC-Ag levels is helpful for early detection or prediction of relapse in patient with cervical cancer.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferrandina G, Legge F, Fagotti A, Fanfani F, Distefano M, Morganti A, et al. Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: safety, outcome, and prognostic measures. Gynecol Oncol. 2007;107(1 Suppl 1):S127–S132. doi: 10.1016/j.ygyno.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Classe JM, Rauch P, Rodier JF, Morice P, Stoeckle E, Lasry S, et al. Surgery after concurrent chemoradiotherapy and brachytherapy for the treatment of advanced cervical cancer: morbidity and outcome: results of a multicenter study of the GCCLCC (Groupe des Chirurgiens de Centre de Lutte Contre le Cancer) Gynecol Oncol. 2006;102:523–529. doi: 10.1016/j.ygyno.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 4.Lai CH. Management of recurrent cervical cancer. Chang Gung Med J. 2004;27:711–717. [PubMed] [Google Scholar]
- 5.Chou HH, Wang CC, Lai CH, Hong JH, Ng KK, Chang TC, et al. Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:442–448. doi: 10.1016/s0360-3016(01)01628-5. [DOI] [PubMed] [Google Scholar]
- 6.Bodurka-Bevers D, Morris M, Eifel PJ, Levenback C, Bevers MW, Lucas KR, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000;78:187–193. doi: 10.1006/gyno.2000.5860. [DOI] [PubMed] [Google Scholar]
- 7.Strauss HG, Laban C, Lautenschlager C, Buchmann J, Schneider I, Koelbl H. SCC antigen in the serum as an independent prognostic factor in operable squamous cell carcinoma of the cervix. Eur J Cancer. 2002;38:1987–1991. doi: 10.1016/s0959-8049(02)00159-4. [DOI] [PubMed] [Google Scholar]
- 8.Bolli JA, Doering DL, Bosscher JR, Day TG, Jr, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 9.Bolger BS, Dabbas M, Lopes A, Monaghan JM. Prognostic value of preoperative squamous cell carcinoma antigen level in patients surgically treated for cervical carcinoma. Gynecol Oncol. 1997;65:309–313. doi: 10.1006/gyno.1997.4619. [DOI] [PubMed] [Google Scholar]
- 10.Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol. 1994;12:2309–2316. doi: 10.1200/JCO.1994.12.11.2309. [DOI] [PubMed] [Google Scholar]
- 11.Micke O, Prott FJ, Schafer U, Tangerding S, Potter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000;20:5113–5115. [PubMed] [Google Scholar]
- 12.Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001;19:3960–3966. doi: 10.1200/JCO.2001.19.19.3960. [DOI] [PubMed] [Google Scholar]
- 13.Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002;84:7–11. doi: 10.1006/gyno.2001.6497. [DOI] [PubMed] [Google Scholar]
- 14.Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002;52:23–32. doi: 10.1016/s0360-3016(01)01805-3. [DOI] [PubMed] [Google Scholar]
- 15.Micke O, Bruns F, Schafer U, Prott FJ, Willich N. The impact of squamous cell carcinoma (SCC) antigen in patients with advanced cancer of uterine cervix treated with (chemo-)radiotherapy. Anticancer Res. 2005;25:1663–1666. [PubMed] [Google Scholar]
- 16.Rose PG, Baker S, Fournier L, Nelson BE, Hunter RE. Serum squamous cell carcinoma antigen levels in invasive cervical cancer: prediction of response and recurrence. Am J Obstet Gynecol. 1993;168(3 Pt 1):942–946. doi: 10.1016/s0002-9378(12)90850-9. [DOI] [PubMed] [Google Scholar]
- 17.Choi DH, Kim ES, Nam KH. A study of relationship between the level of serum SCC antigen and recurrence patterns after treatment of uterine cervix cancer. J Korean Soc Ther Radiol Oncol. 1999;17:120–128. [Google Scholar]
- 18.Yoon SM, Shin KH, Kim JY, Seo SS, Park SY, Moon SH, et al. Use of serum squamous cell carcinoma antigen for follow-up monitoring of cervical cancer patients who were treated by concurrent chemoradiotherapy. Radiat Oncol. 2010;5:78. doi: 10.1186/1748-717X-5-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008;66:10–20. doi: 10.1016/j.critrevonc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Forni F, Ferrandina G, Deodato F, Macchia G, Morganti AG, Smaniotto D, et al. Squamous cell carcinoma antigen in follow-up of cervical cancer treated with radiotherapy: evaluation of cost-effectiveness. Int J Radiat Oncol Biol Phys. 2007;69:1145–1149. doi: 10.1016/j.ijrobp.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 21.Jeong BK, Choi DH, Huh SJ, Park W, Bae DS, Kim BG. The role of squamous cell carcinoma antigen as a prognostic and predictive factor in carcinoma of uterine cervix. Radiat Oncol J. 2011;29:191–198. doi: 10.3857/roj.2011.29.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]