Abstract
This review examines sex differences in health and survival, with a focus on the Nordic countries. There is a remarkable discrepancy between the health and survival of the sexes: men are physically stronger and have fewer disabilities, but have substantially higher mortality at all ages compared with women: the so-called male-female health-survival paradox. A number of proposed explanations for this paradox are rooted in biological, social, and psychological interpretations. It is likely to be due to multiple causes that include fundamental biological differences between the sexes such as genetic factors, immune system responses, hormones, and disease patterns. Behavioral differences such as risk-taking and reluctance to seek and comply with medical treatment may also play a role. Another consideration is that part of the difference may be due to methodological challenges, such as selective non-participation and under-reporting of health problems, and delayed seeking of treatment by men. The Nordic countries provide a unique opportunity for such studies, as theyhave good-quality data in their national health registers, which cover the whole population, and a long tradition of high participation rates in surveys.
Keywords: Health, mortality, Nordic countries, review, sex differences
INTRODUCTION
In the 8 April 2006 issue of the British Medical Journal, an editorial announced “Life expectancy: women now on top everywhere”. During 2006, even in the poorest countries, women could expect to outlive men (1). However, there is a remarkable discrepancy between the health and survival of men versus women. According to a recent report on health differences in 21 European countries, men rated their health higher than women in all but one country, Finland, with significant differences in 13 countries - among them three other Nordic countries, Sweden, Norway and Denmark (2). Research literature generally suggests that men are physically stronger, report fewer diseases and have fewer limitations in the activities of daily living at older ages. Nonetheless, female death rates are substantially lower than those for males at all ages. That is, in terms of mortality, women are healthier than men.
Interpreting this apparent contradiction - that women live longer than men but experience worse health - is complicated by several factors, and a number of explanations have been proposed that are rooted in biological, social and psychological interpretations. The most commonly proposed explanations are biological risks, risks acquired through social roles, lifestyle and illness behaviors, and differential healthcare access, treatment and use (3–8).
This review provides data documenting sex differences in health and survival, with a focus on the Nordic countries. It is followed by a section on methodological challenges and a final section on possible explanations for the paradox and suggestions for future research.
OBSERVED SEX DIFFERENCES IN HEALTH AND MORTALITY
Sex differences in mortality
As far back as the mid-18th century there was a female advantage in survival (9) and, with economic development and improved living conditions for women, the sex gap increased in the first three quarters of the 20th century in most Western countries (3, 4, 7), including Denmark and other Nordic countries (10, 11). However, in developed countries, the sex differences in mortality development were mixed in the last quarter of the 20th century. Female-male differences in life expectancy narrowed in most European countries and in the US over the period 1980–1996, but became larger in other countries, namely Greece, Hungary, the Russian Federation and Japan (12, 13). There is general agreement that cigarette smoking is the single largest identifiable factor, and also that this alone cannot explain the trajectories in sex differential mortality, which is illustrated by the sex difference in survival among those who have never smoked (14).
Sex differences in mortality in Nordic countries
This section describes the trends of sex differences in mortality in the four Nordic countries, Denmark, Finland, Norway, and Sweden, for 1950–2004, based on the Human Mortality Database (15) and the World Health Organization (WHO) Mortality Database (16). To ensure comparability, the mortality rates in the four Nordic countries were standardized according to the European standard population. Most of the disease-specific comparisons were based on truncated age groups (35–74 years) to minimize the risk of different coding practices, especially among the elderly.
During the period 1850–1950, the sex difference in life expectancy in Denmark, Norway and Sweden was 2–4 years (Fig. 1). The difference increased to 6–7 years between 1950 and 1980, and then decreased to 4.5–5 years. The sex ratio for all-cause mortality (ratio between age-standardized mortality rates) showed the same pattern since 1950 as the sex difference in life expectancy (Fig. 1). This pattern was slightly different for Finland: an increase in the sex gap began in the 1920s, and was somewhat greater than in the other Nordic countries and more affected by World War II.
Fig. 1.
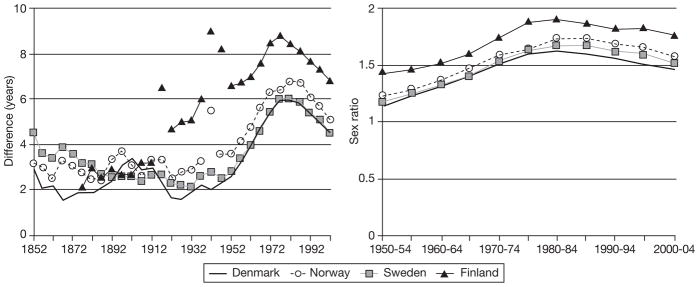
Difference in life expectancy between females and males (1850–2005) and sex ratio for all-cause mortality (ratio between age-standardized mortality rates) in four Nordic countries (1950–2005).
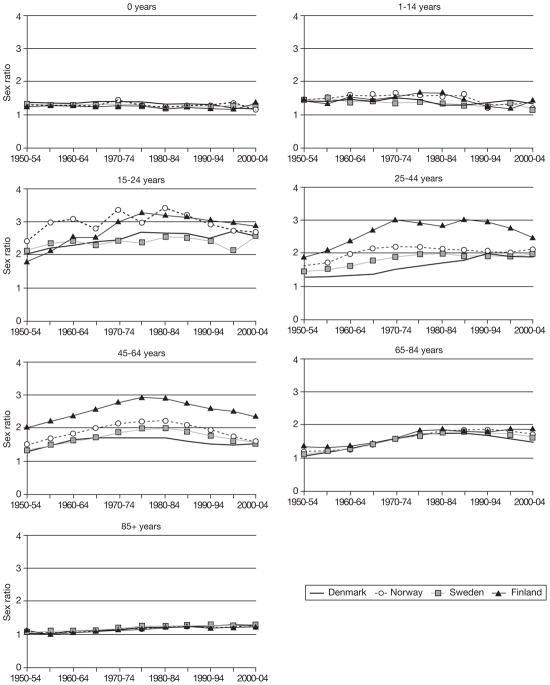
The age-specific trajectories of mortality rates for 1950–2005 (Fig. 2) show that infant mortality was consistently 20–30% higher for boys than girls. Sex differences were slightly greater among 1–14-year olds, and varied very little in the 50-year period. Men aged 15–24 years had about twice the mortality of women in the early 1950s. Thereafter, the sex ratio increased slightly in all four countries. Mortality differences in the 25–44-year and, especially, 45–64-year age groups followed nearly the same pattern as the difference in life expectancy. In the 65–84-year age group, the sex ratio was small in the 1950s, but increased in all four countries until 1980. Lastly, in the oldest age group, there was almost no sex difference in mortality rates at the beginning of the period but, since 1975, it has been constant and similar in all four countries, with men having about an increased mortality risk of 17–29% (which is a substantial absolute difference, given high mortality rates at age 85 or over).
Fig. 2.
Sex ratio for all-cause age-specific mortality (ratio between age-standardized mortality rates) in four Nordic countries, 1950–2005.
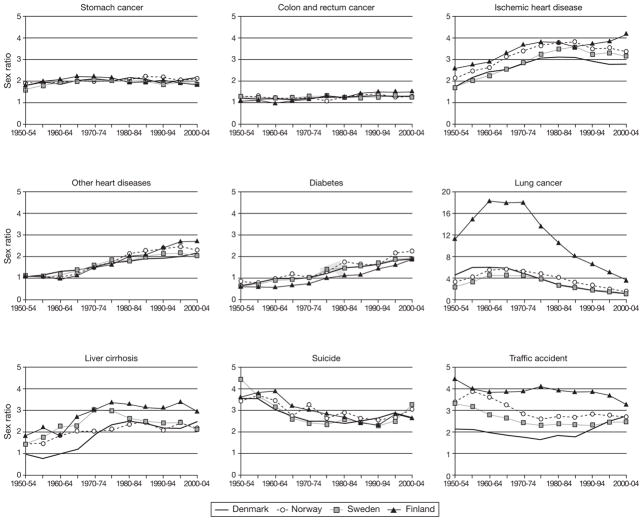
There were substantial variations in sex differential mortality at the disease-specific level (Fig. 3). Sex ratios for mortality from stomach, colon and rectum cancer were similar in all countries and over time. In contrast, sex differences in mortality from ischemic heart disease, other heart diseases and diabetes substantially increased since 1950 and clearly contributed to the change in the sex difference in life expectancy in all four countries. There were huge sex differences in mortality from lung cancer, traffic accidents and suicide. Since 1970, the differences from lung cancer have decreased in all four countries, but the decline was much steeper in Finland. Sex ratios for liver cirrhosis and traffic accidents also changed greatly since 1950, although in the opposite direction.
Fig. 3.
Sex ratios for cause-specific mortality rates (ratio between age-standardized mortality rates) in four Nordic countries (35–74 years), 1950–2005.
In conclusion, all Nordic countries have had similar patterns of age-specific sex differences in all-cause mortality since 1950, except that Finnish men have had higher excess mortality than the other three countries in the 25–44-and 45–64-year age groups. The most marked changes in sex differences over 1950–2004 were in the 25–44-, 45–64-and 65–84-year age groups, suggesting that these groups have been responsible for most changes in sex differences in life expectancy since 1950. Trajectories of sex differences in cause-specific mortality had similar patterns in the four countries. Notable changes in the sex ratio for all-cause mortality within the last 50 years can mainly be attributed to diseases and causes of deaths closely related to lifestyle and health behaviors interacting with living conditions, i.e., ischemic heart disease, other heart diseases, lung cancer, liver cirrhosis, traffic accidents, and suicide.
Sex differences in self-reported health, activities of daily living and physical performance tests
Most research papers consistently find poorer self-reported health and a lower quality of life among women compared with men, although some studies have been unable to detect substantial sex differences in self-reported health (17–19). Women also have more difficulties in performing activities of daily living(ADL), due to a higher incidence and prevalence of disability than men at all ages (20, 21). Case and Paxson showed that men and women with the same conditions had similar self-reported health, but men had higher mortality from these conditions, indicating greater severity (8). The “male advantage” in physical health has also been revealed with physical performance tests. Grip strength predicts disability, morbidity and mortality in both sexes, and the mean grip strength of elderly men is still comparable to that of middle-aged women (22). There are large statistically significant sex differences in physical health in populations as diverse as the Danish and Chinese oldest-old populations (19, 23). The female disadvantage is larger for nonagenarians and centenarians than octogenarians, suggesting that sex differences in disability become more pronounced at advanced age. In conclusion, men are physically stronger and have fewer limitations in ADL, factors that are significantly associated with better survival in both sexes, yet men have higher mortality than women at all ages.
Sex differences in morbidity
The issue of sex differences in morbidity is more complex than the pattern of sex differences in ADL and physical performance tests. The complexity is due to variations in definitions of diseases, diagnostic procedures and age-related changes in incidence rates for many diseases. For example, the incidence of coronary heart disease (CHD) starts rising about 10 years earlier for men than women and, in middle-age, it is about twice as high for men than women. However, the male excess of CHD incidence and mortality declines after 60, and after 80 the difference is small (24–26). Studies generally show that women have a significantly higher mean number of reported symptoms, prevalence of migraine, musculoskeletal and autoimmune diseases, whereas men have an earlier and higher incidence of cardiovascular diseases (27, 28). A study of different-sex twins, which controls for childhood environment and, to some extent, genetic factors, showed that men reported more very life-threatening conditions, such as heart insufficiency, angina pectoris, thrombosis in leg, and others, whereas women reported more total health conditions, non-life-threatening conditions, and physical and psychological symptoms (29). This suggests that excessive morbidity in women depends highly on a selected disease or illness indicator.
Severity of diseases may also interfere with female-male differences. Some studies found substantially higher risk of severecoronary artery calcification score and intima-media thickness in men of most ages, but the risk of severity was similar in women and men when ankle-arm index and degree of aortic calcification where used (30). Acute myocardial infarction was more severe in women for some severity measures, but when other indicators were applied, men were rated to be as sick as, or sicker than women (31).
The frequency and outcomes of percutaneous coronary interventions (PCI) and coronary artery bypass graft surgery (CABG) may also serve as proxies for disease severity. Considerably more men undergo PCI and CABG than women, and gender inequity increases with age (32, 33). Such differences in disease management are partially due to differences in symptom presentation, patient preferences for surgical or conservative treatment, selective referral for invasive procedures favoring men, and more beneficial results of invasive management in men than women (34, 35). A recent review pointed out that modern technological advances (e.g., PCI accompanied by stent insertion, development of drug-eluting stents, and atherectomy devices) diminished or eliminated sex differences in the operative and 1-year mortality rates (36). However, the male advantage in hospital and post-operative mortality after PCI and CABG surgery is still debatable.
To sum up, it is well-established that, in terms of mortality, women are healthier than men at all ages, but have higher disability levels than men. Sex differences in morbidity remain unclear, as they depend on disease definition, severity measure used, and age trajectories of particular diseases.
METHODOLOGICAL CHALLENGES IN STUDIES OF SEX DIFFERENCES IN HEALTH AND MORTALITY
Sex differences in reporting
The research literature on sex differences in the accuracy of self-reports is divisive. In the Longitudinal Aging Study of Amsterdam, the accuracy of information provided by patients was moderate to high for most selected chronic conditions: men tended to under-report health problems, whereas women more frequently over-reported malignancies and arthritis (37). Other studies in the US revealed that women’s self reports were in greater agreement with medical records for myocardial infarction, diabetes, hypertension and stroke (38). Zhu et al. reported that men stated more histories of urinary tract infection, sexually transmitted diseases and some other conditions than were identified in medical records (39). In a study of middle-aged men, the self-reports agreed well with general physician (GP) records for angina, and the disagreement was mainly from over-reporting of angina (40).
The Seattle Longitudinal Study suggests that, for women, there is lower agreement between self-reported drugs and pharmacy data, and more errors in both sources of medication use when compared with men (41). The recall accuracy among elderly women was highest for antihypertensive and statin medications and lowest for anti-depressants (42). Other Dutch and US studies found no significant sex differences in recall accuracy for alimentary, central nervous system, cardiovascular system and non-steroid anti-inflammatory medications (43, 44). Such discrepancies in results regarding sex differences in reporting patterns are expected, considering differences in sample age structure, sample size, and methodological approaches employed in studies.
Sex differences in participation
Empirical evidence on differences in survey participation by sex of the respondent is inconsistent. There were lower participation rates for women in several population-based studies of the elderly in Denmark (45, 46). Some Nordic studies could not detect sex differences in survey participation rates (47–49). A review on attrition rates in longitudinal studies of the elderly reported that few studies found higher non-response among women (50). Furthermore, in the Finnish and Dutch working-age populations, the participation rate was higher for women than men (51, 52). Jacomb et al. found that the sex differential in response pattern changed as their study progressed (53). In the baseline survey, elderly women were more likely to refuse study participation, although no sex difference in participation rates was observed during follow-up surveys.
Regarding age differences in response rate, the literature predominantly reports lower participation rates with increased age (50). However, some studies found no differences in the response pattern with age (54) and others reported lower response rates among younger subjects (51, 55). Furthermore, one US and one Danish study demonstrated that, among younger individuals, a non-response was more common among men, whereas men over 60 years had more active participation in surveys than women (56, 57).
There has been much effort on assessing the representativeness of study samples by comparing the health status of participants with that of the general population and non-respondents. Many studies report that participants were more likely to have higher cognitive status (49, 53), higher ratings of general health, lower levels of physical disabilities (58) and to be free from chronic illness (59) compared with non-respondents. This suggests that participants were healthier than non-participants.
Other studies of non-responses used healthcare utilization as the operational measure of morbidity. Among Danes aged 70–75, a higher proportion of non-respondents were hospitalized within one year prior to study, but there were no differences in healthcare use indicated by the response pattern in the 12 years prior to the interview (47). Using the national Danish register data, Kjoller and Thoning analyzed differences in hospital admission rates by response status at 5 years before, 6 months before and 2 years after data collection. The only statistically significant differences favoring participants were in the hospitalization rate 6 months prior to the survey (57). Other population-based studies in Denmark and the Netherlands did not detect differences in the prevalence or number of diagnosed somatic disorders by response pattern, but psychological problems were significantly more frequent among non-respondents (48, 60). Similarly, none of the healthcare use variables were predictive of non-response among US Medicare beneficiaries 65 years old and older in a multivariate analysis (61).
Investigators in Switzerland and Sweden found that the proportion of people with healthcare expenditures greater than “zero” was higher among participants than non-respondents (54, 55). Those with higher healthcare use, such as more frequent visits to GPs, specialists or alternative medical practitioners, were more likely to participate in a health examination survey in the Netherlands (59). No differences in the use of inpatient care were indicated by the response pattern, although for all other types of healthcare, users were more likely to be participants (56, 62). This suggests that “worried well”, i.e., healthy individuals making frequent use of health care services, were more likely to respond.
A potential source of sex bias in surveys may be the exclusion of nursing home populations, use of proxy respondents, and interviewers themselves. In a systematic literature review of attrition rates in longitudinal studies of the elderly, only 2 out of 19 studies included the residents of nursing homes and sheltered accommodation (50). It has been demonstrated that proxy respondents are mainly women, and that female interviewers elicited more information than male interviewers (63). Some studies suggested that lay proxy respondents tend to over-report physical disabilities and cognitive function and under-report the quality of life, compared with the respondents themselves (64, 65). However, little is known about whether the exclusion of institutionalized populations, who generally have poorer health than same-age non-institutionalized counterparts, and of proxies, who are often spouses, confounds the analysis of sex differences in response patterns and health assessments in surveys.
As illustrated in this short literature overview, the health characteristics of non-respondents to surveys were mixed and dependent on age, cohort, country and sex. It is not clear whether the sex differences in participation and reporting are important for the differences in health between men and women.
EXPLANATIONS FOR SEX DIFFERENCES IN HEALTH AND MORTALITY
The most widely cited explanations for the male-female health-survival paradox include biological endowments, risks acquired through social roles and behaviors (including illness and health reporting behaviors), physicians’ diagnostic patterns, and differential healthcare access, treatment and use.
Biological explanations
The most prominent biological explanations for the health-survival paradox are hormonal, autoimmune and genetic (66). The increase in cardiovascular disease in men approximately 10 years before women, combined with the favorable effect of estrogen on serum lipids (67) and its protective effect on brain cells (68) and consequent prevention of degenerative processes, has led to the hypothesis that estrogen is a central factor in the paradox. Endogenous estrogen decreases serum low-density lipoprotein cholesterol and increases high-density lipoprotein cholesterol levels, lowering the CHD risk in women of reproductive age. The widely accepted opinion that hormonal replacement therapy (HRT) decreases the CHD risk for post-menopausal women has been challenged since publication of the principal results of randomized clinical trials of HRT in post-menopausal women (69, 70). The Women’s Health Initiative (WHI) trial revealed an excessive risk of CHD events, pulmonary embolism, stroke and breast cancer among post-menopausal women receiving estrogen plus progestin compared with a placebo group, although the protective effect of HRT for colorectal and endometrial cancers and osteoporosis was also indicated (69, 70). An ancillary substudy of the WHI trial indicated that young post-menopausal women with estrogen treatment alone had lower coronary artery calcification and, thus, risk of coronary events, compared with a placebo group (71). The researchers suggested that estrogen therapy reduced the calcified plaque burden of coronary arteries in young post-menopausal women who were still free from atherosclerosis. However, due to its complex effects on the cardiovascular system, estrogen may elevate the CHD risk due to increased likelihood of thrombosis and plaque rupture in older women with advanced atherosclerosis (72). Recently, Barrett-Connor argued against the timing hypothesis, commenting that the evidential support from clinical trials was weak (73). The estrogen hypothesis does not explain the sex differences at older ages, when menopause occurred decades ago and where the sex differences in cardiovascular diseases are very modest. More research is needed to reveal stronger evidence that estrogen partially explains sex differences in health and mortality.
The evidence of risks and benefits of testosterone replacement therapy in men is under debate (74). The latest research indicates no beneficial or harmful effects of low-dose testosterone replacement on body composition, physical performance, insulin sensitivity or quality of life in men (75).
The “immunocompetence” hypothesis is that increased male mortality throughout life may partly be due to the greater susceptibility of men to infections (76, 77). Indeed, some data indicate that men have higher mortality due to parasitic and infectious diseases. Conversely, because testosterone causes immunosuppression, it has been suggested that men have a lower likelihood of producing autoantibodies and, hence, of having autoimmune diseases, which predominantly occur in women (76).
According to the X-chromosome hypothesis, the lack of a second X chromosome in men is associated with increased mortality. Studies of peripheral blood cells from elderly monozygotic female twins show a strong tendency for the same cell line to become predominant in two co-twins, which suggests that X-linked genetic factors influence human hematopoietic stem-cell kinetics and, potentially, organism survival. The fact that women have two cell lines with different potentials may be one reason why they live longer than men (66, 78).
Sex differences in health transitions
Studies of younger elderly people suggest that the rate-of-change in physical and cognitive functioning is associated with longevity (79). Women aged 65 years and over had a greater rate of decline in physical function and were less likely to recover from disability than men (80). The sex differences in recovery rates were largest among nonagenarians (21). However, the data are sparse among the oldest-old, due to logistical challenges (high mortality and non-response), but available data suggest that, although the level of functioning is predictive of survival, the rate of decline is also important. The decline-mortality association is central in ‘terminal decline research’, and it has been found that poor cognitive functioning among elderly individuals is associated with impending death (81). The effects of level and rate-of-change are mixed and may vary with age. The inconsistent results may be a consequence of the fact that most of these studies had few participants with three or more assessments, which creates difficulties in studying sex differences by means of latent growth curve modeling and transition models.
Little is known about sex differences in the rate-of-change in physical and cognitive function and recovery rates in the oldest population. It is unclear to what extent the health-survival paradox is due to different transition rates from an ‘unhealthy state’ to death or a ‘healthy state’ for men and women. It is possible that ‘unhealthy’ men have higher mortality rates and that the sex difference in transition rates depends on how ‘unhealthy’ is defined (disabilities vs diseases, self-report vs measured vs healthcare use). There is evidence that incidence, recovery and mortality influence the sex difference in disability prevalence, and that incidence has the greatest impact (21). It is also not clear whether level or rate-of-change (relative or absolute) is the best predictor of subsequent survival. If absolute rate-of-change is the most predictive, this may explain part of the health-survival paradox, as men have the largest absolute decline.
Sex differences in lifestyle behavior
Research has consistently demonstrated that men engage more frequently in higher risk-taking behaviors, such as cigarette smoking, alcohol consumption, more frequent use of psychoactive substances, and less safe driving, which all increase the risks of CHD, lung cancer, chronic obstructive pulmonary diseases, liver cirrhosis, and accident fatalities in comparison with women (7, 13). Although the prevalence of smoking has declined in recent years in both sexes in most European countries, the decrease was smaller in women than men (82). The US data for 1999–2004 suggest that the increasing trend of being overweight was more pronounced among men and was leveling off in women (83). Similarly, in Sweden in 1985–2002, the percentage of overweight individuals was always higher in men than women, and the increase in body mass index was more pronounced in men, whereas abdominal obesity increased mainly in women (84). Several studies have shown that women are more likely than men to choose low-fat foods, consume less meat and more fruit and fiber, and limit salt intake (85, 86), but men are more physically active than women (87). Although unhealthy behaviors contribute to the increased risk of cardiovascular and other chronic diseases and mortality in men, they cannot fully explain sex differences in health and mortality. This is suggested by sex differential mortality in the studies restricted to populations with a particular health profile, such as old Amish (88) and Mormons (89, 90).
Sex differences in social roles and health behavior
Wingard argued that sex differences in morbidity and mortality may be partially attributed to sex differences in risks acquired through social roles and behaviors, such as reporting, illness and help-seeking behaviors (5). Since women were traditionally more responsible for family health and knowledgeable about pathological signs, they had a higher propensity to use healthcare services than men. Gender stereotypes and related social norms made it culturally more acceptable for women to be sick, report more health problems and get advice about illness, suggesting that sex differences in health could be partially attributed to gender role expectations and responsibilities. Several studies found that women reported significantly higher mean numbers of symptoms (91), had more interest in health and slightly more absent days from work compared with men (92). Women reported more trivial and often medically unexplained symptoms (93) and all types of symptoms (94). A recent UK study showed that men with higher ‘femininity’ scores had a lower risk of death from CHD, whereas there was no similar relationship for women, suggesting that men with more stereotypical ‘masculine’ behavior were at higher risk of premature mortality (95).
To contrast the hypothesis that the sick role is more compatible with women, it has been proposed that a woman’s illness is more detrimental for the entire family, due to the greater burden of household responsibilities on women (96). In addition, there was little or no evidence of sex differentials in the number of reported physical symptoms (e.g., painful joints) and seeking medical care for diseases requiring prompt medical intervention, such as cancer (27). More frequent reporting by women occurred only in the number of malaise-type symptoms (e.g., sleep problems or concentration difficulties). A UK study found no evidence that women more readily reported trivial or mental health problems compared with men (97). Some authors suggest that occupational and socio-economic status and conditions are important factors influencing help-seeking behavior when individuals face illness. The Whitehall II study showed that a lower employment grade was associated with higher rates of short- and long-term absence, and that 50% of women were employed in the clerical and office support grades compared with 9% of men (98). The adjustment for employment grade revealed excess absenteeism in men in several disease categories. Other scholars found that employed, married parents had better health than unemployed single women and men without children (99). Additionally, contemporary industrial societies with gender-equality oriented policies have broken the traditional distribution of sex roles, and have more fathers with household responsibilities and caring for children and more women working.
Sex differences in healthcare utilization
Previous research has indicated the higher utilization of healthcare services by women compared with men, although sex differences fall for more serious health problems or hospital admissions (93, 100). Although differences in reproductive biology are important in explaining sex differences in healthcare utilization, being a woman predicts higher use of health services if sex-specific conditions are removed (6, 92). Women had significantly higher mean numbers of visits to primary care and diagnostic clinics compared with men, although the mean number of hospitalizations was similar (101, 102). Analysis of the national Danish Registry data showed that men had a lower rate of primary care use, but a higher hospitalization rate than women (103), suggesting that Danish men disregarded early signs of disease and the importance of preventive measures. They seem to postpone going to a physician until the later stages of disease development, which require more complex interventions, and are less effective for long-term survival and more costly. A review also suggested a trend of delayed help-seeking behavior in men (104).
The use of prescription medicines was more common among women in the Nordic and other populations, even when reproduction-related medications were excluded (105–107). Women used more anti-anxiety, anti-depressant, diuretic, and non-steroid anti-inflammatory drugs, but less pulmonary medications compared with men. Cardiovascular agents were the most commonly used medications in both genders at age 65 and over, although men used cardiovascular system medication, except diuretics, more often and from earlier ages than did women.
CONCLUSIONS
Although the male-female health-survival paradox has been studied for decades, we still do not fully understand either the reasons for it or its mechanisms (66). There are probably multiple causes, including fundamental biological differences between the sexes, such as genetic factors, immune system response, hormones, and disease patterns. Behavioral differences such as risk-taking or reluctance to seek and comply with medical treatment also probably play a role. Some of the differences may be due to delays in seeking treatment by men, or bias in surveys, if men are more reluctant than women to participate and/or accurately report in surveys, if they have disabilities or diseases. Quantifying the effects of these proposed mechanisms are important research topics that need more attention to shed light on the male-female paradox of health and survival. If further research suggests that heightened male hospitalization and mortality is partially due to delayed treatment seeking, then factors affecting the seeking of medical help should be carefully studied and appropriate measures taken to improve men’s illness behavior. If women have a more rapid transition from ‘healthy’ to ‘unhealthy’ states and a lower probability of recovery compared with men, then future research should focus on identifying contributing factors and designing strategies to prevent disability and improve the quality of life of elderly women. The Nordic countries, with their long tradition of surveys with high participation rates and comprehensive national health registers, are excellent settings for such studies.
Acknowledgments
The authors thank referees for their helpful comments and Susann Backer for valuable language editing of the paper. The work was supported by Grant P01-AG-08761 from the National Institute of Aging and the VELUX Foundation.
Footnotes
Review article based on the 4th Andrus Viidik Lecture in Gerontology, given by K. Christensen at the 19th Nordic Congress of Gerontology, Oslo, Norway, 25–28 May 2008. The Nordic Gerontological Federation (NGF) initiated these lectures when Professor Viidik retired from the NGF, after having been its Secretary (1974–1988) and Chairman (1988–2002).
References
- 1.Barford A, Dorling D, Smith GD, Shaw M. Life expectancy: women now on top everywhere. BMJ. 2006;332:808. doi: 10.1136/bmj.332.7545.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen KM, Dahl S-A. Health differences between European countries. Soc Sci Med. 2007;64:1665–78. doi: 10.1016/j.socscimed.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Preston SH. Studies in population. New York: Academic Press; 1976. Mortality patterns in national populations: with special reference to recorded causes of death. [Google Scholar]
- 4.Nathanson CA. Illness and the feminine role: a theoretical review. Soc Sci Med. 1975;9:57–62. doi: 10.1016/0037-7856(75)90094-3. [DOI] [PubMed] [Google Scholar]
- 5.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. 1984;5:433–58. doi: 10.1146/annurev.pu.05.050184.002245. [DOI] [PubMed] [Google Scholar]
- 6.Verbrugge LM. Gender and health: an update on hypotheses and evidence. J Health Soc Behav. 1985;26:156–82. [PubMed] [Google Scholar]
- 7.Waldron I. What do we know about causes of sex differences in mortality? A review of the literature. Population Bulletin of the United Nations. 1985:59–76. [PubMed] [Google Scholar]
- 8.Case A, Paxson C. Sex differences in morbidity and mortality. Demography. 2005;42:189–214. doi: 10.1353/dem.2005.0011. [DOI] [PubMed] [Google Scholar]
- 9.Kalben BB. Why men die younger: causes of mortality differences by sex. North American Actuarial Journal. 2000;4:83–111. [Google Scholar]
- 10.Helweg-Larsen K, Juel K. Sex differences in mortality in Denmark during half a century, 1943–92. Scand J Public Health. 2000;28:214–21. [PubMed] [Google Scholar]
- 11.Rigby JE, Dorling D. Mortality in relation to sex in the affluent world. J Epidemiol Community Health. 2007;61:159–64. doi: 10.1136/jech.2006.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gjonca A, Tomassini C, Toson B, Smallwood S. Sex differences in mortality, a comparison of the United Kingdom and other developed countries. Health Stat Q. 2005:6–16. [PubMed] [Google Scholar]
- 13.Waldron I. Recent trends in sex mortality ratios for adults in developed countries. Soc Sci Med. 1993;36:451–62. doi: 10.1016/0277-9536(93)90407-u. [DOI] [PubMed] [Google Scholar]
- 14.Preston SH, Wang H. Sex mortality differences in the United States: the role of cohort smoking patterns. Demography. 2006;43:631–46. doi: 10.1353/dem.2006.0037. [DOI] [PubMed] [Google Scholar]
- 15.HMD. Human Mortality Database. University of California, Berkeley and Max Planck Institute for Demographic Research. Available at www.mortality.org.
- 16.WHOMD. WHO Mortality Database. Available at http://www.who.int/healthinfo/morttables/en/index.html.
- 17.Jylha M, Guralnik JM, Ferrucci L, Jokela J, Heikkinen E. Is self-rated health comparable across cultures and genders? J Gerontol B Psychol Sci Soc Sci. 1998;53:S144–52. doi: 10.1093/geronb/53b.3.s144. [DOI] [PubMed] [Google Scholar]
- 18.Benyamini Y, Blumstein T, Lusky A, Modan B. Gender differences in the self-rated health-mortality association: is it poor self-rated health that predicts mortality or excellent self-rated health that predicts survival? Gerontologist. 2003;43:396–405. doi: 10.1093/geront/43.3.396. [DOI] [PubMed] [Google Scholar]
- 19.Yi Z, Yuzhi L, George LK. Gender differentials of the oldest old in China. Res Aging. 2003;25:65–80. [Google Scholar]
- 20.Arber S, Cooper H. Gender differences in health in later life: the new paradox? Soc Sci Med. 1999;48:61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- 21.Leveille SG, Penninx BW, Melzer D, Izmirlian G, Guralnik JM. Sex differences in the prevalence of mobility disability in old age: the dynamics of incidence, recovery, and mortality. J Gerontol B Psychol Sci Soc Sci. 2000;55:S41–50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- 22.Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8,342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–62. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: The Danish 1905 Cohort Survey. J Am Geriatr Soc. 2001;49:601–9. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- 24.Hjertestatistik 2004/Heart Statistics 2004. Copenhagen: The Danish Heart Foundation and the Danish National Institute of Public Health.
- 25.Wingard DL, Cohn BA, Kaplan GA, Cirillo PM, Cohen RD. Sex differentials in morbidity and mortality risk examined by age and cause in the same cohort. Am J Epidemiol. 1989;130:601–10. doi: 10.1093/oxfordjournals.aje.a115374. [DOI] [PubMed] [Google Scholar]
- 26.Rich-Edwards JW, Manson JE, Hennekens CH, Buring JE. The primary prevention of coronary heart disease in women. N Engl J Med. 1995;332:1758–66. doi: 10.1056/NEJM199506293322607. [DOI] [PubMed] [Google Scholar]
- 27.Macintyre S, Hunt K, Sweeting H. Gender differences in health: are things really as simple as they seem? Soc Sci Med. 1996;42:617–24. doi: 10.1016/0277-9536(95)00335-5. [DOI] [PubMed] [Google Scholar]
- 28.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 29.Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci. 2002;57:S168–76. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- 30.Kardys I, Vliegenthart R, Oudkerk M, Hofman A, Witteman JCM. The female advantage in cardiovascular disease: do vascular beds contribute equally? Am J Epidemiol. 2007;166:403–12. doi: 10.1093/aje/kwm115. [DOI] [PubMed] [Google Scholar]
- 31.Iezzoni LI, Ash AS, Shwartz M, Mackiernan YD. Differences in procedure use, in-hospital mortality, and illness severity by gender for acute myocardial infarction patients: are answers affected by data source and severity measure? Med Care. 1997;35:158–71. doi: 10.1097/00005650-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Shaw M, Maxwell R, Rees K, et al. Gender and age inequity in the provision of coronary revascularisation in England in the 1990s: is it getting better? Soc Sci Med. 2004;59:2499–507. doi: 10.1016/j.socscimed.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 33.Bowling A, Bond M, McKee D, et al. Equity in access to exercise tolerance testing, coronary angiography, and coronary artery bypass grafting by age, sex and clinical indications. Heart. 2001;85:680–6. doi: 10.1136/heart.85.6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anand SS, Xie CC, Mehta S, Franzosi MG, et al. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol. 2005;46:1845–51. doi: 10.1016/j.jacc.2005.05.091. [DOI] [PubMed] [Google Scholar]
- 35.Vaccarino V, Lin ZQ, Kasl SV, et al. Gender differences in recovery after coronary artery bypass surgery. J Am Coll Cardiol. 2003;41:307–14. doi: 10.1016/s0735-1097(02)02698-0. [DOI] [PubMed] [Google Scholar]
- 36.Chambers TA, Bagai A, Ivascu N. Current trends in coronary artery disease in women. Curr Opin Anaesthesiol. 2007;20:75–82. doi: 10.1097/ACO.0b013e3280146455. [DOI] [PubMed] [Google Scholar]
- 37.Kriegsman DMW, Penninx BWJH, Van Eijk JTM, Boeke AJP, Deeg DJH. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly: a study on the accuracy of patients’ self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–17. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- 38.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Zhu K, McKnight B, Stergachis A, Daling JR, Levine RS. Comparison of self-report data and medical records data: results from a case-control study on prostate cancer. Int J Epidemiol. 1999;28:409–17. doi: 10.1093/ije/28.3.409. [DOI] [PubMed] [Google Scholar]
- 40.Lampe FC, Walker M, Lennon LT, Whincup PH, Ebrahim S. Validity of a self-reported history of doctor-diagnosed angina. J Clin Epidemiol. 1999;52:73–81. doi: 10.1016/s0895-4356(98)00146-2. [DOI] [PubMed] [Google Scholar]
- 41.Caskie GI, Willis SL, Warner Schaie K, Zanjani FA. Congruence of medication information from a brown bag data collection and pharmacy records: findings from the Seattle longitudinal study. Exp Aging Res. 2006;32:79–103. doi: 10.1080/03610730500326341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boudreau DM, Daling JR, Malone KE, Gardner JS, Blough DK, Heckbert SR. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol. 2004;159:308–17. doi: 10.1093/aje/kwh038. [DOI] [PubMed] [Google Scholar]
- 43.Van den Brandt PA, Petri H, Dorant E, Goldbohm RA, Van de Crommert S. Comparison of questionnaire information and pharmacy data on drug use. Pharm Weekbl Sci. 1991;13:91–6. doi: 10.1007/BF01974987. [DOI] [PubMed] [Google Scholar]
- 44.West SL, Savitz DA, Koch G, Strom BL, Guess HA, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 45.Nybo H, Gaist D, Jeune B, et al. The Danish 1905 Cohort: a genetic-epidemiological nationwide survey. J Aging Health. 2001;13:32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- 46.Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. J Aging Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- 47.Osler M, Schroll M. Differences between participants and non-participants in a population study on nutrition and health in the elderly. Eur J Clin Nutr. 1992;46:289–95. [PubMed] [Google Scholar]
- 48.Drivsholm T, Eplov LF, Davidsen M, et al. Representativeness in population-based studies: a detailed description of non-response in a Danish cohort study. Scand J Public Health. 2006;34:623–31. doi: 10.1080/14034940600607616. [DOI] [PubMed] [Google Scholar]
- 49.von Strauss E, Fratiglioni L, Jorm AF, Viitanen M, Winblad B. Attitudes and participation of the elderly in population surveys: data from a longitudinal study on aging and dementia in Stockholm. J Clin Epidemiol. 1998;51:181–7. doi: 10.1016/s0895-4356(97)00242-4. [DOI] [PubMed] [Google Scholar]
- 50.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58:13–9. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Van Loon AJM, Tijhuis M, Picavet HSJ, Surtees PG, Ormel J. Survey non-response in the Netherlands: effects on prevalence estimates and associations. Ann Epidemiol. 2003;13:105–10. doi: 10.1016/s1047-2797(02)00257-0. [DOI] [PubMed] [Google Scholar]
- 52.Korkeila K, Suominen S, Ahvenainen J, et al. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol. 2001;17:991–9. doi: 10.1023/a:1020016922473. [DOI] [PubMed] [Google Scholar]
- 53.Jacomb P, Jorm A, Korten A, Christensen H, Henderson AS. Predictors of refusal to participate: a longitudinal health survey of the elderly in Australia. BMC Public Health. 2002;2:4. doi: 10.1186/1471-2458-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Etter J-F, Perneger TV. Analysis of non-response bias in a mailed health survey. J Clin Epidemiol. 1997;50:1123–8. doi: 10.1016/s0895-4356(97)00166-2. [DOI] [PubMed] [Google Scholar]
- 55.Carlsson F, Merlo J, Lindstroem M, Oestergren P-O, Lithman T. Representativity of a postal public health questionnaire survey in Sweden, with special reference to ethnic differences in participation. Scand J Public Health. 2006;34:132–9. doi: 10.1080/14034940510032284. [DOI] [PubMed] [Google Scholar]
- 56.Lamers LM. Medical consumption of respondents and non-respondents to a mailed health survey. Eur J Public Health. 1997;7:267–71. [Google Scholar]
- 57.Kjoller M, Thoning H. Characteristics of non-response in the Danish Health Interview Surveys, 1987–1994. Eur J Public Health. 2005;15:528–35. doi: 10.1093/eurpub/cki023. [DOI] [PubMed] [Google Scholar]
- 58.Hoeymans N, Feskens EJM, Van Den Bos GAM, Kromhout D. Non-response bias in a study of cardiovascular diseases, functional status and self-rated health among elderly men. Age Ageing. 1998;27:35–40. doi: 10.1093/ageing/27.1.35. [DOI] [PubMed] [Google Scholar]
- 59.Boshuizen HC, Viet AL, Picavet HSJ, Botterweck A, van Loon AJM. Non-response in a survey of cardiovascular risk factors in the Dutch population: determinants and resulting biases. Public Health. 2006;120:297–308. doi: 10.1016/j.puhe.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 60.van den Akker M, Buntinx F, Metsemakers JF, Knottnerus JA. Morbidity in responders and non-responders in a register-based population survey. Fam Pract. 1998;15:261–3. doi: 10.1093/fampra/15.3.261. [DOI] [PubMed] [Google Scholar]
- 61.Grotzinger KM, Stuart BC, Ahern F. Assessment and control of nonresponse bias in a survey of medicine use by the elderly. Med Care. 1994;32:989–1003. doi: 10.1097/00005650-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Reijneveld SA, Stronks K. The impact of response bias on estimates of health care utilization in a metropolitan area: the use of administrative data. Int J Epidemiol. 1999;28:1134–40. doi: 10.1093/ije/28.6.1134. [DOI] [PubMed] [Google Scholar]
- 63.Nathanson CA. Sex roles as variables in the interpretation of morbidity data: a methodological critique. Int J Epidemiol. 1978;7:253–62. doi: 10.1093/ije/7.3.253. [DOI] [PubMed] [Google Scholar]
- 64.Magaziner J, Zimmerman SI, Gruber-Baldini AL, Hebel JR, Fox KM. Proxy reporting in five areas of functional status: comparison with self-reports and observations of performance. Am J Epidemiol. 1997;146:418–28. doi: 10.1093/oxfordjournals.aje.a009295. [DOI] [PubMed] [Google Scholar]
- 65.Yasuda N, Zimmerman S, Hawkes WG, Gruber-Baldini AL, Hebel R, Magaziner J. Concordance of proxy-perceived change and measured change in multiple domains of function in older persons. J Am Geriatr Soc. 2004;52:1157–62. doi: 10.1111/j.1532-5415.2004.52315.x. [DOI] [PubMed] [Google Scholar]
- 66.Austad S. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 67.Waldron I. Contributions of biological and behavioral factors to changing sex differences in ischemic heart disease mortality. In: Lopez AD, Caselli G, Valkonen T, editors. Adult Mortality in Developed Countries: From Description to Explanation. Oxford: Oxford University Press; 1995. pp. 167–78. [Google Scholar]
- 68.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 69.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 70.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 71.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 72.Manson JE, Bassuk SS. Invited Commentary: Hormone therapy and risk of coronary heart disease: why renew the focus on the early years of menopause? Am J Epidemiol. 2007;166:511–7. doi: 10.1093/aje/kwm213. [DOI] [PubMed] [Google Scholar]
- 73.Barrett-Connor E. Hormones and heart disease in women: the timing hypothesis. Am J Epidemiol. 2007;166:506–10. doi: 10.1093/aje/kwm214. [DOI] [PubMed] [Google Scholar]
- 74.Vastag B. Many questions, few answers for testosterone replacement therapy. JAMA. 2003;289:971–2. doi: 10.1001/jama.289.8.971. [DOI] [PubMed] [Google Scholar]
- 75.Nair KS, Rizza RA, O’Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–59. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 76.Owens IPF. Ecology and evolution: sex differences in mortality rate. Science. 2002;297:2008–9. doi: 10.1126/science.1076813. [DOI] [PubMed] [Google Scholar]
- 77.Crimmins EM, Finch CE. Commentary: Do older men and women gain equally from improving childhood conditions? Int J Epidemiol. 2006;35:1270–1. doi: 10.1093/ije/dyl194. [DOI] [PubMed] [Google Scholar]
- 78.Christensen K, Kristiansen M, Hagen-Larsen H, et al. X-linked genetic factors regulate hematopoietic stem-cell kinetics in females. Blood. 2000;95:2449–51. [PubMed] [Google Scholar]
- 79.Deeg DJH, Hofman A, van Zonneveld RJ. The association between change in cognitive function and longevity in Dutch elderly. Am J Epidemiol. 1990;132:973–82. doi: 10.1093/oxfordjournals.aje.a115740. [DOI] [PubMed] [Google Scholar]
- 80.Beckett LA, Brock DB, Lemke JH, et al. Analysis of change in self-reported physical function among older persons in four population studies. Am J Epidemiol. 1996;143:766–78. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- 81.Laukka EJ, MacDonald SWS, Backman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology. 2006;66:833–8. doi: 10.1212/01.wnl.0000203112.12554.f4. [DOI] [PubMed] [Google Scholar]
- 82.Costanza M, Salamun J, Lopez A, Morabia A. Gender differentials in the evolution of cigarette smoking habits in a general European adult population from 1993–2003. BMC Public Health. 2006;6:130. doi: 10.1186/1471-2458-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 84.Berg C, Rosengren A, Aires N, Lappas G, et al. Trends in overweight and obesity from 1985 to 2002 in Goteborg, West Sweden. Int J Obes Relat Metab Disord. 2005;29:916–24. doi: 10.1038/sj.ijo.0802964. [DOI] [PubMed] [Google Scholar]
- 85.Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27:107–16. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- 86.Prattala R, Paalanen L, Grinberga D, Helasoja V, Kasmel A, Petkeviciene J. Gender differences in the consumption of meat, fruit and vegetables are similar in Finland and the Baltic countries. Eur J Public Health. 2006:ckl265. doi: 10.1093/eurpub/ckl265. [DOI] [PubMed] [Google Scholar]
- 87.Simpson EEA, O’Connor JM, Livingstone MBE, et al. Health and lifestyle characteristics of older European adults: the ZENITH study. Eur J Clin Nutr. 2005;59:S13–21. doi: 10.1038/sj.ejcn.1602292. [DOI] [PubMed] [Google Scholar]
- 88.Post W, Bielak LF, Ryan KA, et al. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation. 2007;115:717–24. doi: 10.1161/CIRCULATIONAHA.106.637512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lyon JL, Wetzler HP, Gardner JW, Klauber MR, Williams RR. Cardiovascular mortality in Mormons and non-Mormons in Utah, 1969–1971. Am J Epidemiol. 1978;108:357–66. doi: 10.1093/oxfordjournals.aje.a112632. [DOI] [PubMed] [Google Scholar]
- 90.Merrill RM, Lyon JL. Cancer incidence among Mormons and non-Mormons in Utah (United States) 1995–1999. Prev Med. 2005;40:535–41. doi: 10.1016/j.ypmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Ladwig KH, Marten-Mittag B, Formanek B, Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16:511–8. doi: 10.1023/a:1007629920752. [DOI] [PubMed] [Google Scholar]
- 92.Green CA, Pope CR. Gender, psychosocial factors and the use of medical services: a longitudinal analysis. Soc Sci Med. 1999;48:1363–72. doi: 10.1016/s0277-9536(98)00440-7. [DOI] [PubMed] [Google Scholar]
- 93.Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Women Health. 1987;12:103–45. doi: 10.1300/J013v12n02_07. [DOI] [PubMed] [Google Scholar]
- 94.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60:150–5. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Hunt K, Lewars H, Emslie C, Batty GD. Decreased risk of death from coronary heart disease amongst men with higher ‘femininity’ scores: a general population cohort study. Int J Epidemiol. 2007;36:612–20. doi: 10.1093/ije/dym022. [DOI] [PubMed] [Google Scholar]
- 96.Parsons T, Fox R. Illness, therapy, and the modern urban American family. Journal of Social Issues. 1952;8:2–4. [Google Scholar]
- 97.Macintyre S, Ford G, Hunt K. Do women “over-report” morbidity? Men’s and women’s responses to structured prompting on a standard question on long standing illness. Soc Sci Med. 1999;48:89–98. doi: 10.1016/s0277-9536(98)00292-5. [DOI] [PubMed] [Google Scholar]
- 98.Feeney A, North F, Head J, Canner R, Marmot M. Socioeconomic and sex differentials in reason for sickness absence from the Whitehall II Study. Occup Environ Med. 1998;55:91–8. doi: 10.1136/oem.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verbrugge LM. Multiple roles and physical health of women and men. J Health Soc Behav. 1983;24:16–30. [PubMed] [Google Scholar]
- 100.Waldron I. Sex differences in human mortality: the role of genetic factors. Soc Sci Med. 1983;17:321–33. doi: 10.1016/0277-9536(83)90234-4. [DOI] [PubMed] [Google Scholar]
- 101.Redondo-Sendino A, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. Gender differences in the utilization of health-care services among the older adult population of Spain. BMC Public Health. 2006;6:155. doi: 10.1186/1471-2458-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–52. [PubMed] [Google Scholar]
- 103.Juel K, Christensen K. Are men seeking medical advice too late? Contacts to general practitioners and hospital admissions in Denmark 2005. J Public Health. 2007:1–2. doi: 10.1093/pubmed/fdm072. [DOI] [PubMed] [Google Scholar]
- 104.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–23. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 105.Roe CM, McNamara AM, Motheral BR. Gender- and age-related prescription drug use patterns. Ann Pharmacother. 2002;36:30–9. doi: 10.1345/aph.1A113. [DOI] [PubMed] [Google Scholar]
- 106.Jorgensen T, Johansson S, Kennerfalk A, Wallander MA, Svardsudd K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann Pharmacother. 2001;35:1004–9. doi: 10.1345/aph.10351. [DOI] [PubMed] [Google Scholar]
- 107.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivela S-L, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55:809–17. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]