Abstract
Background
While some studies have found an association between delayed graft function (DGF) after kidney transplantation and worse long-term outcomes, a causal relationship remains controversial. We investigated this relationship using an instrumental variables model (IVM), a quasi-randomization technique for drawing causal inferences.
Methods
We identified 80,690 adult, deceased-donor, kidney-only transplant recipients from the Scientific Registry of Transplant Recipients between 1997 and 2010. We used cold ischemia time (CIT) as an instrument to test the hypothesis that DGF causes death-censored graft loss and mortality at 1 and 5 years post-transplant, controlling for an array of characteristics known to affect patient and graft survival. We compared our IVM results to a multivariable linear probability model (LPM).
Results
DGF occurred in 27% of our sample. Graft loss rates at 1 and 5 years were 6% and 22%, respectively, and 1-year and 5-year mortality rates were 5% and 20%, respectively. In the LPM, DGF was associated with increased risk of both graft loss and mortality at 1 and 5 years (p<0.001). In the IVM, we found evidence suggesting a causal relationship between DGF and death-censored graft loss at both 1 year (13.5% increase; p<0.001) and 5 years (16.2% increase; p<0.001), and between DGF and mortality at both 1 year (7.1% increase; p<0.001) and 5 years (11.0% increase; p<0.01). Results were robust to exclusion of lower-quality as well as pumped kidneys and use of a creatinine-based definition for DGF.
Conclusion
Instrumental variables analysis supports a causal relationship between DGF and both graft loss and mortality.
Keywords: delayed graft function, kidney transplantation, outcomes, cold ischemia time, allograft failure
Introduction
Delayed graft function (DGF) is an early complication of kidney transplantation that may reflect acute allograft injury and suboptimal early allograft function. DGF results in increased hospital length of stay and costs in the short term (1), but it is unclear if DGF is associated with poor long-term kidney transplant recipient outcomes. Some studies have found that DGF is associated with increased risk of graft loss (2–6) or mortality (7, 8) while others have not found a significant effect (9–13). Determining whether DGF causes worse long-term outcomes would have important implications for the use of DGF as a valid surrogate outcome in transplant clinical trials and for development of therapies to treat DGF.
Plausible biological mechanisms have been described to explain how DGF leads to allograft failure or worse allograft function. Ischemic injury may cause increased HLA expression (14), precipitating rejection and subsequent graft loss. Additionally, maladaptive repair of parenchymal and tubular cells after allograft injury may promote fibrosis and permanent loss of filtration function (15). Alternatively, a relationship between DGF and poor allograft survival might not be causal, since DGF is reversible (16); this relationship could instead be confounded by unobservable characteristics, such as lower intrinsic kidney quality, that cause both DGF and allograft loss.
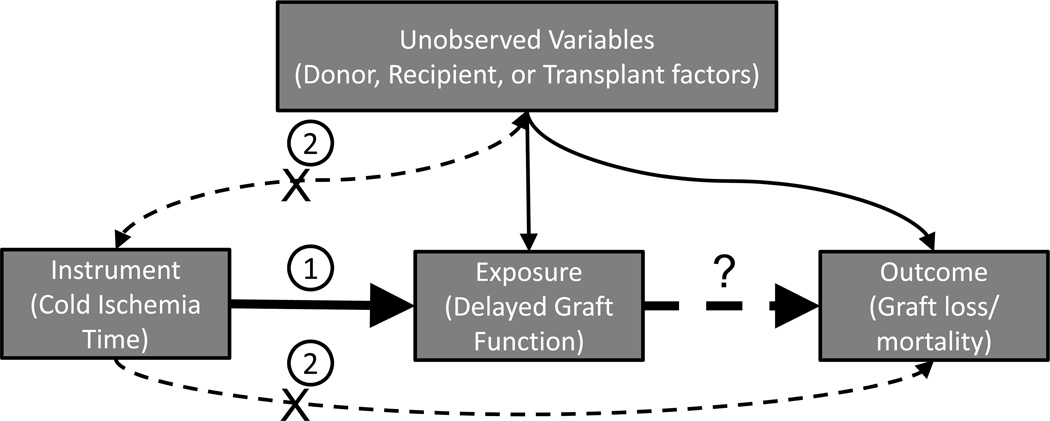
The use of instrumental variables in multivariable analyses allows one to draw causal inferences with observational data. This technique was developed in economics and has been widely applied in medical and epidemiological literature (17–22), although rarely in transplantation studies. In traditional linear regression, unobserved variables affecting both exposure and outcomes can confound relationships between these variables (Figure 1). The use of an instrumental variables model (IVM) can overcome this problem by isolating only the variation in the exposure that is not otherwise associated with the outcome. In an IVM, an exogenous instrumental variable is used to predict variation in the exposure variable. These predicted values of the exposure are then used in a regression to determine whether there is a relationship with the outcome of interest. In order for an instrumental variable to be valid, it must be both relevant and exogenous: a relevant instrument is strongly associated with the exposure, and an exogenous instrument is not associated with the outcome except through the pathway of the exposure (23).
Figure 1. Instrumental variables analysis.
- There must be a relevant association between the instrument and the exposure. This bold line indicates that the instrument is relevant.
- There must not be a relationship between the instrument and the dependent variable either directly or through association with unobserved variables. The “X” through the thin dotted lines indicates that the instrument is exogenous. Any relationship between the instrument and the dependent variable may only exist through the independent variable.
This study investigated the relationship between DGF and recipient outcomes using cold ischemia time (CIT) as an instrument. DGF stems from ischemic injury to an organ during procurement and transport as well as reperfusion injury in the peri-operative period (24). CIT, a measure of the transport and storage time of an organ, is a relevant instrument for predicting DGF since it has been shown to be an independent risk factor for DGF (25). Additionally, CIT should function as an exogenous instrument, given that CIT is primarily dependent on transport and procurement practices which are unlikely to be related to graft failure or mortality other than through the pathway of DGF. The aim of this study was therefore to determine whether the use of CIT as an instrumental variable supported a causal association between DGF and the outcomes of allograft failure and mortality.
Results
Descriptive summary statistics classified by median CIT are detailed in Tables 1–3. CIT in our sample ranged widely and had a median of 17.55 hours (interquartile range 12, 24). Many donor and recipient variables which affect DGF were relatively balanced across median CIT.
Table 1.
Recipient and donor characteristics (categorical variables), stratified by median cold ischemia time
| Variable | n (% of total) | n CIT<17.55 hrs (%) | n CIT>=17.55 hrs (%) | |
|---|---|---|---|---|
| Total sample | 73714 (100%) | 36854 (50%) | 36860 (50%) | |
| Recipient characteristics | ||||
| Gender | ||||
| Female | 27942 (38%) | 13951 (50%) | 13991 (50%) | |
| Male | 45772 (62%) | 22903 (50%) | 22869 (50%) | |
| Education | ||||
| None | 504 (1%) | 267 (53%) | 237 (47%) | |
| Grade school (0–8) | 4628 (6%) | 2335 (50%) | 2293 (50%) | |
| High school (9–12) or GED | 29829 (40%) | 15177 (51%) | 14652 (49%) | |
| Attended college/technical school | 14124 (19%) | 7312 (52%) | 6812 (48%) | |
| Associate/bachelor degree | 7981 (11%) | 4117 (52%) | 3864 (48%) | |
| Post-college graduate degree | 3082 (4%) | 1579 (51%) | 1503 (49%) | |
| Unknown | 12337 (17%) | 5629 (46%) | 6708 (54%) | |
| Missing | 1229 (2%) | 438 (36%) | 791 (64%) | |
| Race | ||||
| Other | 1486 (2%) | 864 (58%) | 622 (42%) | |
| White | 31583 (43%) | 16065 (51%) | 15518 (49%) | |
| Black | 26289 (36%) | 12734 (48%) | 13555 (52%) | |
| Asian | 4489 (6%) | 2466 (55%) | 2023 (45%) | |
| Hispanic | 9867 (13%) | 4725 (48%) | 5142 (52%) | |
| Insurance | ||||
| Other | 566 (1%) | 223 (39%) | 343 (61%) | |
| Private | 19179 (26%) | 9742 (51%) | 9437 (49%) | |
| Public | 53969 (73%) | 26889 (50%) | 27080 (50%) | |
| Diabetes Type | ||||
| No | 47930 (65%) | 24114 (50%) | 23816 (50%) | |
| Type I | 1485 (2%) | 827 (56%) | 658 (44%) | |
| Type II | 8650 (12%) | 4683 (54%) | 3967 (46%) | |
| Other Type | 106 (0%) | 58 (55%) | 48 (45%) | |
| Unknown Type | 13743 (19%) | 6491 (47%) | 7252 (53%) | |
| Unknown | 665 (1%) | 281 (42%) | 384 (58%) | |
| Missing | 1135 (2%) | 400 (35%) | 735 (65%) | |
| Donor characteristics | ||||
| Gender | ||||
| Female | 30233 (41%) | 15053 (50%) | 15180 (50%) | |
| Male | 43481 (59%) | 21801 (50%) | 21680 (50%) | |
| Race | ||||
| Other | 725 (1%) | 365 (50%) | 360 (50%) | |
| White | 51888 (70%) | 26254 (51%) | 25634 (49%) | |
| Black | 9927 (13%) | 4776 (48%) | 5151 (52%) | |
| Asian | 1728 (2%) | 921 (53%) | 807 (47%) | |
| Hispanic | 9446 (13%) | 4538 (48%) | 4908 (52%) | |
| History of Hypertension | ||||
| None | 53549 (73%) | 27217 (51%) | 26332 (49%) | |
| Yes, 0–5 years | 9439 (13%) | 4682 (50%) | 4757 (50%) | |
| Yes, 6–10 years | 3227 (4%) | 1530 (47%) | 1697 (53%) | |
| Yes, >10 years | 3587 (5%) | 1629 (45%) | 1958 (55%) | |
| Yes, Unknown duration | 3189 (4%) | 1459 (46%) | 1730 (54%) | |
| Unknown | 643 (1%) | 308 (48%) | 335 (52%) | |
| Missing | 80 (0%) | 29 (36%) | 51 (64%) | |
| History of Diabetes | ||||
| None | 68822 (93%) | 34548 (50%) | 34274 (50%) | |
| Yes, 0–5 years | 2304 (3%) | 1136 (49%) | 1168 (51%) | |
| Yes, 6–10 years | 776 (1%) | 330 (43%) | 446 (57%) | |
| Yes, >10 years | 804 (1%) | 373 (46%) | 431 (54%) | |
| Yes, Unknown duration | 556 (1%) | 269 (48%) | 287 (52%) | |
| Unknown | 368 (0%) | 168 (46%) | 200 (54%) | |
| Missing | 84 (0%) | 30 (36%) | 54 (64%) | |
| Kidney pumped | ||||
| Not pumped | 57111 (77%) | 29709 (52%) | 27402 (48%) | |
| Pumped | 16333 (22%) | 7024 (43%) | 9309 (57%) | |
| Missing | 270 (0%) | 121 (45%) | 149 (55%) | |
| Cause of Death | ||||
| Anoxia | 11330 (15%) | 5718 (50%) | 5612 (50%) | |
| Stroke | 30358 (41%) | 14999 (49%) | 15359 (51%) | |
| Head trauma | 29691 (40%) | 15075 (51%) | 14616 (49%) | |
| CNS tumor | 541 (1%) | 264 (49%) | 277 (51%) | |
| Other | 1730 (2%) | 776 (45%) | 954 (55%) | |
| Missing | 64 (0%) | 22 (34%) | 42 (66%) | |
| Non-Heart Beating Donor | ||||
| Missing | 106 (0%) | 34 (32%) | 72 (68%) | |
| No | 67702 (92%) | 34070 (50%) | 33632 (50%) | |
| Yes | 5906 (8%) | 2750 (47%) | 3156 (53%) | |
CIT = cold ischemia time
Table 3.
Exposure and outcome variables, stratified by median cold ischemia time
| Variable | n (% of total) | n CIT <=17.5 hrs (%) | n CIT >17.5 hrs (%) | |
|---|---|---|---|---|
| DGF | ||||
| No | 53423 (73%) | 28508 (53%) | 24915 (47%) | |
| Yes | 20185 (27%) | 8293 (41%) | 11892 (59%) | |
| One year graft loss | ||||
| Graft functional | 66329 (94%) | 33562 (51%) | 32767 (49%) | |
| Graft lost | 4382 (6%) | 1893 (43%) | 2489 (57%) | |
| Five year graft loss | ||||
| Graft functional | 32461 (78%) | 15733 (48%) | 16728 (52%) | |
| Graft lost | 9252 (22%) | 4098 (44%) | 5154 (56%) | |
| One year mortality | ||||
| Alive | 69861 (95%) | 35093 (50%) | 34768 (50%) | |
| Deceased | 3853 (5%) | 1761 (46%) | 2092 (54%) | |
| Five year mortality | ||||
| Alive | 38837 (80%) | 18604 (48%) | 20233 (52%) | |
| Deceased | 9444 (20%) | 4234 (45%) | 5210 (55%) | |
CIT = cold ischemia time
DGF occurred in 20,185 participants, which was 27% of the cohort. Death-censored graft loss occurred in 4,382 (6%) individuals by 1 year and 9,252 (22%) individuals by 5 years. The mortality rates in our sample were 5% at 1 year (n=3,853) and 20% at 5 years (n=9,444).
We assessed the strength of our instruments using the F-test, which examines whether the inclusion of the instrument in the first-stage regression model is relevant. An F-statistic > 10 is suggested as a general rule to classify an instrument as relevant (26). The F-statistics for exclusion of the instrumental variable were 63.56 and 156.80 in the 1-year and 5-year graft loss models, respectively, and 62.48 and 147.02 in the 1-year and 5-year mortality models, respectively. These are all greater than 10, indicating that the instrument was relevant in our models.
DGF and Graft Loss (Table 4)
Table 4.
Effect of DGF on graft loss and mortality at 1 and 5 years using a conventional linear probability model regression versus an instrumental variable analysis
| Dependent variable |
One year graft loss (N=69,922) |
Five year graft loss (N=41,138) |
One year mortality (N=72,879) |
Five year mortality (N=47,604) |
||||
|---|---|---|---|---|---|---|---|---|
| Model | LPM | IVM | LPM | IVM | LPM | IVM | LPM | IVM |
| Coefficient on DGF | 0.10072 | 0.13546 | 0.12475 | 0.16183 | 0.03312 | 0.07112 | 0.05986 | 0.11032 |
| (standard error) | (0.00375) | (0.02224) | (0.00465) | (0.03805) | (0.00233) | (0.01493) | (0.00466) | (0.03529) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
LPM = Linear Probability Model; IVM = Instrumental Variables Model
All standard errors are heteroskedasticity robust and clustered by region
Multiply coefficient by 100 to derive the probability of dependent variable on outcome.
All models control for the following covariates: transplant year, organ procurement organization, age, sex, educational level, race, insurance status, diabetes type, days on waiting list, peak panel reactive antibodies, and number of HLA mismatches at transplant, donor age, donor sex, donor race, donor diabetes, donor hypertension, whether the kidney was pumped, terminal serum creatinine, donor cause of death, and whether the kidney was a donation after circulatory determination of death.
DGF was associated with a 10.07% increase in probability of 1-year graft loss (p<0.001) in the linear probability model (LPM), which corresponds to an approximate relative risk of 3.98. The magnitude of this effect was larger in the IVM, where DGF was associated with a 13.55% increase in probability of 1-year graft loss (p<0.001), corresponding to an approximate relative risk of 5.01. DGF was associated with a 12.48% increase in probability of 5-year graft loss (p<0.001) in the LPM, corresponding to an approximate relative risk of 1.69. The magnitude of this effect was larger in the IVM, where DGF was associated with a 16.18% increase in probability of 5-year graft loss (p<0.001), corresponding to an approximate relative risk of 1.89.
DGF and Mortality (Table 4)
In the IVM, DGF was associated with a 7.11% increase in probability of 1-year mortality (p<0.001), corresponding to an approximate relative risk of 2.72. Additionally, DGF was associated with an 11.03% increase in probability of 5-year mortality (p=0.002) in the IVM, corresponding to an approximate relative risk of 1.64.
Supplemental Results
The rate of DGF as defined by lack of reduction in serum creatinine by 25% in a week was 50.37% (n=28,998) in our sample. Upon repeating our IVM with this alternate specification, we continued to find evidence suggesting a causal relationship between DGF and graft loss at both 1 year (12.00% increase, p<0.001) and 5 years (12.90% increase, p=0.007) and between DGF and mortality at both 1 year (7.68% increase, p < 0.001) and 5 years (8.56% increase, p=0.014). These were similar in magnitude and statistical significance to results using the original specification of DGF, indicating our analysis is robust to this alternate specification.
Upon regressing our outcomes on CIT using only observations without DGF, we found no association between cold ischemia time and 5-year graft loss (p=0.221). While we did find a statistically significant association between CIT and 1-year graft loss (b=0.00022, p=0.036) and mortality at 1 year (b=0.00038, p<0.001) and 5 years (b=0.00061, p=0.035), the magnitude of these coefficients were clinically insignificant suggesting these associations were likely a function of our large sample size only and do not refute our assertion that CIT was exogenous.
Upon repeating our original IVM after exclusion of the 20,479 individuals who had a Kidney Donor Risk Index (KDRI) greater than 1.155, the 75th percentile in our sample, we continued to find evidence suggesting a causal relationship between DGF and graft loss at 1 year (10.52% increase, p<0.001) and 5 years (10.64% increase, p=0.007) and between DGF and mortality at 1 year (5.94% increase, p=0.001) and 5 years (9.90% increase, p=0.002). These were all similar in magnitude and statistical significance to our original results, indicating our analysis is robust to exclusion of individuals receiving lower quality kidneys.
Approximately 22.16% of our sample (n=16,333) received kidneys that were pumped. Upon repeating our IVM analyses stratifying by whether individuals had received pumped kidneys, we continued to find evidence of a causal relationship between DGF and graft loss at 1 year (11.63% increase, p<0.001) and 5 years (16.01% increase, p<0.001) as well as between DGF and mortality at 1 year (5.41% increase, p=0.001) and 5 years (12.94% increase, p=0.001) among non-pumped kidney recipients. Among pumped kidney recipients, we continued to find evidence of a causal relationship between DGF and both graft loss (24.87% increase, p=0.007) and mortality (14.47% increase, p=0.006) at 1 year, though we did not find evidence of a causal relationship between DGF and graft loss (6.07% increase; p=0.569) or mortality (9.57% decrease; p=0.369) at 5 years. Our analysis among non-pumped kidney recipients is concordant with our primary results, but among pumped kidney recipients, a causal effect only persists for 1 year outcomes, likely due to loss of statistical power from excluding the majority of our sample (only 6466 of 41138 observations remain in 5-year graft loss and only 7545 of 47604 observations remain in 5-year mortality models).
Discussion
This study uses instrumental variables analysis, a quasi-randomization technique for drawing causal inferences. We used CIT as an instrument for DGF to find evidence suggesting a causal relationship between DGF and both graft loss and mortality at 1 year and 5 years. These effects were robust to exclusion of individuals with high KDRI and pumped kidneys and use of an alternate specification of DGF.
In our study, evidence suggesting a causal relationship between DGF and graft loss was present at both 1 and 5 years in the IVM. These findings are consonant with a 2009 meta-analysis by Yarlagadda et al., which found that patients with DGF had a significantly higher risk of graft loss (RR 1.41, 95% CI: 1.27–1.56) at a mean of 3.2 years of follow-up (27). However, a more recent study by Kayler et al. that used a paired-kidney analysis to examine the impact of CIT-induced DGF on graft failure did not find a difference in graft survival between paired donor transplants with and without DGF when CIT differences were less than 15 hours (28). Of note, in our study, the magnitude of the increase in probability of graft loss at 1 and 5 years as a result of DGF was greater in our IVM analyses (13.55% and 16.18% at 1 and 5 years, respectively) compared to the LPM analyses (10.07% and 12.48%, at 1 and 5 years, respectively) suggesting that other, unaccounted-for variables may be masking the magnitude of the true effect of DGF on graft loss in non-IVM analyses. For instance, kidney transplant recipients who are sicker at baseline may be more likely to get DGF, yet their risk of graft loss may be somewhat mitigated if they receive closer supervision in their post-transplant care than less sick individuals. Such a mechanism could explain the lack of a statistically detectable effect in kidneys with shorter CIT in Kayler et al. While paired kidney analysis controls for unobserved donor characteristics, it does not adjust for unobserved recipient factors confounding the relationship between DGF and graft loss that may have biased this relationship downwards. IVM analysis may offer advantages over a paired kidney analysis in accounting for the effects of confounders.
Evidence suggesting a causal relationship between DGF and death was also present at both 1 and 5 years in the IVM in our study. The meta-analysis by Yarlagadda et al. pooled data from 8 studies and found no significant increase in risk of mortality among those with DGF (27), though one study included in this review by Fontan et al. in 1996 found a relationship between DGF lasting greater than 3 weeks and an excess in mortality (7). A study by Patel et al. published since this review examined 231 high risk deceased-donor kidney transplant recipients who received routine induction therapy with anti-thymoglobulin and found that DGF was associated with a lower 1 year survival rate (99% non-DGF vs. 91% DGF; p=0.001) (29). Additionally, a more recent study by Tapiawala et al. used observations the US Renal Data system between years 1998 and 2004 to examine the relationship between DGF and mortality among those who died with a functioning graft and found that patients with DGF were significantly more likely to die(adjusted HR: 1.53, 95% CI: 1.45–1.63) (8).
Our evidence suggesting that DGF causes a long-term effect in kidneys has important implications for the prognosis of many individuals, given that DGF is the most common immediate post-transplant complication (30). Our results support that increased attention should be given to individuals with DGF post-transplant to prevent future graft loss. These patients may benefit from modifications in their induction and maintenance immunosuppression regimens and close monitoring of graft function for several years after discharge (31). The potentially causal association of DGF with graft failure should not suggest an increased rate of refusal of marginal quality organs that may benefit transplant candidates on the waiting list out of fear of DGF, but rather should stimulate development of agents for alleviating effects of ischemia-reperfusion injury and graft failure. Trials evaluating such agents could target DGF as an efficient surrogate outcome for early drug development clinical trials, given the ease and immediacy of measurement relative to following kidney transplants for several years in order to accumulate graft loss events. Alternatively, patients with DGF could be randomized in treatment trials for testing of clinical management strategies to prevent progression of graft loss. Furthermore, a potential causal effect of DGF on graft loss has important implications for the long-term effects of other types of AKI, given that histological findings on biopsy in kidneys with DGF mimic those found in acute tubular necrosis in native kidneys (32). If the findings concerning the relationship between DGF and graft loss generalize to other types of kidney injury, the results from our study suggest that AKI can potentially cause future chronic kidney disease and add to the growing literature on the long-term effects of AKI (33, 34).
Nevertheless, our findings need to be interpreted in light of our limitations. The DGF specification we used is specific, but not sensitive, and may thus bias our analysis towards an effect (35). However, some patients may require dialysis after transplant for other indications, despite good allograft function, which would bias our analysis towards the null. Our results were also robust to an alternate, more sensitive specification of DGF. Additionally, the two-stage least squares specification for IVM results in artificially large standard errors in settings where binary outcome variables are congregated near 0 or 1, resulting a higher likelihood of rejecting our alternative hypotheses in favor of the null. [22] However, we overcome this conservative approach to hypothesis testing with our large sample size, and we find a statistically significant effect in all our IVM analyses. Finally, CIT may not have been a completely exogenous instrument. It is possible that CIT relates to outcomes through a non-DGF mechanism, such that worse kidneys may be turned down from more centers and take longer to place. Therefore, poor kidney quality could both prolong CIT and associate with allograft failure. However, we adjust for variables that may reflect kidney quality in our model, which would be expected to lessen the impact of such a mechanism on our results. Additionally, the robustness of our results to exclusion of individuals with a high KDRI, which reflects lower allograft quality, suggests that this mechanism is unlikely. Furthermore, the lack of a clinically significant association between CIT and our outcomes in absence of DGF suggests that the only meaningful relationship between CIT and our outcomes is through DGF. Finally, DGF may have different biological implications among those with pumped kidneys compared to those without pumped kidneys, which may not be completely addressed by simple inclusion of this variable in our primary IVM analyses. However upon stratifying our analyses by whether kidneys were pumped, our findings persisted in all models with the exception of the 5-year outcomes among those with pumped kidneys; this non-significant finding with 5-year mortality outcomes may be due to the exclusion of the majority of our sample in these models.
In conclusion, this paper uses a novel technique for analyzing the relationship between DGF and long-term outcomes. Using IVM analyses, we find highly suggestive evidence of a causal effect of DGF on both graft loss and mortality at 1 and 5 years. In addition to the clinical implications of our results on patient prognosis, the results of this study open up new avenues for research using DGF as a surrogate outcome and improving long-term outcomes by testing therapies to prevent progressive loss in graft function among recipients with DGF.
Materials and Methods
Participants and study design
This study used data from the Scientific Registry of Transplant Recipients (SRTR). We included all 101,565 adult deceased-donor single primary kidney-only transplant recipients from January 1997 to December 2010 without primary graft failure. We excluded 8,870 recipients with missing CIT, 6,976 recipients who were not on dialysis prior to transplant, and 12,005 recipients with 0 HLA mismatches, given the systematically altered allocation practices of such kidneys that affect both CIT and outcomes. Our final sample was 73,714 observations.
Variables
The primary exposure examined was DGF, defined as dialysis in the 1st week post-transplant. The primary outcomes were death-censored graft loss and mortality. Graft loss at 1 and 5 years from transplant was calculated as time to recorded graft failure or to start of chronic maintenance dialysis, whichever was shorter. Mortality at 1 and 5 years was calculated as time to death as reported in the Social Security Master Death File, with inclusion of additional death dates reported by centers to the Organ Procurement Transplantation Network. For analyses of 5-year mortality and graft loss, we only included the sub-cohort of kidney transplant recipients from January 1997 to December 2006, so that all individuals would have opportunity for 5 years of follow-up. The instrumental variable was CIT, in hours.
We also included recipient, donor, and allograft characteristics known to affect outcomes as covariates. Recipient characteristics included transplant year, organ procurement organization (OPO), age, sex, education, race, insurance, diabetes type, days on waiting list, and peak panel reactive antibodies (PRA). Donor characteristics included age, sex, race, history of diabetes, history of hypertension, terminal serum creatinine, cause of death, and whether the kidney was a donation after circulatory determination of death (DCDD). Allograft characteristics included number of HLA mismatches and whether the allograft was pumped.
Statistical analysis
To perform IVM analysis, we used a two-stage least squares regression specification: in the first stage, we used CIT as an instrument to predict DGF independent of outcomes; in the second stage, we examined the relationship between predicted DGF values and outcomes. Given that DGF, graft loss, and mortality are all binary variables and that we have included a large number of covariates, the two-stage least squares regression specification was most appropriate for our IVM because it produces estimates that are very similar to nonlinear specifications, such as logistic or bivariate probit (36), yet these estimates are more robust to potential misspecification of the distribution of error terms (37). For additional information on IVM analysis, please refer to the digital supplemental content (SDC, Detailed Methods). We compared these IVM results to those from a simple linear probability model (LPM) of the relationship between DGF and outcomes. This LPM was calculated using simple least-squares regression with a binary outcome variable regressed on DGF and all of the covariates included in the IVM.
To assess strength of the IVM, we used the F-test, which examines whether the inclusion of the instrument in the first-stage regression model is relevant. All models adjusted for the recipient, donor and allograft characteristics listed above. Missing data in categorical variables was treated as a separate category using a dummy variable. Observations with missing data in quantitative variables were dropped from the regression model, though this accounted for less than 0.5% of the total sample. All standard errors were heteroskedasticity-robust and clustered around OPO. For ease of interpretation, relative graft loss and mortality rates were approximated from absolute risk differences in the IVM and LPM analyses as 1 + the absolute risk difference divided by the graft loss or mortality rates among those without DGF in a manner similar to Stukel at al. (21). All analyses were conducted using STATA 11.0 IC (StataCorp, College Station, TX).
We also conducted several supplemental analyses. In order to remove concerns about the sensitivity of our specification of DGF, we repeated our original analyses using an alternate specification of DGF defined as either dialysis in the 1st week post-transplant or a failure for a recipient’s creatinine to decline by 25% or more in the first 24 hours. In order to remove concerns about an alternate pathway between CIT and outcomes, we examined the association between CIT and outcomes among those without DGF in a multivariate linear probability regression using our fully adjusted model. Additionally, we repeated analyses excluding individuals with the top 25% KDRI (38) and stratified our analyses by whether a kidney was pumped.
Supplementary Material
Table 2.
Recipient and donor characteristics (continuous variables), stratified by median cold ischemia time
| Variable | Mean (SD) | Mean if CIT <17.55 hrs (SD) | Mean if CIT >=17.55 hrs (SD) |
|---|---|---|---|
| Recipient age | 51.21 (12.99) | 51.06 (13.16) | 51.36 (12.84) |
| Days on waiting list | 818.78 (651.89) | 829.31 (652.77) | 808.26 (650.86) |
| Peak PRAa | 2 (15) | 2 (15) | 2 (15) |
| HLA mismatches | 4.22 (1.21) | 4.27 (1.19) | 4.18 (1.23) |
| Donor age | 39.32 (16.69) | 38.93 (16.35) | 39.71 (17.02) |
| Donor serum creatinine | 1.15 (1.11) | 1.10 (1.03) | 1.20 (1.18) |
SD = standard deviation; CIT = cold ischemia time; PRA = panel reactive antibody
Peak PRA reported as median (IQR)
Acknowledgments
The authors would like to acknowledge the insight and guidance of Dr. Amanda Kowalski from the Department of Economics, Yale University.
The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Dr. Parikh was supported by the K24-DK090203 grant from NIH. Dr. Reese was supported by K23 –DK078688-01 from the NIH.
Abbreviations
- AKI
acute kidney injury
- CIT
cold ischemia time
- DCDD
donation after circulatory determination of death
- DGF
delayed graft function
- ECD
expanded criteria donor
- IVM
instrumental variables model
- KDRI
kidney donor risk index
- LPM
linear probability model
- OPO
organ procurement organization
- PRA
panel reactive antibody
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NMB participated in research design, the writing of the paper, the performance of the research, and data analysis; the author declares no conflict of interest.
PPR participated in research design and the writing of the paper, and contributed analytic tools; supported by K23 –DK078688-01 from the NIH; the author declares no conflict of interest.
MDD participated in research design and the writing of the paper, and contributed analytic tools; the author declares no conflict of interest.
CRP participated in research design, the writing of the paper, and data analysis, and contributed analytic tools; supported by the K24-DK090203 grant from NIH; the author declares no conflict of interest.
An abstract of this paper was presented as an oral presentation at the American Transplant Conference 2012.
Statement of Competing Interests
The authors of this study declare no competing interests.
Contributor Information
Neel M. Butala, Yale School of Medicine, New Haven, CT.
Peter P. Reese, Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA; Renal Division, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Mona D. Doshi, Division of Nephrology, Wayne State University School of Medicine, Detroit, MI.
Chirag R. Parikh, Program of Applied Translational Research, Department of Medicine, Yale School of Medicine, New Haven, CT; Section of Nephrology, Department of Medicine, Yale School of Medicine, New Haven, CT; Clinical Epidemiology Research Center, Veterans Affairs Medical Center, West Haven, CT.
References
- 1.Freedland SJ. Economic impact of delayed graft function and suboptimal kidneys. Transplantation Reviews. 1999;13(1):23. [Google Scholar]
- 2.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66(12):1697–1701. doi: 10.1097/00007890-199812270-00022. Epub 1999/01/12. [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011. Epub 1997/04/15. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqi N, McBride MA, Hariharan S. Similar risk profiles for post-transplant renal dysfunction and long-term graft failure: UNOS/OPTN database analysis. Kidney international. 2004;65(5):1906–1913. doi: 10.1111/j.1523-1755.2004.00589.x. Epub 2004/04/17. [DOI] [PubMed] [Google Scholar]
- 5.Asderakis A, Dyer P, Augustine T, Worthington J, Campbell B, Johnson RW. Effect of cold ischemic time and HLA matching in kidneys coming from "young" and "old" donors: do not leave for tomorrow what you can do tonight. Transplantation. 2001;72(4):674–678. doi: 10.1097/00007890-200108270-00020. Epub 2001/09/07. [DOI] [PubMed] [Google Scholar]
- 6.Moreso F, Seron D, Gil-Vernet S, Riera L, Fulladosa X, Ramos R, et al. Donor age and delayed graft function as predictors of renal allograft survival in rejection-free patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14(4):930–935. doi: 10.1093/ndt/14.4.930. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 7.Perez Fontan M, Rodriquez-Carmona A, Bouza P, Garcia Falcon T, Moncalian J, Oliver J, et al. Outcome of grafts with long-lasting delayed function after renal transplantation. Transplantation. 1996;62(1):42–47. doi: 10.1097/00007890-199607150-00009. Epub 1996/07/15. [DOI] [PubMed] [Google Scholar]
- 8.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. Journal of the American Society of Nephrology : JASN. 2010;21(1):153–161. doi: 10.1681/ASN.2009040412. Epub 2009/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcen R, Orofino L, Pascual J, de la Cal MA, Teruel JL, Villafruela JJ, et al. Delayed graft function does not reduce the survival of renal transplant allografts. Transplantation. 1998;66(4):461–466. doi: 10.1097/00007890-199808270-00008. Epub 1998/09/12. [DOI] [PubMed] [Google Scholar]
- 10.Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, et al. One-year posttransplant renal function is a strong predictor of long-term kidney function: results from the Neoral-MOST Observational Study. Transplantation proceedings. 2003;35(8):2863–2867. doi: 10.1016/j.transproceed.2003.10.070. Epub 2003/12/31. [DOI] [PubMed] [Google Scholar]
- 11.Troppmann C, Gillingham KJ, Gruessner RW, Dunn DL, Payne WD, Najarian JS, et al. Delayed graft function in the absence of rejection has no long-term impact. A study of cadaver kidney recipients with good graft function at 1 year after transplantation. Transplantation. 1996;61(9):1331–1337. doi: 10.1097/00007890-199605150-00008. Epub 1996/05/15. [DOI] [PubMed] [Google Scholar]
- 12.Woo YM, Jardine AG, Clark AF, MacGregor MS, Bowman AW, Macpherson SG, et al. Early graft function and patient survival following cadaveric renal transplantation. Kidney international. 1999;55(2):692–699. doi: 10.1046/j.1523-1755.1999.00294.x. Epub 1999/02/13. [DOI] [PubMed] [Google Scholar]
- 13.Oppenheimer F, Aljama P, Asensio Peinado C, Bustamante Bustamante J, Crespo Albiach JF, Guirado Perich L. The impact of donor age on the results of renal transplantation. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(Suppl 3):iii11–iii15. doi: 10.1093/ndt/gfh1008. Epub 2004/06/12. [DOI] [PubMed] [Google Scholar]
- 14.Shoskes DA, Parfrey NA, Halloran PF. Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation. 1990;49(1):201–207. doi: 10.1097/00007890-199001000-00045. Epub 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet. 2004;364(9447):1814–1827. doi: 10.1016/S0140-6736(04)17406-0. Epub 2004/11/16. [DOI] [PubMed] [Google Scholar]
- 16.Chatziantoniou C, Dussaule JC. Is kidney injury a reversible process? Current opinion in nephrology and hypertension. 2008;17(1):76–81. doi: 10.1097/MNH.0b013e3282f1bb69. Epub 2007/12/20. [DOI] [PubMed] [Google Scholar]
- 17.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA : the journal of the American Medical Association. 1994;272(11):859–866. Epub 1994/09/21. [PubMed] [Google Scholar]
- 18.Rizzo JA, Coady MA, Elefteriades JA. Procedures for estimating growth rates in thoracic aortic aneurysms. Journal of clinical epidemiology. 1998;51(9):747–754. doi: 10.1016/s0895-4356(98)00050-x. Epub 1998/09/10. [DOI] [PubMed] [Google Scholar]
- 19.Gifford AL, Bormann JE, Shively MJ, Wright BC, Richman DD, Bozzette SA. Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. J Acquir Immune Defic Syndr. 2000;23(5):386–395. doi: 10.1097/00126334-200004150-00005. Epub 2000/06/24. [DOI] [PubMed] [Google Scholar]
- 20.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(4):1064–1070. doi: 10.1200/JCO.2001.19.4.1064. Epub 2001/02/22. [DOI] [PubMed] [Google Scholar]
- 21.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA : the journal of the American Medical Association. 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. Epub 2007/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. The New England journal of medicine. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571. Epub 2008/02/22. [DOI] [PubMed] [Google Scholar]
- 23.Cameron AC, Trivedi PK. Microeconometrics : Methods and Applications. 2005 [Google Scholar]
- 24.Siedlecki A. Delayed Graft Function in the Kidney Transplant. American journal of transplantation. 2011;11(11):2279. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doshi MD, Garg N, Reese PP, Parikh CR. Recipient risk factors associated with delayed graft function: a paired kidney analysis. Transplantation. 2011;91(6):666–671. doi: 10.1097/TP.0b013e318209f22b. Epub 2011/02/15. [DOI] [PubMed] [Google Scholar]
- 26.Staiger D, Stock J. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557–586. [Google Scholar]
- 27.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. Epub 2008/12/24. [DOI] [PubMed] [Google Scholar]
- 28.Kayler LK, Srinivas TR, Schold JD. Influence of CIT-induced DGF on kidney transplant outcomes. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(12):2657–2664. doi: 10.1111/j.1600-6143.2011.03817.x. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 29.Patel SJ, Duhart BT, Jr, Krauss AG, Moore LW, Egidi MF, Amiri HS, et al. Risk factors and consequences of delayed graft function in deceased donor renal transplant patients receiving antithymocyte globulin induction. Transplantation. 2008;86(2):313–320. doi: 10.1097/TP.0b013e31817ef190. Epub 2008/07/23. [DOI] [PubMed] [Google Scholar]
- 30.Yarlagadda SG, Klein CL, Jani A. Long-term renal outcomes after delayed graft function. Advances in chronic kidney disease. 2008;15(3):248–256. doi: 10.1053/j.ackd.2008.04.005. Epub 2008/06/21. [DOI] [PubMed] [Google Scholar]
- 31.Pascual M. Strategies to improve long-term outcomes after renal transplantation. The New England journal of medicine. 2002;346(8):580. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 32.Smith KD, Wrenshall LE, Nicosia RF, Pichler R, Marsh CL, Alpers CE, et al. Delayed graft function and cast nephropathy associated with tacrolimus plus rapamycin use. Journal of the American Society of Nephrology : JASN. 2003;14(4):1037–1045. doi: 10.1097/01.asn.0000057542.86377.5a. Epub 2003/03/28. [DOI] [PubMed] [Google Scholar]
- 33.Ishani A. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology. 2009;20(1):223. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wald R. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA (Chicago, Ill) 2009;302(11):1179. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 35.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. Epub 2008/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angrist JD. Estimation of limited dependent variable models with dummy endogenous regressors. Journal of Business & Economic Statistics. 2001;19(1):2–28. [Google Scholar]
- 37.Chiburis RDJ, Lokshin M. A Practical Comparison of the Bivariate Probit and Linear IV Estimators. World Bank Policy research Working Paper no 5601. 2011 [Google Scholar]
- 38.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. Epub 2009/07/23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.