Abstract
Pharmacological data suggest that delta opioid receptors modulate learning and memory processes. In the present study, we investigated whether inactivation of the delta opioid receptor modifies hippocampus (HPC)- and striatum-dependent behaviors. We first assessed HPC-dependent learning in mice lacking the receptor (Oprd1−/− mice) or wild-type (WT) mice treated with the delta opioid antagonist naltrindole using novel object recognition, and a dual-solution cross-maze task. Second, we subjected mutant animals to memory tests addressing striatum-dependent learning using a single-solution response cross-maze task and a motor skill-learning task. Genetic and pharmacological inactivation of delta opioid receptors reduced performance in HPC-dependent object place recognition. Place learning was also altered in Oprd1−/− animals, whereas striatum-dependent response and procedural learning were facilitated. Third, we investigated the expression levels for a large set of genes involved in neurotransmission in both HPC and striatum of Oprd1−/− mice. Gene expression was modified for several key genes that may contribute to alter hippocampal and striatal functions, and bias striatal output towards striatonigral activity. To test this hypothesis, we finally examined locomotor effects of dopamine receptor agonists. We found that Oprd1−/− and naltrindole-treated WT mice were more sensitive to the stimulant locomotor effect of SKF-81297 (D1/D5), supporting the hypothesis of facilitated striatonigral output. These data suggest, for the first time, that delta receptor activity tonically inhibits striatal function, and demonstrate that delta opioid receptors modulate learning and memory performance by regulating the HPC/striatum balance.
Keywords: opioid receptors, knockout mice, learning and memory, hippocampus-striatum balance, associative/procedural learning
INTRODUCTION
The role played by endogenous opioid peptides and their receptors in modulating learning and memory processes elicits sustained interest in the literature. Delta opioid receptors are expressed in the hippocampus (HPC), dorsal and ventral striatum, amygdala, and prefrontal cortex (Le Merrer et al, 2009; Scherrer et al, 2006), all key brain substrates for memory function (White and McDonald, 2002). Pharmacological data support the notion that stimulating or inactivating delta opioid receptors impacts memory performance, although the exact implication of delta receptor activity remains unclear. Delta opioid receptor agonists administered peripherally either facilitate (Martinez et al, 1984; Pavone et al, 1990; Yang et al, 2003) or impair (Jutkiewicz et al, 2003; Martinez et al, 1984; Schulteis and Martinez, 1990; Ukai et al, 1997) avoidance or operant learning, while the preferential delta antagonist ICI 174,864 improves retrieval of avoidance conditioning in mice (Ilyutchenok and Dubrovina, 1995; Schulteis and Martinez, 1990). However, a major concern when using pharmacology is the possible cross-reactivity of delta agonists and antagonists at other opioid receptors (Hutcheson et al, 2001; Scherrer et al, 2004). Therefore, gene targeting represents a unique approach to address the specific role of each opioid receptor in vivo.
To our knowledge, learning and memory have not been assessed in mice lacking delta opioid receptors (Oprd1−/− mice). Others and we showed that morphine-induced place conditioning is reduced in these animals (Chefer and Shippenberg, 2009; Le Merrer et al, 2012b; Le Merrer et al, 2011). Our results (Le Merrer et al, 2012b; Le Merrer et al, 2011) further suggested that this phenotype may reflect deficient ability to form drug-context associations rather than deficient reward processes, leading us to speculate that hippocampal function is altered in Oprd1−/− mice. Finally, previous experiments showed increased break points for morphine self-administration under a progressive-ratio schedule of reinforcement (Le Merrer et al, 2011), as well as increased motor impulsivity (Olmstead et al, 2009) in Oprd1−/− mice, suggesting that striatal function may also be modified in these animals. We therefore designed the present study to characterize learning and memory abilities in Oprd1−/− animals, and examined both HPC-dependent and striatum-dependent responses. We show that mutant mice display decreased ability to resolve HPC-dependent tasks and facilitated striatum-dependent responses. In support to behavioral data, expression analysis of key hippocampal and striatal genes suggests modified neurotransmission in the two brain structures, and pharmacology shows modified responses to D1 (striatonigral pathway) and D2 (striatopallidal pathway) dopamine receptor agonists. Together our data concur to demonstrate that delta opioid receptors modulate learning and memory performance by regulating both hippocampal and striatal function.
MATERIALS AND METHODS
Subjects
Male and female Oprd1−/− mice (Filliol et al, 2000) and their wild-type (WT) (Oprd1+/+) controls were bred in-house on a hybrid 50% 129SVPas—50% C57BL/6J background. Animals studied in rotarod experiments were aged 7 weeks at the beginning of the test; other behavioral experiments were performed in animals aged 8–12 weeks. Animals were group housed (2–5 mice per cage) on a 12 h light/dark cycle (lights on at 0700 hours) at controlled temperature (22±1 °C). A cardboard igloo (Dietex, Saint Gratien, France) was provided in each cage. Food and water were available ad libitum throughout all experiments, unless otherwise stated. Experimental procedures were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and approved by the Comité Régional d'Ethique en Matière d'Expérimentation Animale de Strasbourg (CREMEAS, 2003-10-08-[1]-58).
Behavioral Experiments
Behavioral testing was performed using independent cohorts of naïve animals for each experiment, except for rotarod and runway experiments (see detailed protocols in Supplementary Information). Anxiety levels were evaluated under low light conditions in the elevated plus-maze (preliminary experiment, see Supplementary Information and Supplementary Figure S1). Recognition of novel objects and their spatial location was assessed using a novel object recognition task (experiments 1−2). Place learning was evaluated using a dual-solution task (experiment 3), and response learning was measured using a single-solution task (experiment 4) in the cross-maze. Motor abilities were assessed using a motor skill-learning task on the accelerating rotarod (experiment 5). Sensitivity to dopamine receptor activation was evaluated by measuring locomotor activity under pharmacological challenge (experiment 6). Finally, we adapted a runway task and a novelty-suppressed feeding task to explore motivation for food in WT and Oprd1−/− mice (experiment 7−8, see Supplementary Information).
Quantitative Real-Time PCR Analysis
Brains were removed and placed into a brain matrix (ASI Instruments, Warren, MI, USA). Caudate putamen, nucleus accumbens, and HPC were punched or dissected out from 1 mm-thick slices. Tissues were immediately frozen on dry ice and kept at −80 °C until use. As the delta opioid receptor gene knockout influenced hippocampal and striatal-dependent performance similarly in male and female mice, tissue from one male and one female mouse was pooled in the same sample for each structure of interest and each genotype (n=5 samples/genotype). RNA was extracted and purified using the MIRNeasy mini-kit (Qiagen, Courtaboeuf, France). cDNA was synthetized using the first-strand Superscript II kit (Invitrogen, Life Technologies, Saint Thomas, France)(Le Merrer et al, 2012a). Quantitative real-time -PCR (qRT-PCR) was performed in quadruplets on a LightCycler 480 RT- PCR (Roche, Manheim, Germany) using iQ-SYBR Green supermix (Bio-Rad, Marnes-la-Coquette, France) kit with 0.25 μl cDNA in a 12.5 μl final volume. Gene-specific primers were designed using Primer3 software to obtain a 100−150 bp product (see Supplementary Table S1). Relative expression ratios were normalized to the level of actin and the 2−ΔΔCt method was applied to evaluate differential expression level.
Statistical Analyses
Behavioral experiments
Detailed statistical methods are found in Supplementary Information. Analysis of variance was used to assess the effects of gender, genotype, experimental condition, or treatment as between-subject factors and phase, trial or session as within-subject factors (Statistica 9.0, StatSoft, Maisons Alfort). Statistical significance was set at p<0.05 for all tests.
Gene expression
QRT-PCR data were transformed before statistical analysis to obtain a symmetrical distribution centered on 0 (corresponding to no change in gene expression) using the following formula: if x<1, y=1-1/x; if x>1, y=x-1 (x: qPCR data; y: transformed data) (Le Merrer et al, 2012a). A Student's t-test was then performed to assess their statistical significance. Calculated p-values indicated probability for a regulation to differ from 0. Only regulations over +1.20 or below −1.20 (fold-change) were retained as significant.
RESULTS
Genetic and Pharmacological Inactivation of Delta Opioid Receptors Impairs Recognition of a Displaced Object
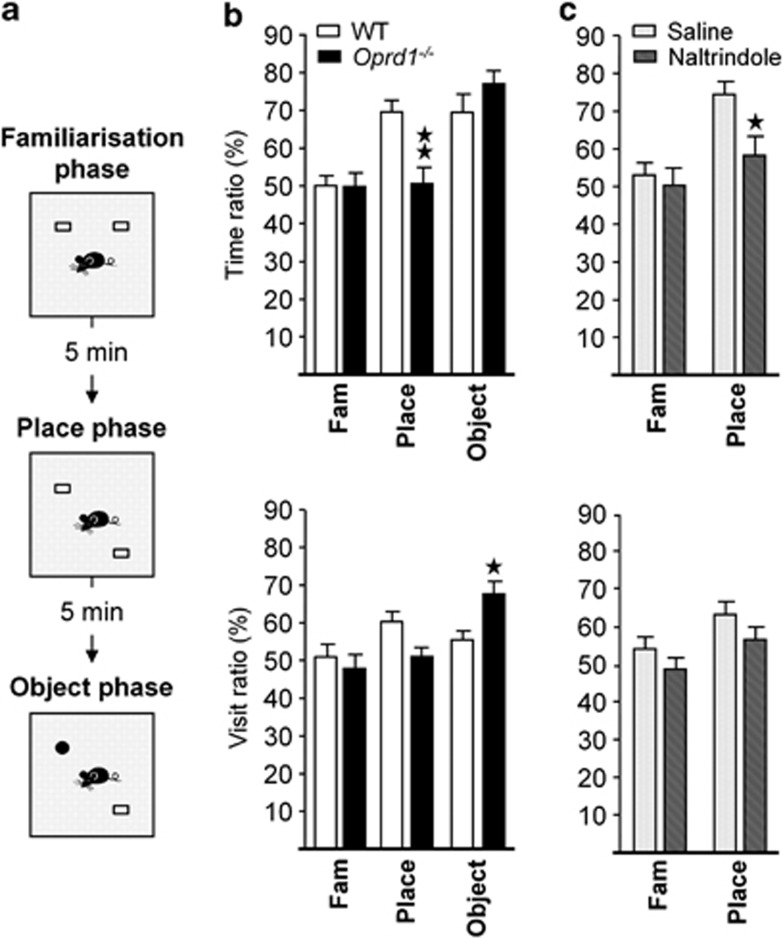
To tackle hippocampal function in the absence of delta receptors, we used a novel object recognition task and assessed the ability to discriminate either novel objects or their spatial location in WT vs Oprd1−/− mice (experiment 1) or in WT animals treated with the delta opioid receptor antagonist naltrindole (experiment 2) (Supplementary Information). Indeed, detection of changes in object location is critically dependent on hippocampal integrity (Ennaceur et al, 1997; Mumby et al, 2002; Oliveira et al, 2010). In trial 1, mice were presented with two copies of an unfamiliar object (familiarization); in trial 2, one of the two objects was displaced to a novel location (place phase); in trial 3, the unmoved object was replaced by a novel object (object phase).
In experiment 1 (WT—n=eight females, eight males; Oprd1−/−—n=seven females, eight males), the number of visits to objects (genotype: F1,27=3.72, NS; gender: F1,27=3.26, NS; phase: F1,54=25.05, p<0.0001), and time spent exploring the objects (genotype: F1,27<1; gender: F1,27<1; phase: F1,54=3.65, p<0.05) decreased similarly across phases in both mouse lines (Table 1). However, Oprd1−/− mice differed from WT mice in their visit ratio (genotype: F1,27<1; gender: F1,27<1; phase: F1,54=6.24, p<0.01; phase × genotype: F1,54=5.28, p<0.01), and time ratio (genotype: F1,27=1.59, NS; gender: F1,27<1; phase: F1,54=17.59, p<0.0001; phase × genotype: F1,54=6.08, p<0.01) across phases of the object recognition task (Figure 1b). Subsequent analysis performed for place phase revealed a significant deficit in time ratio (genotype: F1,27=12.44, p<0.01), but not visit ratio (genotype: F1,27=3.41, NS) in mutant as compared with WT animals. Conversely, during object phase, visit ratio (genotype: F1,27=6.40, p<0.05), but not time ratio (genotype: F1,27=1.63, NS) was increased in Oprd1−/− animals as compared with controls. Mutant mice, therefore, showed impaired memory for object location but higher interest for the novel object.
Table 1. Genetic Deletion of the Delta Opioid Receptors does not Modify Exploration Parameters in the Novel Object Recognition Task, whereas Pharmacological Blockade Reduced Exploration of Objects in WT Mice.
| Experiment | Genotype | Treatment |
Familiarization phase |
Place phase |
Object phase |
|||
|---|---|---|---|---|---|---|---|---|
| Time | Visits | Time | Visits | Time | Visits | |||
| Experiment 1 | WT | None | 11.38±1.36 | 17.75±1.92 | 7.84±2.55 | 9.94±1.72 | 12.54±3.14 | 9.50±1.81 |
| Oprd1−/− | None | 14.39±1.77 | 23.27±2.22 | 9.61±1.88 | 13.73±1.73 | 12.52±3.29 | 13.47±2.46 | |
| Experiment 2 | WT | Saline | 13.69±2.38 | 17.67±2.97 | 6.79±1.51 | 8.50±1.41 | n.a. | n.a. |
| WT | NTI 0.3 mg/kg | 7.64±1.87 | 11.14±2.13 | 3.39±0.60* | 5.36±0.65* | n.a. | n.a. | |
Parameters of object exploration (time and number of visits for both objects) decreased similarly over phases in WT and Oprd1−/− mice. In contrast, WT animals injected peripherally with naltrindole (0.3 mg/kg) spent less time exploring objects and approached less these objects than saline-injected mice. Parameters of exploration were presented as total number of visits and total time exploring the objects over 10 min phases (mean±SEM). *p<0.05; **p<0.01; ***p<0.001 (genotype effect: one-way ANOVA). n.a.: non assessed.
Figure 1.
Genetic or pharmacological inactivation of delta opioid receptors impairs object place recognition. (a) Schematic representation of the protocol. (b) In the novel object recognition task, Oprd1−/− mice fail to display preference for the displaced object during the place phase, as shown by their low time ratio (percent time exploring displaced vs still object). In contrast, mice approach novel object more often during the object phase, as indicated by their high visit ratio (percent number of visits to novel vs familiar object). (c) WT mice treated with naltrindole (0.3 mg/kg) display a similar deficit as Oprd1−/− animals in recognizing a displaced object. Object preference is expressed as a percentage of time spent exploring (time ratio) or visits (visit ratio) to novel/displaced object vs unmoved/familiar object (mean±SEM). 1 star: p 0.05; 2 stars: p 0.01 (one-way analysis of variance).
In experiment 2 (Saline—n=six females, eight males; naltrindole—n=six females, six males), systemic naltrindole treatment (0.3 mg/kg) reduced the number of visits to objects (treatment: F1,27=4.98, p<0.05; gender: F1,27<1; phase: F1,54=22.05, p<0.001) and time spent exploring the objects (treatment: F1,27=5.92, p<0.05; gender: F1,27=1.48, NS; phase: F1,54=15.64, p<0.001) over the two phases of the task in WT mice (Table 1), consistent with anxiogenic effects (Perrine et al, 2006). Besides, delta opioid receptor blockade diminished visit (treatment: F1,27=4.42, p<0.05; gender: F1,27=1.45, NS; phase: F1,54=5.02, p<0.05) and time (treatment: F1,27=4.77, p<0.05; gender: F1,27<1; phase: F1,54=9.70, p<0.01) ratios during place phase (Figure 1c). Pharmacological inactivation of delta opioid receptors thus mimicked the effects of genetic deletion on object place recognition, suggestive of altered hippocampal function.
Oprd1 −/− Mice Show Delayed Acquisition of a Place Strategy and Facilitated Acquisition of Response Learning
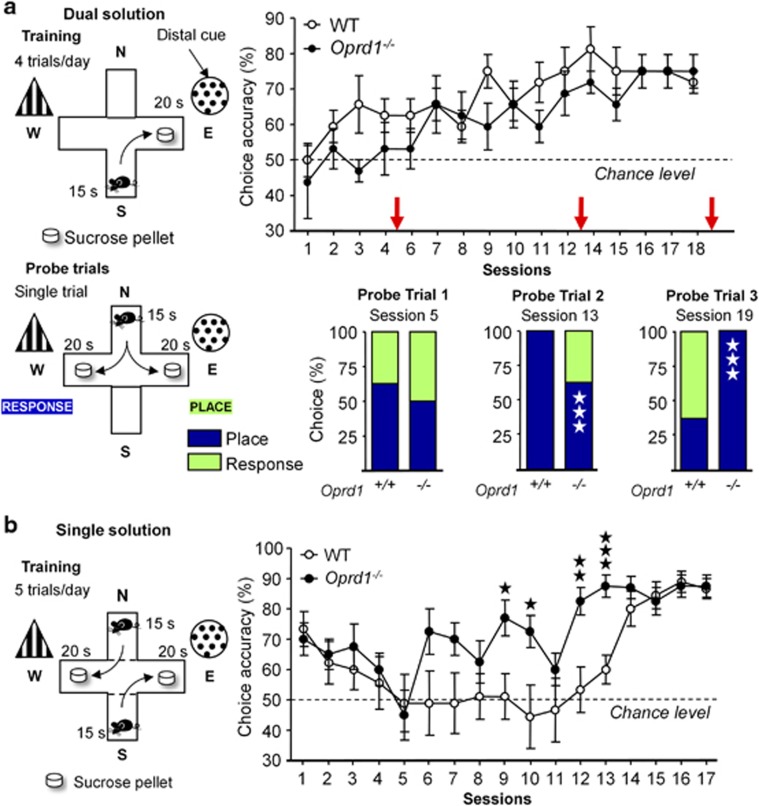
To further examine whether delta opioid receptors has a role in HPC-dependent behavior, and evaluate potential implication in striatum-dependent behavior, we tested WT and Oprd1−/− mice in two cross-maze tasks (Supplementary Information). We first used a dual-solution task, in which performance at early stages of training rely preferentially on a HPC-mediated allocentric strategy (experiment 3; place learning). Second, we used a single-solution response task (experiment 4), which solely requires a striatal-dependent egocentric strategy (response learning, see (Packard, 2009)).
In experiment 3 (WT—n=four females, four males; Oprd1−/−—n=three females, five males), mutant and WT animals similarly acquired the dual-solution cross-maze task, as observed by comparable choice accuracies along sessions (genotype: F1,12=2.46, NS; gender: F1,12<1; session: F15,180=5.26, p<0.0001; Figure 2a). Choice latency decreased over sessions as animals from both lines learned the task (genotype: F1,12=3.51, NS; gender: F1,12=5.65, p<0.05; session: F15,180=13.29, p<0.0001; Supplementary Figure S2). Performance on probe trials, though, revealed that 16 training sessions (probe trial 3) were needed for Oprd1−/− mice to adopt a place strategy, when 11 sessions (probe trial 2) were sufficient in controls (probe trial 1: χ2=2.92, NS; probe trial 2: χ2=46.91, p<0.0001; probe trial 3: χ2=53.63, p<0.001; Figure 2a). Thus, acquisition of an allocentric strategy to solve the cross-maze task was delayed in Oprd1−/− mice, consistent with the hypothesis of altered hippocampal function in these animals.
Figure 2.
Oprd1−/− mice show delayed acquisition of a place strategy in the dual-solution cross-maze task, and facilitated acquisition of a response strategy in the single-solution response task. (a) WT and mutant animals similarly acquire a dual-solution cross-maze task. Probe trials reveal, however, that mutant mice need more sessions (16 vs 11) to adopt an allocentric strategy in this task. (b) In contrast, Oprd1−/− mice develop an egocentric strategy more rapidly in the single-solution response task. Performance in cross-maze tasks is expressed as percentage of correct choices (mean±SEM). Three open stars: p<0.001 (Pearson's χ2 test). one solid star: p 0.05; two solid stars: p 0.01; Three solid stars: p<0.001 (one-way analysis of variance).
In experiment 4 (WT—n=five females, four males; Oprd1−/−—n=five females, three males), pretraining initially biased all animals towards a response strategy; their performance dropped, however, as they shifted rapidly to an early place strategy (spontaneous alternation). From there, Oprd1−/− mice acquired a proper response strategy more rapidly than controls, as shown by earlier increase in choice accuracy along sessions (genotype: F1,13=9.36, p<0.01; gender: F1,13=2.06, NS; session: F16,208=6.99, p<0.0001; Figure 2b). Choice latency decreased more rapidly over sessions in mutant compared with WT mice (genotype: F1,13=1.38, NS; gender: F1,13<1; session: F16,208=16.31, p<0.0001; session × genotype: F16,208=2.41, p<0.01; session × gender: F16,208=2.92, p<0.001; Supplementary Figure S2). Oprd1−/− mice, therefore, acquired the single-solution response task faster, suggesting that acquisition of a dorsal striatal-dependent response strategy was facilitated in mutant animals.
Together, results from cross-maze experiments support the hypothesis of deficient hippocampal function in Oprd1−/− mice, and suggest that, in contrast, striatal function is facilitated in these animals.
Oprd1 −/− Mice Show Enhanced Motor Skill Learning
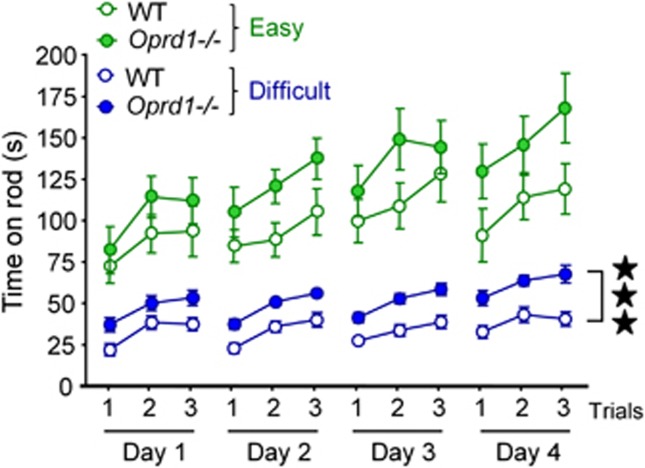
Because increased motivation for food may partially explain facilitation of striatal-mediated responses in the cross-maze experiments (Supplementary Information and Supplementary Figure S3), we further tested striatal-dependent learning under conditions that do not involve food seeking (experiment 5). We used a rotarod task in WT and mutant mice to assess skill motor learning, a form of procedural learning that was demonstrated to strongly rely on dorsal striatum functional integrity (Dang et al, 2006; Durieux et al, 2009). Mice were trained to run on an accelerating rotarod for 3 trials a day during 4 consecutive days, under either easy (WT—n=five females, five males; Oprd1−/−—n=five females, five males) or difficult (WT—n=seven females, seven males; Oprd1−/−—n=six females, six males) conditions (Supplementary Information and Figure 3). Oprd1−/− mice stayed significantly longer than WT animals on the rod, especially under ‘difficult' conditions (genotype: F1,38=9,76, p<0.01; gender: F1,38=1.00, NS; condition: F1,38=96.25, p<0.0001; session: F3,114=30.00, p<0.0001; trial: F2,76=176,82, p<0.0001; session × genotype: F3,114=3.30, p<0.05; session × condition: F3,114=12.10, p<0.0001; trial × genotype: F2,76=3.38, p<0.005; trial × condition: F2,76=16.12, p<0.0001). Indeed, mutant mice performed significantly better than controls in the ‘difficult' version of the task (genotype: F1,22=30.61, p<0.0001; gender: F1,22=2.81, NS; session: F3,66=3.27, p<0.05; trial: F2,44=17.48, p<0.0001; trial × session: F6,132=15.15, v<0.0001). Under those conditions, ameliorated performance in Oprd1−/− mice was detectable since the first day of training (genotype: F1,22=8.06, p<0.01; gender: F1,22<1; trial: F2,44=21.45, p<0.001). There was no significant difference in the ‘easy' version of the task (genotype: F1,16=2.92, NS; gender: F1,16=1.49, NS; session: F3,48=6.26, p<0.01; trial: F2,32=32.10, p<0.0001; session × trial: F6,96=9.95, p<0.0001), but a similar trend was observed. Therefore, striatal-dependent motor skill learning was facilitated in Oprd1−/− mice.
Figure 3.
Acquisition of a motor skill learning task is facilitated in Oprd1−/− mice. The average latency (mean±SEM) to fall from the accelerating rotarod is longer in Oprd1−/− as compared with WT mice. This difference reaches significance under ‘difficult' conditions. Three stars: p<0.001 (three-way analysis of variance).
Oprd1 −/− Mice Show Modified Expression of Key Signaling Genes in Dorsal HPC and Dorsal Striatum
To identify potential molecular and cellular mechanisms underlying impaired hippocampal and facilitated striatal function in Oprd1−/− mice, we quantified the expression of 67 genes in the dorsal HPC and the dorsal striatum (caudate putamen) using qRT-PCR (Table 2). Genes were selected as to represent key actors of GABA, glutamate, and monoamine signaling pathways (transporters, receptors, and receptor subunits, enzymes), or were known neuronal markers. We also analyzed the expression of these genes in the ventral striatum (Nucleus Accumbens) to test for regional specificity of striatal modifications in gene expression (Supplementary Table S2).
Table 2. Difference of Gene Expression Levels in the HPC and Caudate Putamen (Dorsal Striatum) of WT and Oprd1 −/− Mice.
|
Hippocampus |
Caudate putamen |
||||||
|---|---|---|---|---|---|---|---|
| Gene function | RefSeq | Gene title | Protein encoded | median±SEM | p value | median±SEM | p value |
| GABA signaling | |||||||
| NM_172890 | Slc6a11 | GAT4 | −1.01±0.19 | 0.884 | 1.42±0.15 | 0.031 | |
| NM_133661 | Slc6a12 | GAT2 | 1.81±0.10 | 0.000 | 1.12±0.18 | 0.250 | |
| Glutamate signaling | |||||||
| NM_177407 | Camk2a | CamKIIa | −1.59±0.28 | 0.057 | −1.83±0.27 | 0.000 | |
| NM_013540 | Gria2 | GluR2 | 1.13±0.08 | 0.579 | −1.47±0.13 | 0.005 | |
| NM_001177656 | Grin1 | GluN1 | −1.83±0.47 | 0.043 | −1.34±0.06 | 0.004 | |
| NM_008170 | Grin2a | GluN2A | −1.35±0.12 | 0.016 | −1.10±0.21 | 0.227 | |
| NM_008171 | Grin2b | GluN2B | −1.07±0.08 | 0.173 | 1.29±0.14 | 0.026 | |
| NM_016976 | Grm1 | mGluR1 | 1.33±0.10 | 0.003 | −1.01±0.04 | 0.977 | |
| NM_001013385 | Grm4 | mGluR4 | −1.05±0.13 | 0.535 | 1.27±0.03 | 0.000 | |
| NM_182993 | Slc17a7 | VGlut1 | −1.06±0.05 | 0.170 | 1.59±0.25 | 0.041 | |
| NM_009200 | Slc1a6 | EAAT4 | −1.35±0.10 | 0.015 | −1.05±0.57 | 0.276 | |
| Monoamine signaling | |||||||
| NM_009891 | Chat | CHAT | 1.61±0.17 | 0.009 | 1.00±0.24 | 0.506 | |
| NM_173740 | Maoa | MAOA | 1.29±0.10 | 0.010 | −1.17±0.08 | 0.008 | |
| NM_010484 | Slc6a4 | SERT | 1.13±0.40 | 0.487 | −1.54±0.18 | 0.017 | |
| Neuronal markers | |||||||
| NM_028755 | Arpp21 | ARPP21 | −1.23±0.12 | 0.011 | −1.30±0.06 | 0.003 | |
| NM_021399 | Bcl11b | CTIP2 | −1.42±0.16 | 0.031 | −1.53±0.13 | 0.002 | |
| NM_007699 | Chrm4 | M4R | 1.05±0.10 | 0.862 | −1.24±0.05 | 0.001 | |
| NM_199058 | Gpr6 | GPR6 | −1.90±0.27 | 0.006 | −1.52±0.11 | 0.002 | |
| NM_010471 | Hpca | HPCA | −1.26±0.15 | 0.033 | −1.15±0.09 | 0.048 | |
| NM_011866 | Pde10a | PDE10A | −1.40±0.04 | 0.000 | −1.10±0.09 | 0.216 | |
| NM_018863 | Pdyn | PDyn | −1.45±0.17 | 0.021 | 1.35±0.14 | 0.050 | |
| NM_001002927 | Penk | PEnk | −1.50±0.18 | 0.041 | 1.03±0.15 | 0.490 | |
| NM_009311 | Tac1 | Subst P | −1.21±0.29 | 0.008 | −1.37±0.07 | 0.002 | |
A total of 67 genes encoding actors of GABA, glutamate or monoamine signaling, and neuronal markers (see Supplementary Table S2), were tested and only genes showing a significant fold-change difference are shown. Data are presented as fold-change Oprd1−/− vs WT mice (median±SEM). Student's t-tests were performed on transformed data (Supplementary Information) to determine whether fold changes differed from 0 (no regulation; corresponds to±1 in table). Significant regulations are highlighted in bold.
In the HPC, Oprd1−/− mice showed altered expression of 16 genes, coding for GABA and glutamate transporters, receptors or receptor subunits (up: Slc6a12, Grm1; down: Slc1a6, Grin1, and Grin2a), enzymes involved in monoamine metabolism (up: Chat, Maoa) and several neuronal markers (up: Arpp21, Bcl11, Foxp1, GPR6, Hpca, Pde10a, Pdyn, Penk, and Tac1). Within the dorsal striatum (caudate putamen), the expression of 12 genes was different in Oprd1−/− as compared with WT mice, which code for glutamate transporters, receptor subunits, and an element of the postsynaptic signaling cascade (up: Slc1a3, Grin2b, and Slc17a7; down: Camk2a; Grin1, and Gria2), a monoamine transporter (up: Slc6a3), and neuronal markers (down: Chrm4, Tac1, Gpr6, Bcl11b, and Arpp21). Delta receptor deletion produced differential changes of gene expression between the dorsal and ventral (nucleus accumbens) striatal regions (Supplementary Table S2), stressing the regional specificity of these modifications. Together, gene expression data reveal altered expression of several key genes for neurotransmission. Modifications observed in the striatum overall suggested that striatal dysfunction may result from an imbalance of striatonigral/striatopallidal output, which we tested further.
Genetic and Pharmacological Inactivation of Delta Opioid Receptors Increases Sensitivity to the Locomotor Effects of D1 Receptor Agonist SKF-81297
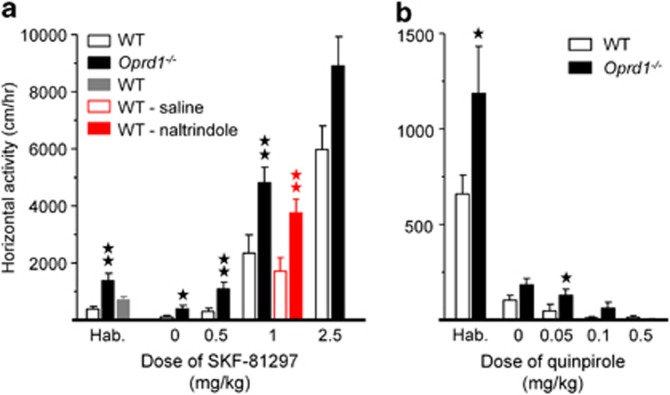
Because modified striatal function had not been reported earlier in Oprd1−/− mice, we examined the reactivity of striatonigral and striatopallidal output pathways. Striatonigral medium spiny neurons preferentially express D1 dopamine receptors, whereas striatopallidal neurons express predominantly D2 dopamine receptors. We thus assessed stimulant locomotor effects of the dopamine D1/D5-type receptor agonist SKF-81297 in WT and Oprd1−/− mice as well as in WT mice treated with either saline or naltrindole. We also tested the inhibitory locomotor effects of D2/D3-type receptor agonist quinpirole in WT and Oprd1−/− animals (experiment 6; animal numbers in Supplementary Table S3).
Consistent with our previous observations (Filliol et al, 2000), locomotor activity during habituation was higher in mutant animals as compared with controls (SKF-81297, genotype: F1,62=13.55, p<0.0001; gender: F1,62=5.33, p<0.05—Quinpirole, genotype: F1,46=4.87, p<0.05; gender: F1,46<1, NS; Figure 4a and b). Mutant mice were more sensitive to the stimulant effects of SKF-81297 on locomotor activity than WT animals (genotype: F1,50=21.10, p<0.0001; gender: F1,50=16.20, p<0.0001; dose: F1,50=90.08, p<0.0001; genotype × dose: F1,50=3.16, p<0.05; gender × dose: F1,50=6.46, p<0.001; Figure 4a). Similarly, pretreatment with systemic naltrindole (0.3 mg/kg) facilitated the stimulant effects of SKF-81297 on locomotor activity in WT animals (treatment: F1,16=11.76, p<0.01; gender: F1,16=6.45, p<0.05; Figure 4a; see also Supplementary Figure S4). Besides, Oprd1−/− mice remained slightly more active than controls under quinpirole treatment (genotype: F1,54=4.45, p<0.05; gender: F1,54<1, NS; dose: F1,54=9.16, p<0.0001; Figure 4b). These data show that lack of delta opioid receptors modifies the striatonigral/striatopallidal balance in favor of the striatonigral output.
Figure 4.
Locomotor activity under pharmacological challenge by dopamine receptor agonists is biased towards prominent activity of the striatonigral pathway in Oprd1−/− mice. (a) Mutant animals were more sensitive to the stimulant locomotor effects of the D1/D5 dopamine receptor agonist SKF-81297, for doses ranging from 0.5–2.5 mg/kg. WT mice injected with naltrindole (0.3 mg/kg) similarly showed increased sensitivity to the locomotor effects of SKF-81297 (1 mg/kg). (b) Oprd1−/− mice tended to be less sensitive to the inhibitory effects of the D2/D3 dopamine receptor agonist quinpirole on locomotion, at doses from 0.05–0.5 mg/kg. Mutant mice were more active during habituation than WT animals, in agreement with previous results (Filliol et al, 2000). Data are presented as distance traveled (mean±SEM) per hour. One star: p 0.05; 2 stars: p 0.01 (one-way analysis of variance). Hab.: habituation.
DISCUSSION
For the first time we report that disruption of delta opioid receptor function modifies learning and memory abilities in mice. We had previously observed that delta opioid receptor knockout mice show reduced ability to form drug-context but not drug-cue associations in a place-conditioning task, suggesting that hippocampal-dependent learning may be deficient in these animals (Le Merrer et al, 2012b; Le Merrer et al, 2011). Here, we demonstrate a key role for delta receptors in modulating HPC- and striatum-dependent behaviors, and further reveal potential neural substrates engaging delta receptors in these processes.
In a novel object recognition task, Oprd1−/− mice failed to show preference for the displaced object during place phase. This was unlikely due to insufficient overall exploration of objects, as mutant mice spent equal time visiting the objects, and approached these objects as often as WT controls. Specific deficit in object place recognition has been reported after hippocampal lesions (Ennaceur et al, 1997; Mumby et al, 2002; Oliveira et al, 2010), suggesting deficient hippocampal function in Oprd1−/− mice. Peripheral injection of the delta opioid receptor antagonist naltrindole at a low dose previously shown to blunt morphine-induced place conditioning (Chefer and Shippenberg, 2009) similarly impaired recognition of object location in WT mice. Therefore, the deficit observed in Oprd1−/− animals did not result from developmental adaptations. Mutant mice otherwise visited the novel object more often during the object phase (experiment 1). Not only is this observation compatible with impaired hippocampal function (Oliveira et al, 2010), but also the facilitated novel object recognition suggests that novelty is more attractive to these animals (Ennaceur, 2010). In the cross-maze, Oprd1−/− mice performed as their WT counterparts to acquire a dual-solution task, but needed more time to adopt an allocentric strategy. Delayed acquisition of spatial learning in the cross-maze is a landmark of hippocampal dysfunction in rats and mice (Deipolyi et al, 2008; Packard, 2009; Packard and McGaugh, 1996), further confirming the hypothesis of altered hippocampal function in mutants.
Impaired performance of Oprd1−/− mice in HPC-dependent tasks may result from disrupted delta opioid receptor signaling in the HPC itself, or at multiple levels in the related brain circuitry, where these receptors are expressed (entorhinal and prefrontal cortices, subiculum,and septal area). Our behavioral results support local alteration of hippocampal function. Accordingly, anatomical and pharmacological data show that local delta opioid receptors can modulate hippocampal activity. These receptors are abundantly expressed in the HPC (Le Merrer et al, 2009), primarily in GABAergic interneurons (Rezai et al, 2012; Scherrer et al, 2006; Svoboda et al, 1999), and their pharmacological activation on hippocampal slices was found to disinhibit principal glutamatergic cells via interneuron inhibition (Lupica, 1995). Moreover, enkephalins, among endogenous ligands of delta receptors, are released in the lateral perforant path, where delta receptor activation is required for high frequency-induced LTP (Bramham et al, 1991; Chavkin et al, 1985). Therefore, inactivation of delta opioid receptors in the HPC might prevent their endogenous ligands from inhibiting GABAergic interneurons, favoring the inhibition of principal cells, and thus reducing the probability for LTP, a plausible mechanism for impaired hippocampal function in Oprd1−/− mice.
Analysis of qRT-PCR data suggests further mechanisms through which long-term disruption of delta opioid receptor signaling may locally alter hippocampal function. Transcript levels of Grin1 and Grin2a, coding for GluN1 (NR1) and GluN2A (NR2A) subunits of NMDA glutamate receptors, respectively, were low in Oprd1−/− mice. Decreased expression of these two subunits may compromise spatial learning in mutant mice (Bannerman et al, 2008; Korotkova et al, 2010; Place et al, 2012). We also detected low hippocampal mRNA levels for several neuronal markers, among which Pdyn, Penk, and Tac1, encoding dynorphin, enkephalin, and substance P, respectively, and reduced the expression of these neuropeptides may also participate in altering hippocampal activity (McDermott and Schrader, 2011; McQuiston, 2011; Ogier et al, 2008).
In the cross-maze, Oprd1−/− mice developed a response strategy more rapidly than WT animals under a single-solution response paradigm. This result suggests that control of response learning is facilitated in mutants (Packard, 2009; Packard and McGaugh, 1996), most likely via the lateral dorsal striatum (Lovinger, 2010). Increased performance of mutant mice in the single-solution response task, however, could also result from their increased motivation to gain a food reward (Experiments 7–8, (Le Merrer et al, 2011)). We thus further assessed striatal-dependent behavior using a motor skill learning task, which does not engage food seeking. Oprd1−/− mice indeed performed better than controls on the accelerating rotarod. Lateral dorsal striatal circuits are critically involved during motor skill learning (Lovinger, 2010; Yin et al, 2009). Therefore, our data concur to indicate that dorsal striatal function is facilitated in mice lacking delta opioid receptors.
Decreased hippocampal activity is known to facilitate striatal-based skills when the two systems compete to drive behavior (Middei et al, 2004; Schroeder et al, 2002). Therefore, impaired hippocampal function in Oprd1−/− mice may account for their ameliorated performance in response and skill learning tasks, via altered HPC-striatum balance in favor of the striatum (Ciamei and Morton, 2009). Meanwhile, the local absence of delta opioid receptors likely further contributes to modify striatal function. In this region, delta receptors are prominently expressed in cholinergic interneurons and inhibit their activity (Gazyakan et al, 2000; Le Moine et al, 1994; Scherrer et al, 2006). A small proportion of these receptors is also detected in GABAergic (inter)neurons (Scherrer et al, 2006) or presynaptic glutamatergic terminals (Jiang and North, 1992). Consequently, delta receptor deletion has multiple potential consequences on striatal function. Receptor knockout in cholinergic interneurons should facilitate their depolarization and subsequent acetylcholine release. Disinhibiting cholinergic interneurons, however, was demonstrated to bias striatal networks towards increased striatopallidal activity (Ding et al, 2010), which is not consistent with present behavioral results. Lack of delta opioid receptor tone in GABAergic interneurons and/or glutamatergic terminals may, therefore, underlie facilitated striatal function.
QRT-PCR data from Oprd1−/− mice caudate putamen samples suggest several molecular mechanisms through, which delta receptor deletion could affect striatal output neuron activity. Our gene expression analysis reveals potential molecular abnormalities in both striatonigral and striatopallidal pathways. On one hand, low mRNA levels of Camk2a and chrm4 (coding for the alpha isoform of the calcium/calmodulin-dependent protein kinase II—a critical effector of muscarinic receptor 4—and the cholinergic muscarinic receptor 4, respectively), and high mRNA levels of grin2b (NR2B subunit of NMDA glutamate receptors) may potentiate the striatonigral output (Gomeza et al, 1999; Guo et al, 2010; Jocoy et al, 2011; Tzavara et al, 2004). On the other hand, increased expression of Grm4 (metabotropic glutamate receptors mGluR4) and Pdyn (dynorphin and related peptides), and decreased expression of Tac1 (substance P) and Gpr6 (G-protein-coupled receptor GPR6) would rather blunt striatopallidal activity (Govindaiah et al, 2010; Hopkins et al, 2009; Lobo et al, 2007; Perreault et al, 2007). The relative weights of striatonigral and striatopallidal outputs, therefore, may be modified in Oprd1−/− mice.
In order to test the reactivity of the striatonigral and striatopalllidal pathways, we challenged WT and Oprd1−/− mice with agonists of the D1/D5 or D2/D3 dopamine receptors (which expression levels are unchanged in mutant mice). We observed higher sensitivity to D1/D5 activation in Oprd1−/− mice, providing pharmacological evidence that striatal function in these animals is tilted towards facilitated striatonigral output (Figure 5). Naltrindole injection in WT mice similarly produced a facilitation of the locomotor effects of a D1/D5 dopamine receptor agonist. Thus, disruption of delta opioid receptor function facilitates striatonigral activity independently from neurodevelopmental adaptations. Remarkably, the overall behavioral phenotype of Oprd1−/− mice recapitulates symptoms associated with prominent activity of the striatonigral pathway (Durieux et al, 2009). These symptoms encompass increased object exploration and reactivity to novelty, increased locomotor activity with deficient habituation to contexts, increased early performance in a rotarod skill learning task, and increased food and drug seeking (present study, Le Merrer et al, 2011; Roberts et al, 2001). Hence, facilitated acquisition of striatal-dependent tasks in Oprd1−/− animals is a likely consequence of potentiated striatonigral activity.
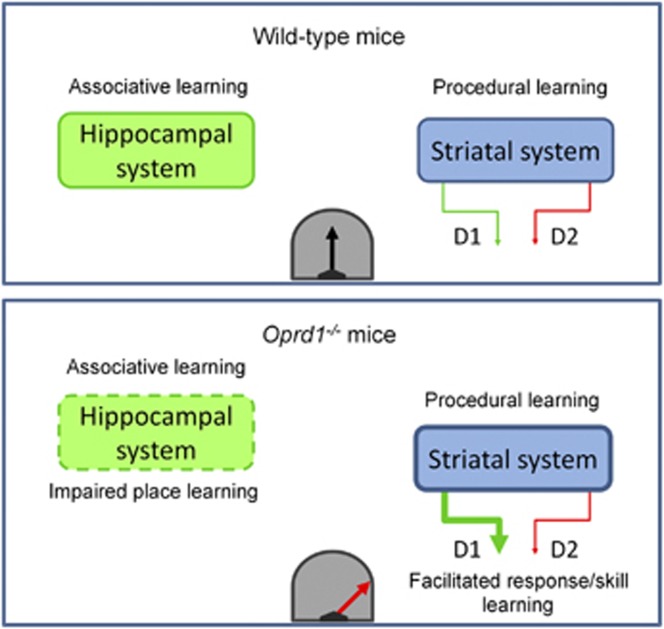
Figure 5.
HPC-striatum balance is tilted towards facilitated striatal function in mice lacking delta opioid receptors. This imbalance is revealed by impaired performance in HPC-dependent tasks (associative learning), but facilitated acquisition of striatum-dependent tasks (procedural learning) in these animals. Facilitated striatal function in Oprd1−/− mice is a likely consequence of potentiated striatonigral activity.
In conclusion, the present study demonstrates that both genetic and pharmacological invalidation of delta opioid receptor function impairs hippocampal-dependent behaviors, and facilitates striatal-dependent performance. These findings provide a basis that may explain other behavioral deficits previously reported for Oprd1−/− animals. Compromised hippocampal function can account for their deficit in place preference conditioning when spatial cues are prominent (Chefer and Shippenberg, 2009; Le Merrer et al, 2012b; Le Merrer et al, 2011), whereas enhanced dorsal striatal activity may underlie increased motor impulsivity in these mutants (Olmstead et al, 2009). Further, the transcriptional modifications observed in the HPC and striatum of Oprd1−/− mice are indicative of potential molecular mechanisms through, which delta receptor activity may influence neurotransmission in these brain regions. Delta opioid receptor-associated activity, therefore, represents a promising candidate pathway for the treatment of neurodegenerative or psychiatric diseases affecting hippocampal and/or striatal function, such as Alzheimer or Parkinson disease, or obsessive/compulsive disorder, and addiction.
Acknowledgments
We thank A Matifas, G Duval and D Memetov for animal care. This work was supported by the Center National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM) and Université de Strasbourg. We also thank the National Institutes of Health (NIAAA no.16658, NIDA no.16768, NIDA no.005010) for financial support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, et al. NMDA receptor subunit NR2A is required for rapidly acquired spatial working memory but not incremental spatial reference memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Milgram NW, Srebro B. Delta opioid receptor activation is required to induce LTP of synaptic transmission in the lateral perforant path in vivo. Brain Res. 1991;567:42–50. doi: 10.1016/0006-8993(91)91433-2. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Shoemaker WJ, McGinty JF, Bayon A, Bloom FE. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci. 1985;5:808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciamei A, Morton AJ. Progressive imbalance in the interaction between spatial and procedural memory systems in the R6/2 mouse model of Huntington's disease. Neurobiol Learn Mem. 2009;92:417–428. doi: 10.1016/j.nlm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci USA. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deipolyi AR, Fang S, Palop JJ, Yu GQ, Wang X, Mucke L. Altered navigational strategy use and visuospatial deficits in hAPP transgenic mice. Neurobiol Aging. 2008;29:253–266. doi: 10.1016/j.neurobiolaging.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Ding JB, Guzman JN, Peterson JD, Goldberg JA, Surmeier DJ. Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron. 2010;67:294–307. doi: 10.1016/j.neuron.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113:509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gazyakan E, Hennegriff M, Haaf A, Landwehrmeyer GB, Feuerstein TJ, Jackisch R. Characterization of opioid receptor types modulating acetylcholine release in septal regions of the rat brain. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:32–40. doi: 10.1007/s002100000253. [DOI] [PubMed] [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, et al. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaiah G, Wang Y, Cox CL. Substance P selectively modulates GABA(A) receptor-mediated synaptic transmission in striatal cholinergic interneurons. Neuropharmacology. 2010;58:413–422. doi: 10.1016/j.neuropharm.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Guo ML, Mao LM, Wang JQ. Modulation of M4 muscarinic acetylcholine receptors by interacting proteins. Neurosci Bull. 2010;26:469–473. doi: 10.1007/s12264-010-0933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR, Lindsley CW, Niswender CM. mGluR4-positive allosteric modulation as potential treatment for Parkinson's disease. Future Med Chem. 2009;1:501–513. doi: 10.4155/fmc.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Matthes HW, Valjent E, Sanchez-Blazquez P, Rodriguez-Diaz M, Garzon J, et al. Lack of dependence and rewarding effects of deltorphin II in mu-opioid receptor-deficient mice. Eur J Neurosci. 2001;13:153–161. [PubMed] [Google Scholar]
- Ilyutchenok RY, Dubrovina NI. Memory retrieval enhancement by kappa opioid agonist and mu, delta antagonists. Pharmacol Biochem Behav. 1995;52:683–687. doi: 10.1016/0091-3057(95)00099-i. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocoy EL, Andre VM, Cummings DM, Rao SP, Wu N, Ramsey AJ, et al. Dissecting the contribution of individual receptor subunits to the enhancement of N-methyl-d-aspartate currents by dopamine D1 receptor activation in striatum. Front Syst Neurosci. 2011;5:28. doi: 10.3389/fnsys.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol. 2003;14:509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, et al. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012a;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012b;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Kieffer B, Gaveriaux-Ruff C, Befort K, Bloch B. Delta-opioid receptor gene expression in the mouse forebrain: localization in cholinergic neurons of the striatum. Neuroscience. 1994;62:635–640. doi: 10.1016/0306-4522(94)90464-2. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Cui Y, Ostlund SB, Balleine BW, Yang XW. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–1397. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR. Delta and mu enkephalins inhibit spontaneous GABA-mediated IPSCs via a cyclic AMP-independent mechanism in the rat hippocampus. J Neurosci. 1995;15:737–749. doi: 10.1523/JNEUROSCI.15-01-00737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, Olson K, Hilston C. Opposite effects of Met-enkephalin and Leu-enkephalin on a discriminated shock-escape task. Behav Neurosci. 1984;98:487–495. doi: 10.1037//0735-7044.98.3.487. [DOI] [PubMed] [Google Scholar]
- McDermott CM, Schrader LA. Activation of kappa opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J Physiol. 2011;589:3517–3532. doi: 10.1113/jphysiol.2011.211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR. Mu opioid receptor activation normalizes temporo-ammonic pathway driven inhibition in hippocampal CA1. Neuropharmacology. 2011;60:472–479. doi: 10.1016/j.neuropharm.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middei S, Geracitano R, Caprioli A, Mercuri N, Ammassari-Teule M. Preserved fronto-striatal plasticity and enhanced procedural learning in a transgenic mouse model of Alzheimer's disease overexpressing mutant hAPPswe. Learn Mem. 2004;11:447–452. doi: 10.1101/lm.80604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier R, Wrobel LJ, Raggenbass M. Action of tachykinins in the hippocampus: facilitation of inhibitory drive to GABAergic interneurons. Neuroscience. 2008;156:527–536. doi: 10.1016/j.neuroscience.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Hawk JD, Abel T, Havekes R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn Mem. 2010;17:155–160. doi: 10.1101/lm.1625310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Ouagazzal AM, Kieffer BL. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One. 2009;4:e4410. doi: 10.1371/journal.pone.0004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Exhumed from thought: basal ganglia and response learning in the plus-maze. Behav Brain Res. 2009;199:24–31. doi: 10.1016/j.bbr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Pavone F, Populin R, Castellano C, Kreil G, Melchiorri P. Deltorphin, a naturally occurring peptide with high selectivity for delta opioid receptors, improves memory consolidation in two inbred strains of mice. Peptides. 1990;11:591–594. doi: 10.1016/0196-9781(90)90063-b. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Scattolon S, Wang Y, Szechtman H, Foster JA. Cotreatment with the kappa opioid agonist U69593 enhances locomotor sensitization to the D2/D3 dopamine agonist quinpirole and alters dopamine D2 receptor and prodynorphin mRNA expression in rats. Psychopharmacology (Berl) 2007;194:485–496. doi: 10.1007/s00213-007-0855-3. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place R, Lykken C, Beer Z, Suh J, McHugh TJ, Tonegawa S, et al. NMDA signaling in CA1 mediates selectively the spatial component of episodic memory. Learn Mem. 2012;19:164–169. doi: 10.1101/lm.025254.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai X, Faget L, Bednarek E, Schwab Y, Kieffer BL, Massotte D. Mouse delta opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell Mol Neurobiol. 2012;32:509–516. doi: 10.1007/s10571-011-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, et al. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–1256. [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Wingard JC, Packard MG. Post-training reversible inactivation of hippocampus reveals interference between memory systems. Hippocampus. 2002;12:280–284. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Martinez JL. ICI 174,864, a selective delta opioid antagonist, reverses the learning impairment produced by [leu]enkephalin. Psychopharmacology (Berl) 1990;100:102–109. doi: 10.1007/BF02245798. [DOI] [PubMed] [Google Scholar]
- Svoboda KR, Adams CE, Lupica CR. Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J Neurosci. 1999;19:85–95. doi: 10.1523/JNEUROSCI.19-01-00085.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, et al. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. Faseb J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Ukai M, Takada A, Sasaki Y, Kameyama T. Stimulation of delta1- and delta2-opioid receptors produces amnesia in mice. Eur J Pharmacol. 1997;338:1–6. doi: 10.1016/s0014-2999(97)01310-1. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Yang S, Kawamura Y, Yoshikawa M. Effect of rubiscolin, a delta opioid peptide derived from Rubisco, on memory consolidation. Peptides. 2003;24:325–328. doi: 10.1016/s0196-9781(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.