Abstract
Smaller hippocampal volume is reported in major depressive disorder (MDD). We hypothesize that it may be related to fewer granule neurons (GN) in the dentate gyrus (DG), a defect possibly reversible with antidepressants. We studied age-, sex-, and postmortem interval-matched groups: no major psychopathology (controls); unmedicated-MDD; and MDD treated with serotonin reuptake inhibitors (MDD*SSRI) or tricyclics (MDD*TCA). Frozen right hippocampi were fixed, sectioned (50 μm), immunostained with neuronal nuclear marker (NeuN), and counterstained with hematoxylin. GN and glial number, and DG and granule cell layer (GCL) volumes were stereologically estimated. Fewer GNs in the anterior DG were present in unmedicated-MDDs compared with controls (p=0.013). Younger age of MDD onset correlated with fewer GNs (p=0.021). Unmedicated-MDDs had fewer mid-DG GNs than MDD*SSRIs (p=0.028) and controls (p=0.032). Anterior GCL glial number did not differ between groups. Anterior/mid GCL volume was smaller in unmedicated-MDDs vs controls (p=0.008) and larger in MDD*SSRIs vs unmedicated-MDDs (p<0.001), MDD*TCAs (p<0.001), and controls (p<0.001). Anterior GCL volume and GN number (r=0.594, p=0.001), and mid DG volume and GN number (r=0.398, p=0.044) were correlated. Anterior DG capillary density correlated with GN number (p=0.027), and with GCL (p=0.024) and DG (r=0.400, p=0.047) volumes. Posterior DG volume and GN number did not differ between groups. Fewer GNs in unmedicated-MDD without fewer neuronal progenitor cells, as previously reported, suggests a cell maturation or survival defect, perhaps related to MDD duration. This may contribute to a smaller hippocampus and is potentially reversed by SSRIs. Postmortem studies are correlative and animal studies are needed to test implied causal relationships.
Keywords: NeuN, postmortem, stereology, plasticity, psychopharmacology, serotonin
INTRODUCTION
Major depressive disorder (MDD) is the second leading cause of disability adjusted life years (DALYs) in those aged 15–44 years and affects about 120 million people worldwide. MDD is projected to be the second biggest cause of DALYs for all ages by 2020 and the leading cause of disease burden worldwide by 2030 (World Health Organization, 2012). Despite its growing importance, the pathogenesis of MDD remains unclear.
Smaller hippocampal volume in MDD has been reported, although there are conflicting reports (see meta-analysis by McKinnon et al, 2009). Disease duration and treatment status contribute to the findings in both recurrent and first episode MDD (McKinnon et al, 2009). Seven studies of only first episode MDD, found smaller hippocampal volume bilaterally, with sex and disease duration effects shown in some but not all studies (Cole et al, 2011). Smaller hippocampal volume may precede MDD onset or it may develop shortly thereafter. Assuming longer exposure to MDD is correlated with hippocampal volume loss, identifying that the structural contributors to hippocampal volume reduction may clarify the pathogenesis of MDD.
Abnormal neurogenesis is hypothesized in MDD pathogenesis (Kempermann and Kronenberg, 2003). Neurogenesis impairment has been reported in rodent stress-induced models of depression (Pham et al, 2003) and there are consistent reports of smaller dentate gyrus (DG) and cornu ammonis 3 (CA3) subfield volumes in patients with post-traumatic stress disorder, with sparing of other hippocampal subfields (Wang et al, 2010). Determinants of hippocampal volume in MDD are not known. Like others (Reif et al, 2006), we did not detect fewer mitotic cells (Boldrini et al, 2009) or neural progenitor cells (NPCs) in the DG postmortem (Boldrini et al, 2009, 2012) in untreated MDD subjects (<58–60 y old) compared to those with no major psychopathology. Notably, blocking (Santarelli et al, 2003) or blunting (Vollmayr et al, 2003) hippocampal proliferation in adulthood is not sufficient to induce depression-like behavior in mice. Therefore, deficits in an early phase of adult neurogenesis may not explain MRI findings of a smaller hippocampus in MDD.
We therefore sought to determine whether fewer mature DG granule neurons (GNs) contribute to smaller hippocampal volume in MDD, and whether this effect may be appear to be reversed in antidepressant-treated MDD. We compared GN number in the DG of antidepressant-treated and untreated MDD subjects (MDDs), with subjects with no major psychopathology (control group) (Boldrini et al, 2009, 2012). We further compared GN number and DG volume between MDDs treated with serotonin reuptake inhibitors (MDD*SSRI) and tricyclics (MDD*TCA), because SSRIs are serotonergic, and TCAs affect both serotonin and norepinephrine reuptake.
A functional dissociation of the anterior and posterior hippocampus exists in rats (Bannerman et al, 2004) and non-human primates (Strange and Dolan, 2001). The anterior hippocampus in primates (Thierry et al, 2000) and the corresponding ventral hippocampus in rodents (Risold and Swanson, 1996) have connections with prefrontal cortex and amygdala and are implicated in emotion regulation (Nettles et al, 2000). The posterior primate hippocampus and the dorsal rodent hippocampus are mainly implicated in memory (Bannerman et al, 2004; Risold and Swanson, 1996). The role of the anterior hippocampus (Sahay and Hen, 2007) in emotion regulation would be tied more closely to neurogenesis if effects on neurogenesis were confined to anterior DG. We found that antidepressant-treated MDDs have more mitotic cells and NPCs selectively in the anterior DG (Boldrini et al, 2009, 2012). Therefore, we hypothesized that changes in GN number associated with MDD or antidepressant treatment will be localized to the anterior DG.
MATERIALS AND METHODS
Brain Collection
Tissue obtained from the Macedonian/New York State Psychiatric Institute Brain Collection was studied with IRB approval. At autopsy, 2 cm-thick coronal blocks of the right hemisphere were frozen in dichlorodifluoromethane (−30 °C) and stored at −80 °C. The left hemisphere was fixed. Selected brain areas were used for neuropathology, and cerebellar tissue for brain pH and toxicology (see Boldrini et al, 2012 for details). All cases and the control group underwent psychological autopsy, as described elsewhere (Boldrini et al, 2012; Kelly and Mann, 1996).
Subjects
We studied the right hippocampus from 17 subjects with no Axis I or Axis II psychiatric diagnosis (controls), 15 untreated MDDs, and 10 antidepressant-treated MDDs, of whom five MDDs were treated with tricyclics (MDD*TCA) and five MDDs treated with selective serotonin reuptake inhibitors (MDD*SSRI). Treated subjects were prescribed antidepressants within 3 months of death and had positive brain toxicology for the antidepressant at the time of death. Groups did not differ on demographic variables (Table 1) such as age or sex, and postmortem interval (PMI).
Table 1. Demographic and Clinical Characteristics of Subjects.
| Group | Age (years) | PMI (h) | Brain pH | Sex (M:F) | Suicide (n) | Cause of Death | Axis I | Toxicology (n) |
|---|---|---|---|---|---|---|---|---|
| NC (N=17) | 44±4 | 15.4±1.1 | 6.00±0.09 | 10:7 | 0 | Cardiovascular (9) Trauma (6) Pulmonary (1) Gastrointestinal (1) | None (17) | None (10) Lidocaine (4) Benzodiazepine (1) Theophylline (1) Antiarrhythmic (1) |
| MDD (N=15) | 51±5 | 13.4±1.9 | 6.49±0.08 | 9:6 | 12 | Suicide (12): Poisoning (3) Hanging (3) Jumping (2) Gunshot (2) Drowning (2) Nonsuicide (3): Respiratory failure (1) Gastrointestinal (1) Trauma (1) | MDD (15) | None (14) Lidocaine (1) Benzodiazepine (1) |
| MDD*SSRI (N=5) | 40±7 | 17.2±3.4 | 6.49±0.20 | 3:2 | 4 | Suicide (4): Jumping (2) Hanging (1) Trauma (1) Nonsuicide (1): Respiratory failure (1) | MDD (4) MDD+ Bul/Anor (1) | Fluoxetine (3) Sertraline (2) |
| MDD*TCA (N=5) | 54±10 | 12.4±2.6 | 6.40±0.05 | 2:3 | 2 | Suicide (2): Jumping (2) Nonsuicide(3): Cardiac (2) Poisoning (1) | MDD (4) MDD+ Bul/Anor (1) | Nortriptyline (2) Clomipramine (2) Mianserin (1) |
Inclusion criteria for depressed subjects were history of and a depressive episode within 4 months before death; unmedicated depressed subjects (MDD) had negative toxicology and no psychotropic drug prescription in the last 3 months of life; antidepressant-treated MDDs had toxicology positive for SSRIs (MDD*SSRI) or TCAs (MDD*TCA) and same drug prescription for at least 3 months before death; controls (NC) died by accident or sudden natural causes, had no psychotropic drug prescription or psychiatric history, and negative toxicology for psychotropic drugs. Exclusion criteria for all groups were: alcohol or drug abuse or dependence, positive toxicology for alcohol or drugs of abuse, mental retardation, AIDS, positive brain neuropathology, undetermined death, resuscitation with prolonged (>10 min) hypoxia. Among untreated MDDs, 11 had no lifetime antidepressant prescription, one had fluoxetine prescription 3 years before death (lasting 4 months); one had perphenazine prescription 18 years before death (lasting 2 months); one had paroxetine and amitriptyline prescription 1 year before death (lasting 1 month); one had amitriptyline and haloperidol prescription 14 years before death (lasting 4 years). We have no information about past treatment compliance.
Tissue Preparation
The hippocampal formation was dissected with a single-edge blade from two or three consecutive frozen coronal blocks of the right hemisphere, fixed in 4% paraformaldehyde (4 °C for 7 days) (Boldrini et al, 2009), cryoprotected in 30% sucrose, sectioned coronally at 50 μm on a freezing microtome (Microm HM 440E, Walldorf, Germany), and stored in 36-well boxes at −20 °C in cryoprotectant (30% ethylene glycol in 0.1 M phosphate buffer). At the time of sectioning, sections at 1 mm intervals were stained with cresyl violet and numbered.
Immunohistochemistry
Sections were immunostained at 2 mm intervals with anti-NeuN mouse monoclonal antibody (1 : 100 000; Chemicon, Temecula, CA) with a modification of our previously used protocol (Boldrini et al, 2009) that darkly stains both nuclei and cytoplasm, rendering the densely packed GNs uncountable (Figure 1 in Boldrini et al, 2009). In the modified assay, we used a biotinylated primary antibody, instead of a biotinylated secondary antibody and obtained lightly stained, clearly defined GN nuclei, amenable to accurate counting. NeuN-immunolabeled sections were stained with hematoxylin to identify glial nuclei in the DG (Figure 1).
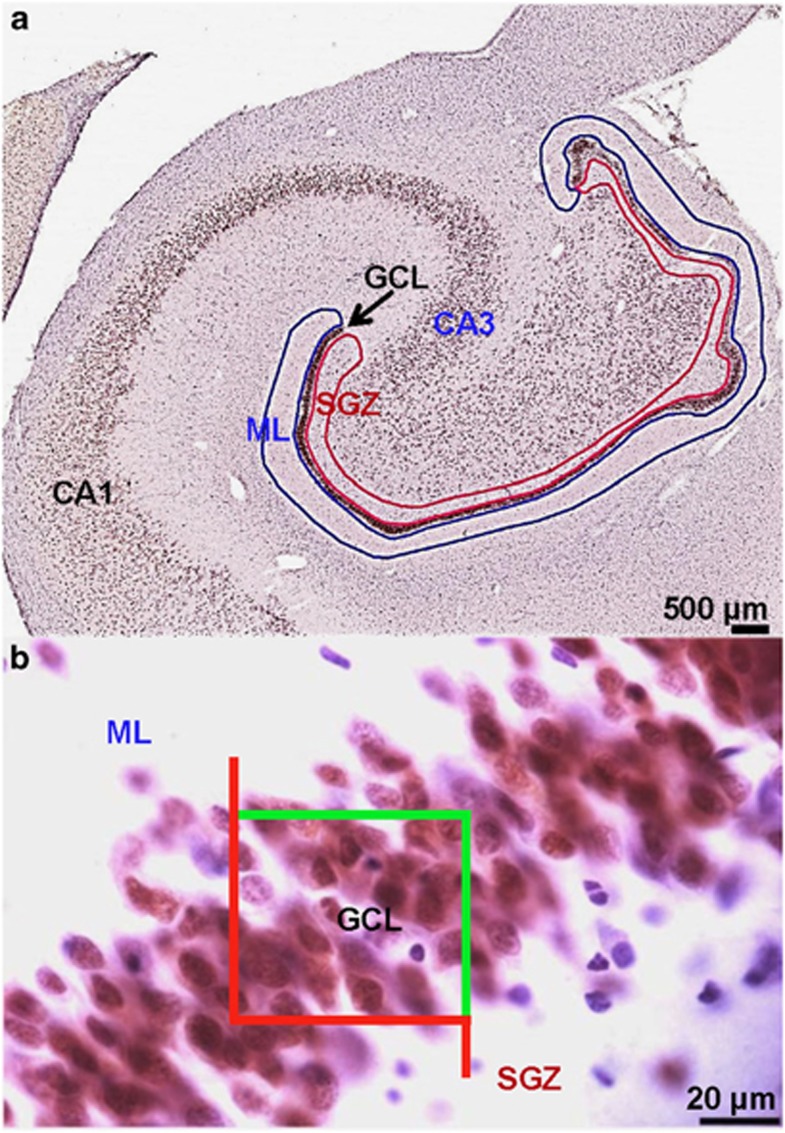
Figure 1.
Granule neurons are identified by immunocytochemistry and cell number and region volumes were estimated by stereology. (a) Neuronal nuclear marker NeuN labels granule neurons (GNs) in the dentate gyrus (DG) and neurons in the hilus and cornu ammonis (CA) regions of the human hippocampus. The region of interest identified for GN counting by stereology is the granule cell layer (GCL; indicated by arrow); volume measures were taken for the GCL as well as the full DG, including also the subgranular zone (SGZ, red) and molecular layer (ML, blue). DG and GCL area were outlined on coronal human hippocampal sections at regular interval along the anterior-posterior axis to estimate DG volume. The anterior, mid and posterior DG was identified as follows: the anterior DG courses along the optic tract for ∼15 mm from the center of the posterior margin of the anterior commissure to the end of the optic tract, including medially the uncal hippocampus and laterally the anterior body. The mid DG spans ∼9 mm to the end of the lateral geniculate. The posterior DG starts when the lateral geniculate disappears, extending ∼16 mm posteriorly and is contiguous to the lateral and inferior pulvinar. We did not analyze the supracallosal continuation of the hippocampus posterior body, which constitutes the induseum griseum. (b) Magnification of the GCL shown in (a); GCL, SGZ, and ML layers are indicated. Nuclei of glial cells are seen in blue, stained with hematoxylin. The optical disector with fractionator defines the sampling frames across the entire region of interest. GNs are visualized at 600 × magnification for cell counting. Cells touching the green line are included in the counting while cells touching the red line are not.
Stereology
Immunostained sections along the rostrocaudal axis of the DG were matched with intercalated Nissl-stained sections using a stereoscope (Leica, Wild M3Z, Heerburg, Switzerland). The boundaries of the DG and granule cell layer (GCL) (Figure 1a) were defined at low magnification using a 1.6 × objective. To estimate total GN number (N) in anterior, mid, and posterior DG we used the optical disector approach with the fractionator method (West and Gundersen, 1990):  where
where 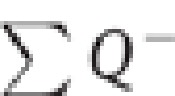 is the total number of cells counted, t the mean section thickness, h the height of the optical disector, adjusting for the guard zones (3 μm) above and below the disector, asf the area sampling fraction (area of counting frame divided by area of x, y step), and ssf the section sampling fraction (1/section interval). A Leica Diaplan microscope (Leitz Wetzlar, Germany) equipped with motorized stage (Ludl Electronic Products, Hawthorne, NY) and color camera (MicroFire CCD, Optronics, Goleta, CA), and connected to a computer running Windows XP was used with the Stereo Investigator software (MBF Biosciences, Williston, VT). Sampling was performed with a 60 × objective (Figure 1b). The sampling parameters were asf=0.02, ssf=0.05 with grid size: 210 × 210 μm2 and counting frame: 30 × 30 μm2. Average
is the total number of cells counted, t the mean section thickness, h the height of the optical disector, adjusting for the guard zones (3 μm) above and below the disector, asf the area sampling fraction (area of counting frame divided by area of x, y step), and ssf the section sampling fraction (1/section interval). A Leica Diaplan microscope (Leitz Wetzlar, Germany) equipped with motorized stage (Ludl Electronic Products, Hawthorne, NY) and color camera (MicroFire CCD, Optronics, Goleta, CA), and connected to a computer running Windows XP was used with the Stereo Investigator software (MBF Biosciences, Williston, VT). Sampling was performed with a 60 × objective (Figure 1b). The sampling parameters were asf=0.02, ssf=0.05 with grid size: 210 × 210 μm2 and counting frame: 30 × 30 μm2. Average 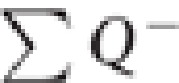 was 672 in anterior, 367 in mid, and 424 in posterior DG. The first slide sampled for GN number was the most anterior one in which the DG appeared and subsequent sections were assayed at 2 mm intervals thereafter toward the posterior end of the DG, until it disappeared, with an average of 12 sections per subject. Glia were counted only in the anterior DG of a subsample of five MDD*SSRI, five matched untreated MDDs, and five matched controls. Anterior, mid, and posterior GCL and DG volumes were calculated with the Cavalieri method (Cavalieri, 1966), as previously described (Boldrini et al, 2009). We measured section thickness at every disector location and calculated average section thickness per subject.
was 672 in anterior, 367 in mid, and 424 in posterior DG. The first slide sampled for GN number was the most anterior one in which the DG appeared and subsequent sections were assayed at 2 mm intervals thereafter toward the posterior end of the DG, until it disappeared, with an average of 12 sections per subject. Glia were counted only in the anterior DG of a subsample of five MDD*SSRI, five matched untreated MDDs, and five matched controls. Anterior, mid, and posterior GCL and DG volumes were calculated with the Cavalieri method (Cavalieri, 1966), as previously described (Boldrini et al, 2009). We measured section thickness at every disector location and calculated average section thickness per subject.
Statistical Analysis
Data analysis was performed using SPSS (version 18.0.3 for Mac). Regression analysis tested correlations of GN number, GCL and DG volume with age, PMI, pH and with measures of nestin-immunoreactive capillaries assessed in an earlier study in a subset (12 controls, 12 MDDs, 5 MDD*TCA, 5 MDD*SSRI) of the same subjects (Boldrini et al, 2012). We used ANOVA analysis of variance with Tukey post hoc analysis for between-group (17 controls, 15 untreated MDDs, five MDD*TCA and five MDD*SSRI) comparisons of dependent variables: GN number, GCL and DG volume, and with age as covariate. A Kruskal–Wallis test was used as a nonparametric test of differences in GN number in anterior, mid, and posterior DG between controls, MDD, MDD*SSRI, and MDD*TCA. MANOVAs were performed to test effects of sex and smoking status. Group and sex, or group and smoker status were fixed factors and GN number, GCL and DG volume were dependent variables. We used repeated-measures MANOVA with repeated measurements in anterior and mid level of GCL and DG volumes, and group and sex as fixed factors. We corrected for multiple comparisons and set p<0.05 for significance level. Data are expressed as mean±SEM.
RESULTS
Effect of MDD and Antidepressant Treatment on DG Granule Neuron Number
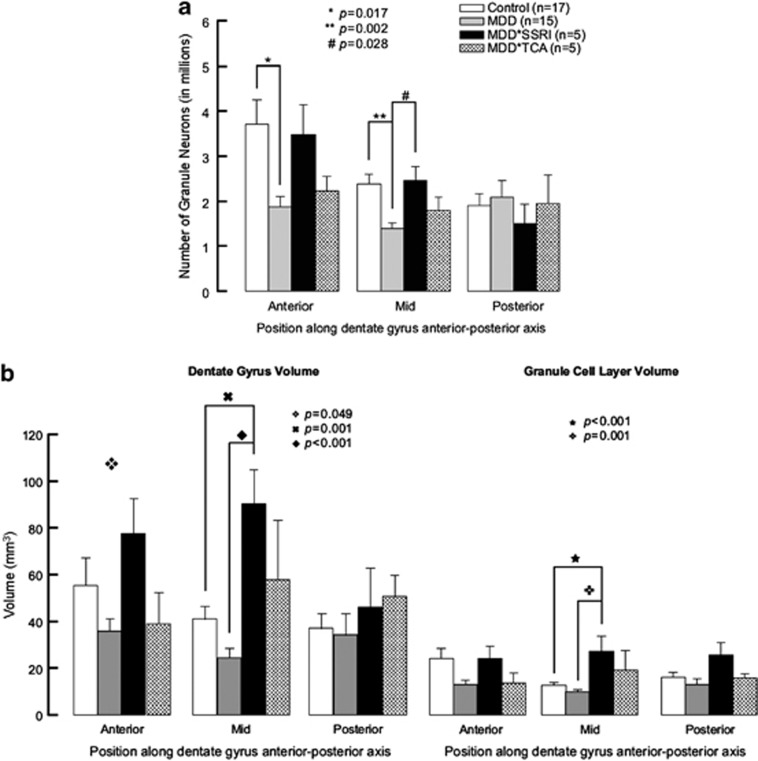
Neurons stained by NeuN were observed in GCL, hilus, and CA regions (Figure 1). GN number differed between groups in anterior DG (controls: 3,133,438±1,173,209; untreated MDD: 1,877,339±836,294; antidepressant-treated MDD: 2,576,142±1,153,361; F=5.644; df=2,37; p=0.007) and mid DG (controls: 2,393,262±838,874; untreated MDD: 1,402,398±436,601; antidepressant-treated MDD: 2,0142,291±642,022; F=7.748; df=2,36; p=0.002). Post hoc, untreated MDDs had fewer GNs vs controls in anterior (p=0.005) and mid (p<0.001) DG. Posterior GN number did not differ between groups. The nonparametric Kruskal–Wallis test confirmed between-group differences in GN number in anterior (p=0.007) and mid (p=0.015) but not posterior (p=0.600) DG, even when the antidepressant-treated MDD group was separated into SSRI and TCA-treated subgroups (Figure 2a). In contrast to untreated MDDs, GN number in treated MDDs did not differ from controls throughout the DG. The SSRI-treated MDD group had mean GN number closer to the controls than untreated MDD, and more GNs compared with untreated MDD statistically only in mid DG (p<028; Figure 2a).
Figure 2.
Granule neuron number and DG and GCL volume along the rostrocaudal axis of the human hippocampus. (a) Untreated subjects with major depressive disorder (MDD) have fewer granule neurons than controls and SRRI-treated MDDs. MDDs show fewer granule neurons (GNs) in the anterior and mid DG compared with controls without psychopathology or treatment. In the mid DG, MDDs treated with selective serotonin reuptake inhibitors (MDD*SSRI) have more GNs than untreated MDDs (MDD). In MDDs treated with tricyclic antidepressants (MDD*TCA), GN number does not differ from the other groups in any DG subregion. (b) Dentate gyrus (DG) and granule cell layer (GCL) volume are larger with antidepressant treatment in major depressive disorder. ANOVA showed anterior DG volume differs between groups, with no post hoc significance change. Mid DG and GCL volumes are larger in MDDs treated with selective serotonin reuptake inhibitors (MDD*SSRI) compared with untreated depressed subjects (MDD) and controls with no psychopathology or treatment. Repeated-measures MANOVA, where anterior and mid position are repeated measures for GCL and DG volumes, showed DG volume is larger in MDD*SSRI vs MDD (p<0.001), MDDs treated with tricyclic antidepressants (MDD*TCA, p=0.020) and controls (p=0.007); GCL volume is smaller in MDD vs controls (p=0.008) and larger in MDD*SSRI vs MDD (p<0.001), MDD*TCA (p<0.001), and controls (p<0.001).
Effect of MDD and Antidepressant Treatment on DG Volume
Volume (mm3) of mid DG differed between groups (controls: 41.28±17.01; MDD: 24.44±13.15; antidepressant-treated MDD: 77.45±30.68; F=13.814; df=2,24; p<0.001). We also examined the GCL. which includes more neuropil, and found a group difference in mid GCL (controls: 12.93±3.46; untreated MDD: 10.02±2.98; antidepressant-treated MDD: 24.30±10.62; F=12.066; df=2,23; p<0.001). Post hoc, mid DG was larger in antidepressant-treated MDD vs untreated MDD (p<0.001) and controls (p=0.004). Mid GCL was also larger in antidepressant-treated MDD vs untreated MDD (p<0.001) and controls (p=0.002). When the antidepressant-treated MDD group was separated into SSRI and TCA-treated subgroups, mid DG and GCL volumes were larger in SSRI-treated MDDs vs untreated MDDs and controls (Figure 2b). Volume of anterior and posterior DG and GCL did not show between-group differences (Figure 2b).
The length (mm) of the fixed hippocampal formation was 29.8±1.2 and did not differ between controls, MDDs, MDD*SSRIs, and MDD*TCAs (F=0.517; df=4,41; p=0.727); neither did the length of the three subregions (defined as described in the stereology method) of the DG (anterior: 7.2±0.6; mid 7.1±0.5; posterior 6.9±0.5). Post-processed section thickness (μm) also did not differ between groups (controls: 22.1±1.2; MDD: 21.9±1.6; MDD*SSRI: 17.5±0.9; MDD*TCA: 23.7±2.7; F=1.242; df:3,41; p=0.308). Thus, volume differences are not explained by differences in length or artifacts in section thickness.
Correlation between GN Number and DG Volume
GN number did not correlate with GCL or DG volume in any subregion in untreated MDD or control groups. In treated MDDs, anterior GN number correlated robustly with anterior GCL (r=0.930, p=0.001) and DG volume (r=0.759, p=0.029).
Effect of MDD and Antidepressant Treatment on Glial Cell Number in Anterior DG
Glia were counted in anterior DG from a subsample of five matched controls, five MDDs and five MDD*SSRI, and no group differences were found (controls: 1,905,779±477,956; MDD: 1,735,147±502,151; MDD*SSRI: 1,416,956±396,910; F=0.289; df=2,12; p=0.754). The percentage of glia (number of glia/[number of glia+number of GN] × 100%) differed between groups (controls: 32.1±11.7; MDDs: 48.5±10.2; MDD*SSRI: 29.4±12.7; F=3.970; df=2,12; p=0.048), but only because GN number differed.
Correlation between Clinical Characteristics and DG Neuron Numbers and Volume
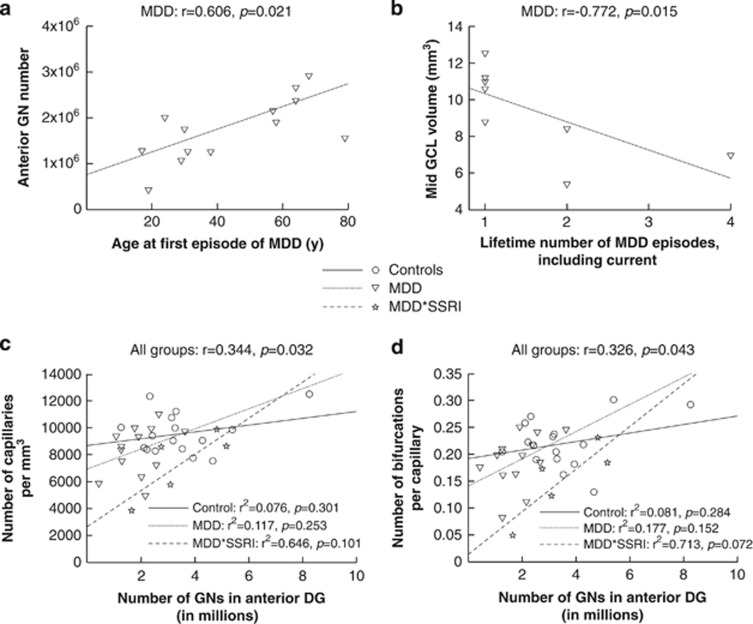
In untreated MDDs, younger age of MDD onset correlated with fewer GNs in anterior DG only (r=0.606, p=0.021; Figure 3a). Although more lifetime depressive episodes correlated with smaller mid GCL volume (r=−0.772, p=0.015; Figure 3b), this finding was driven by only three subjects with two or more lifetime episodes of major depression. These correlations were not present in treated MDDs. There were no correlations of age of onset or lifetime number of episodes with anterior and posterior GCL volume or any DG subregion volume.
Figure 3.
Granule neuron number and dentate gyrus volume correlations with clinical characteristics and capillary characteristics. Subjects that did not have information regarding age of onset of first episode of major depression or lifetime number of episodes of major depression were excluded from the analysis. (a) In untreated MDDs, younger age of MDD onset correlated with fewer granule neurons (GNs) in anterior DG. Age of MDD onset showed no correlation with GN number in mid (r=−0.294) or posterior DG (r=0.149), not shown. (b) More lifetime depressive episodes correlated with smaller mid GCL volume and there was no correlation with anterior (r=−0.099) and posterior (r=−0.159) GCL volume or with any DG subregion volume (not shown). Note: GN=granule neuron; GCL=granule cell layer. (c) GN number in anterior DG correlates with number of capillaries per mm3 in the whole sample. Correlations within groups are not significant. (d) GN number in anterior DG correlates with the number of bifurcations per capillary in the whole sample. Correlations within groups are not significant, including in subjects with major depression treated with tricyclic antidepressants (not shown). Note: Controls=subjects with no Axis I or II psychiatric diagnosis; MDDs=untreated subjects with major depression; MDD*SSRI=subjects with major depression treated with selective serotonin reuptake inhibitors; MDD*TCA=subjects with major depression treated with tricyclic antidepressants.
GN Number and DG Volume Correlate with DG Vascularization
In a recent study, we analyzed DG vascularization in a subset (12 controls, 12 MDDs, 5 MDD*TCA, 5 MDD*SSRI) of the same subjects as the present study (Boldrini et al, 2012). Capillary density (number of capillary per mm3) correlated with GN number in anterior DG (r=0.344, p=0.032; Figure 3c) and mid DG (r=0.461, p=0.015). A comparable correlation was observed between GN number in anterior DG and number of bifurcations per capillary (r=0.326, p=0.043; Figure 3d). Details of the nestin immunohistochemistry methods used can be found in Boldrini et al, 2009, 2012.
Effects of Sex, Smoking Status, Age, PMI, and Brain pH
Sex, age, PMI, or brain pH did not correlate with GN number or DG and GCL volumes in any of the groups. Smokers had smaller posterior DG volume (45.92±0.638) compared with non-smokers (33.80±0.638; F=54.545, df=1, p=0.018). Smokers and non-smokers did not differ on GN number or vascular indices.
DISCUSSION
We found fewer mature GNs in anterior and mid DG in untreated MDD compared with controls and treated MDD. Younger age of onset of MDD was associated with fewer GNs in anterior GCL. MDDs treated with SSRIs had GN number comparable to controls. Volume of anterior DG correlated with GN number and mid DG was smaller only in untreated MDDs compared with MDD*SSRIs and controls. More GNs correlated with higher DG capillary density and bifurcations per capillary, and both correlated with larger DG volume. No glial number differences were observed in a subset of cases quantified in the anterior DG (see Table 2 for summary of results).
Table 2. Summary of GN Number and DG and GCL Volume Results.
| Untreated MDDs | MDD*SRRI | MDD*TCA | |
|---|---|---|---|
| GNs in Anterior DG | ↓ | = | = |
| GNs in Mid DG | ↓ | = | = |
| GNs in Posterior DG | = | = | = |
| Anterior/mid GCL volume | ↓ | ↑ | = |
| Posterior GCL volume | = | = | = |
| Anterior/mid DG volume | = | ↑ | = |
| Posterior DG volume | = | = | = |
Arrows pointing up or down represent deviations compared with the non-psychiatric control group: arrow down=decreased; arrow up=increased. Note: MDDs=subjects with major depression; MDD*SSRI=subjects with major depression treated with selective serotonin reuptake inhibitors; MDD*TCA=subjects with major depression treated with tricyclic antidepressants; GNs=granule neurons; DG=dentate gyrus; GCL=granule cell layer.
Granule Neuron Number in MDD
This is the first published study that we are aware of that compares number of GNs in treated and untreated MDDs and controls. Previously, we did not find fewer NPCs, mitotic cells, or altered vascularization in untreated MDD (Boldrini et al, 2009, 2012), suggesting that fewer GNs in MDD do not result from a deficiency in the early phase of adult neurogenesis. Consistent with this conclusion, we found no correlation (data not shown) between number of GNs and NPCs assessed in a subset of the same subjects in a previous study (Boldrini et al, 2012). Since neurogenesis is much more active in early postnatal period than in adulthood, developmental or early neurogenesis could be altered in MDD, resulting in a GN deficit that may cause a small hippocampus early in life, and contribute to the development of MDD. Another possibility is a deficit of maturation of NPCs or survival of immature neurons in the DG present from childhood and extending into adulthood gradually results in detectably fewer mature GNs in MDD in adulthood. Alternatively, because numbers of mitotic cells (Eriksson et al, 1998) and NPCs are so low in adult humans (Boldrini et al, 2009, 2012), a small difference in neurogenesis throughout adulthood may be hard to detect at one time point, but have detectable long-term effects such as fewer GNs. Of note we found that earlier age of onset of MDD was associated with fewer GNs, consistent with either a small ongoing cumulative effect, or with more severe early damage in early-onset MDD.
DG Volume in MDD
Untreated MDD manifested smaller anterior and mid GCL but normal DG volume compared with controls. Treatment was associated with larger volumes. About half the MRI studies assessing medication-free, first-episode MDDs vs controls found smaller right hippocampus, but none examined substructures such as DG or GCL (McKinnon et al, 2009). Although sample size was very small, making the finding tentative, we also found that number of lifetime depressive episodes in untreated MDD correlated with smaller mid GCL volume. This finding is consistent with reports that cumulative time spent depressed and untreated correlates with smaller hippocampus in vivo (Sheline et al, 1999), and duration of MDD is associated with smaller hippocampus (McKinnon et al, 2009). Perhaps depression-related stress causes dendritic atrophy (McEwen, 1999). Therefore, neuroplastic changes other than GN number, including dendritic arborization, should be examined and may explain hippocampal volume changes in MDD. Alternatively, smaller hippocampal volume is found in first episode MDD (Cole et al, 2011) and so may antedate MDD. We now find evidence that fewer glial cells do not explain a smaller hippocampus in MDD. Although the percentage of glia appears to be slightly elevated in the MDD group, this is explained by the deficiency of GNs, as the number of glia is not higher in that group.
Treatment Effect on Granule Neuron Number
Antidepressant-treated MDDs had GN numbers comparable to controls and more GNs than untreated MDDs in anterior and mid DG. When the treated MDD group was separated into SSRI and TCA-treated subgroups, SSRI-treated MDDs, but not TCA-treated MDDs, showed more GNs than untreated MDDs. This suggests that SSRIs may have a specific effect on GN number, although group sample size was small, and therefore results should be considered preliminary.
Our study is cross-sectional and therefore findings are correlational, but they are consistent with mouse studies finding that SSRIs increase GN number (Santarelli et al, 2003). Antidepressant-induced cell survival has been attributed to specific anti-apoptotic effects, such as the breakpoint cluster-2 gene (Bcl-2; Peng et al, 2008) and brain-derived neurotrophic factor (BDNF; Dias et al, 2003), which also regulates cell survival (Sairanen et al, 2005). In fact, BDNF expression increases in GCL and CA subfields after chronic antidepressant administration (Dias et al, 2003). Serotonin-1A receptor activation supports cell survival, upregulating anti-apoptotic nuclear factor-κB (NF-κB), leading to caspase-3 suppression (Hsiung et al, 2005). Growth factors or downstream pathways of serotonin receptor activation may contribute to more GNs associated with SSRI treatment.
SSRI effect on GN number could also be the result of cognitive and physical activity increase associated with antidepressant benefit. Hippocampal-dependent learning increases the survival of maturing DG cells (Leuner et al, 2004) and both environmental enrichment and physical exercise increase DG neurogenesis in animal studies (Nilsson et al, 1999; van Praag et al, 1999). Activity improves the survival rate of new DG neurons (Deisseroth et al, 2004). Also, fate determination of NPCs (glia vs neurons) is activity-dependent (Liu et al, 2003). Death by suicide was unrelated to antidepressant treatment or GN number as untreated and treated MDDs had a similar percentage of suicides.
Treatment Effect on DG Volume
Mid GCL and DG are larger in SSRI-treated MDDs vs untreated MDDs. Anterior GCL volume correlates with number of anterior GNs in treated MDDs but not in untreated MDDs or controls. Although other cellular and neuropil elements can contribute to volume, GN number correlates with GCL volume. The main determinant of DG volume variance due to SSRI exposure is explained by GN number. These results are consistent with animal and human studies. Antidepressant administration in mice increases dendritic arborization (Wang et al, 2008) and reverses cell death and dendritic shrinkage induced by stress (McEwen, 1999). We previously reported that treated MDDs have larger DG volume (Boldrini et al, 2009, 2012). In vivo, patients on antidepressants have larger mid hippocampal volume than controls or unmedicated MDD (Malykhin et al, 2010). First episode, unmedicated MDD has smaller right hippocampal volume (Cole et al, 2011). Increased bilateral hippocampal volume after electroconvulsive shock therapy is reported in MDD (Nordanskog et al, 2010). In a meta-analysis, 10 studies out of 18 showed smaller hippocampal volume in treated MDDs than in controls, but studies did not compare treated and untreated MDDs (McKinnon et al, 2009). Moreover, studies were cross-sectional, not taking into account antidepressant response, duration of antidepressant exposure, duration of disease, age at onset of MDD, or lifetime episodes of depression, all variables that might affect hippocampal volume. Longitudinal studies separating the effects of treatment from those of disease course and severity are needed, and will require imaging methods that can be used in vivo.
Angiogenesis, Granule Neuron Number and DG Volume
More GNs in anterior and mid DG and larger anterior DG and GCL volumes were associated with higher density of capillaries in the DG and more bifurcations per capillary. This is consistent with the hypothesis that angiogenesis and cell survival or maturation, are regulated by the same factors. Angiogenesis and neurogenesis occur in parallel in the neurogenic niche (Palmer et al, 2000), and both are regulated by vascular endothelial growth factor (VEGF; Jin et al, 2002), BDNF, and nerve growth factor (Kim et al, 2004). Intra-cerebroventricular administration of VEGF in rat increases bromodeoxyuridine (BrdU) labeling of SGZ cells and immature neurons express VEGFR2/Flk-1 receptors (Jin et al, 2002). Moreover, VEGF expression (Lee et al, 2009) and VEGFR2/Flk-1 receptor signaling (Greene et al, 2009) are required for antidepressant behavioral effects and cell proliferation. In MDD treated with SSRIs, more capillaries associated with more NPCs (Boldrini et al, 2012), more GNs, and larger GCL and DG volume, suggest better vascularization may contribute to antidepressant response.
Anterior vs Posterior Hippocampus
Fewer GNs in untreated MDDs and control levels of GNs in SSRI-treated MDDs are found selectively in anterior and mid DG and differences are absent in posterior DG. The length of DG measured in this sample is in agreement with previous reports (Mai et al, 2008), and did not differ between treated and untreated MDD and controls. A smaller anterior and total right hippocampus on MRI is reported in MDD (Malykhin et al, 2010). Chronic administration of agomelatine, a melatonergic agonist and serotonin-2A receptor antagonist, increases cell proliferation and neurogenesis specifically in the ventral DG of rats (Banasr et al, 2006). The anterior DG in human corresponds to the ventral DG in rodent and its functional differentiation (Bannerman et al, 2004; Strange and Dolan, 2001) suggests that neuroplastic changes occurring here affect emotional processing (Nettles et al, 2000). Some MRI studies have divided the hippocampus into anterior, mid, and posterior regions, while most have not (Malykhin et al, 2010). Therefore, studies differed with regard to their definition of the hippocampus, and some studies (Cole et al, 2011) included white matter bundles (ie, alveus and fimbria) in their computation of volume. These methodological differences may have contributed to the heterogeneity of results across in vivo studies. Future studies should focus on the anterior hippocampus as a functionally relevant subregion to investigate volume changes occurring in MDD and with treatment.
Limitations
A limitation of any postmortem study is the cross-sectional design, therefore cause and effect relationships need to be tested in animal studies. We could not control for exercise level in the subjects studied, which affects DG cell number in rodents (van Praag et al, 1999). Benzodiazepines, which may negatively affect GN differentiation and survival (Sinner et al, 2011), did not affect our results as only one control and one MDD case had positive toxicology for benzodiazepines. We did not investigate the effects of stressful life events, and we know that stress affects neurogenesis, cell survival, and hippocampal volume. Stressful life events might have increased within-group variability in our sample. We did not have a measure of MDD severity, although all patients were reported to be depressed close to the time of death. Known and unknown factors might increase within-group variability and reduce the ability to detect differences such as between treated subgroups and controls.
Conclusions
We report for the first time an association of untreated MDD with fewer mature GNs in anterior hippocampal DG, as well as smaller DG and GCL volume. Maturation or survival of neuronal progenitor cells in the DG may be impaired in MDD, and our data are consistent with the hypothesis that antidepressant action may reverse this effect. How cell number and volume relate to clinical characteristics and severity of depression and how antidepressant action affects hippocampal structural plasticity, require in vivo animal studies and in vivo longitudinal studies of hippocampal structure in patients.
Acknowledgments
We thank Mihran J Bakalian, BA, for data management and graphics preparation, Tanya H Butt, BS, for immunohistochemistry and stereology work. This study was supported by MH83862, MH94888, MH64168, the American Foundation for Suicide Prevention and the Diane Goldberg Foundation.
Maura Boldrini, Adrienne Santiago, Andrew Dwork, Gorazd Rosoklija, Hadassah Tamir, and Victoria Arango declare that, except for income received from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Dr Dwork received loans and gifts of equipment and software from Olympus and Visiopharm for research unrelated to this study. Dr René Hen receives compensation as a consultant for Roche and Lundbeck. Dr J John Mann received past unrelated grants from GlaxoSmithKline and Novartis.
References
- Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John MJ, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri B.1966Geometria degli indivisibiliReprint of: Cavalieri B (1635) Geometria indivisibilus continuorum. Bononi: Typis Clemetis FeronijUnione Tipografico–Editrice Torinese: Torino, Italy [Google Scholar]
- Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung SC, Tamir H, Franke TF, Liu KP. Roles of extracellular signal-regulated kinase and Akt signaling in coordinating nuclear transcription factor-kappaB-dependent cell survival after serotonin 1A receptor activation. J Neurochem. 2005;95:1653–1666. doi: 10.1111/j.1471-4159.2005.03496.x. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;5:337–343. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons—adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Li Q, Hempstead BL, Madri JA. Paracrine and autocrine functions of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in brain-derived endothelial cells. J Biol Chem. 2004;279:33538–33546. doi: 10.1074/jbc.M404115200. [DOI] [PubMed] [Google Scholar]
- Lee JS, Jang DJ, Lee N, Ko HG, Kim H, Kim YS, et al. Induction of neuronal vascular endothelial growth factor expression by cAMP in the dentate gyrus of the hippocampus is required for antidepressant-like behaviors. J Neurosci. 2009;29:8493–8505. doi: 10.1523/JNEUROSCI.1321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang J, Zhu D, Fu Y, Lukowiak K, Lu YM. Generation of functional inhibitory neurons in the adult rat hippocampus. J Neurosci. 2003;23:732–736. doi: 10.1523/JNEUROSCI.23-03-00732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Paxinos G, Voss T. Atlas of the Human Brain. Academic Press: New York, NY; 2008. [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB. Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain Res. 2000;858:181–190. doi: 10.1016/s0006-8993(99)02281-7. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, et al. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18:128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of Hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinner B, Friedrich O, Zausig Y, Bein T, Graf BM. Toxic effects of midazolam on differentiating neurons in vitro as a consequence of suppressed neuronal Ca2+-oscillations. Toxicology. 2011;290:96–101. doi: 10.1016/j.tox.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus. 2001;11:690–698. doi: 10.1002/hipo.1084. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Simonis C, Weber S, Gass P, Henn F. Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry. 2003;54:1035–1040. doi: 10.1016/s0006-3223(03)00527-4. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Neylan TC, Mueller SG, Lenoci M, Truran D, Marmar CR, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Gundersen HJG. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2012. Depression: A Global Crisis, World Mental Health Day, October 10, 2012 http://www.wfmh.org/00WorldMentalHealthDay.htm Accessed 26.10.2012.