Abstract
Recently, minocycline, a tetracycline antibiotic, has been reported to improve symptoms of psychiatric disorders and to facilitate sober decision-making in healthy human subjects. Here we show that minocycline also reduces the risk of the ‘honey trap’ during an economic exchange. Males tend to cooperate with physically attractive females without careful evaluation of their trustworthiness, resulting in betrayal by the female. In this experiment, healthy male participants made risky choices (whether or not to trust female partners, identified only by photograph, who had decided in advance to exploit the male participants). The results show that trusting behaviour in male participants significantly increased in relation to the perceived attractiveness of the female partner, but that attractiveness did not impact trusting behaviour in the minocycline group. Animal studies have shown that minocycline inhibits microglial activities. Therefore, this minocycline effect may shed new light on the unknown roles microglia play in human mental activities.
In movies, a female spy often wins the trust of her male target using her physical attractiveness. The male target usually suspects that she is a spy, but because of her attractiveness, he becomes amorously entangled with the female spy despite concerns regarding her trustworthiness. For males, allocating valuable resources to physically attractive females may be evolutionarily adaptive, in that it may increase the probability of producing attractive offspring under natural selection. However, this tendency toward resource allocation to attractive females creates ‘noise’ that complicates decisions in short-term economic exchanges, leading to the tendency to ‘honey trap’ males with this behaviour.
In an economic exchange, attractiveness in a female increases sexual arousal in a male that automatically (without careful evaluation of her trustworthiness) facilitates trusting behaviour. While these traits should be adaptive in terms of mate-choice1, experimental studies have shown that they also affect decisions in social and economic exchange2,3. These traits lead to the question of how males can avoid the honey trap.
Recent studies with human subjects show that minocycline, a commonly used tetracycline antibiotic, may facilitate focus on appropriate environmental cues for social decision-making, possibly by reducing noise and other factors (e.g. personality and arousal) that can obstruct decisions. In an economic exchange, one study showed that subjects treated with minocycline make more sober decisions compared to participants treated with placebo4. In another study, participants were given dextroamphetamine and those treated with minocycline report less of a ‘high’ feeling compared to those who did not receive minocycline4. Minocycline is also known to improve symptoms associated with psychiatric disorders such as schizophrenia and depression5,6,7. There are past studies examining the effects of physical attractiveness on cooperation in social/economic exchange in different sex pairs, but no study has examined the effects of minocycline on such behaviour in different sex pairs. The hypothesis of this study was that minocycline reduces the risk of the honey trap effect and leads to more appropriate decisions in a short-term economic exchange, through a reduction in the noise triggered by physical attractiveness.
In this experiment, 98 healthy males played a trust game with 8 photographed young females after a 4-day oral treatment course of either minocycline or placebo. Looking at a picture showing a female's face, male players decided how much out of 1300 yen (approximately 13 USD) they would give to each female. Males then evaluated how trustworthy each female was and how physically attractive she was using a 11-point Likert Scale (0: Not at all – 10: Perfectly so). Of note, all of the photographed females had actually decided, in advance, to choose ‘betray’ against the male players. Therefore, male participants played with untrustworthy female partners, but were unaware of the deception. The impact of attractiveness and trustworthiness on the amount of money given to female partners was analysed. The independent variables were the evaluations/scores of physical attractiveness and trustworthiness given by the male participants.
Results
Table 1 summarizes the mean scores for the major variables and results of a t-test used to compare the placebo and minocycline conditions. Consistent with previous reports in which trust games were conducted between healthy male participants8,9, the offering rate differed marginally between conditions. The State and Trait Anxiety Inventory (STAI)10 was measured and no significant differences were found for either State or Trait Anxiety scores between conditions.
Table 1. Mean scores and results of t-tests comparing major variables.
| Conditions | t-test | ||||
|---|---|---|---|---|---|
| Item | Placebo (n = 48) | Minocyline (n = 50) | t-value | p | |
| Age (years) | Mean | 21.30 | 21.63 | −1.50 | 0.138 |
| SD | 1.364 | 1.875 | |||
| Offering Rate (0 to 1) | Mean | 0.61 | 0.49 | 1.90 | 0.062 |
| SD | 0.329 | 0.277 | |||
| Mean Attractiveness of All Pictures (0: Not at all – 10: Perfectly) | Mean | 3.08 | 2.78 | 1.37 | 0.175 |
| SD | 1.119 | 1.076 | |||
| Mean Trustworthiness of All Pictures (0: Not at all – 10: Perfectly) | Mean | 5.52 | 5.37 | 0.63 | 0.528 |
| SD | 0.968 | 1.349 | |||
| Mean State Anxiety Score (1: Not at all – 10: Very much so) | Mean | 2.11 | 2.28 | −1.50 | 0.138 |
| SD | 0.514 | 0.573 | |||
| Mean Trait Anxiety Score (1: Not at all – 10: Very much so) | Mean | 2.26 | 2.27 | −0.03 | 0.979 |
| SD | 0.514 | 0.578 | |||
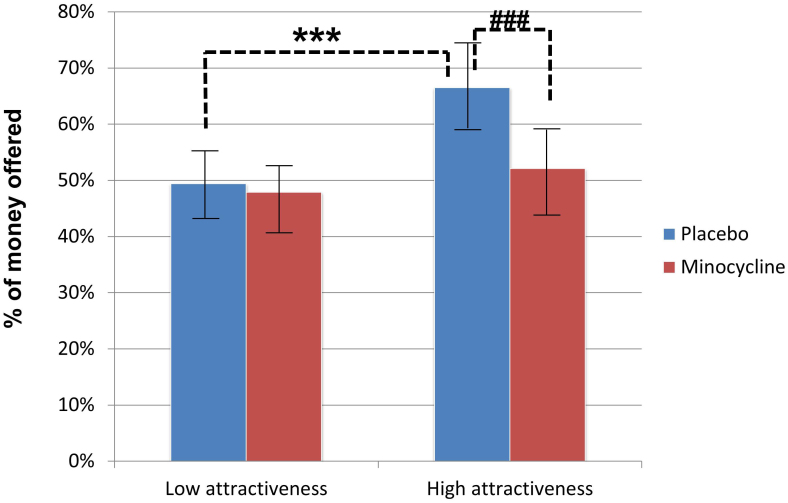
The primary hypothesis of this study was that the minocycline group would be less affected by the attractiveness of pictured females than the placebo group. To test this hypothesis, an ANOVA was performed with condition (minocycline vs. placebo) and attractiveness (high vs. low) as independent variables and the offering rate of money by participants as the dependent variable. The attractiveness score was not normally distributed (P = 0.0004), therefore the score was sub-divided into 2 categories (high vs. low). Figure 1 shows the mean offer rate by condition and the level of attractiveness. There is a significant interaction effect between condition and attractiveness (F (1,776) = 7.78, P = 0.005). Consistent with the primary hypothesis, participants in the placebo group gave larger amounts of money when the partner was more attractive, while participants in the minocycline group did not. According to a simple main effect test, a main effect of attractiveness was detected in the placebo group (P = 0.0004), but not in the minocycline group (P = 0.223). In addition, Figure 1 shows that, for partners with high attractiveness, the offering rate in the placebo group was significantly higher than in the minocycline group (P = 0.0004), but not for less attractive partners (P = 0.590).
Figure 1. Mean Offering Rate (percentage of money offered) by the Male Participants to Less- and More-Attractive Female Partners.
Error bars represent the standard deviation for each condition. *** For the placebo group, the offering rate to highly attractive female partners is higher than that to partners with low attractiveness (P = 0.0004). ### The offering rate to highly attractive partners in the placebo group is higher than that in the minocycline group (P = 0.0004).
Discussion
This study demonstrated that minocycline is the first drug shown to reduce the honey trap effect on young males. A previous report using a trust game with an anonymous male partner showed that minocycline reduces decision-making based on personality and trait9. Rather, minocycline facilitated decision-making based on situational factors such as game structure and evaluation of others' trustworthiness8,9. Consistent with evidence that minocycline attenuates the subjective high feeling associated with dextroamphetamine treatment4, the current results indicate that minocycline may reduce the effect of arousal and lead to sober decision-making. Recent clinical trials suggest that minocycline improves symptoms in patients with schizophrenia and depression5,6,7. In the current experiment, anxiety was measured and no difference was identified between the minocycline and placebo conditions. Future studies should clarify the effects of treatment with minocycline on psychological processes including mood, impulsivity, and cognitive performance in both healthy volunteers and patients with psychiatric disorders.
In rodent models, minocycline is the most commonly used drug for suppressing microglial activity in the brain11,12,13. In addition, a clinical trial with human subjects has shown that a long-term minocycline treatment (200 mg/d) suppresses microglial activation in various areas of the brain including the putamen, thalamus, and frontal cortex14. Microglia are glial cells with immunologic/inflammatory functions that contribute to various brain pathologies, including neurodegenerative diseases15,16,17 and psychiatric disorders (e.g. schizophrenia18,19,20 and autism21,22). Recent animal-model studies have shown that stress increases microglial activation23,24,25 and causes anxiety-like behaviours26. This behavioural change can be modulated with minocycline treatment26. In addition, recent evidence from rodent studies showed that in normal brains, microglia make direct contact with synapses27,28,29,30,31, suggesting that in this study minocycline may change synaptic reactions by suppressing microglial activity. The amygdala, one of the brain regions most affected by minocycline32, is activated during judgments of trustworthiness in human faces33,34. However, no studies have investigated how microglial activation directly contributes to human social decision-making and how these effects are modulated by minocycline. Taken together, these results suggest that microglial activity in the amygdala may modulate cognitive and emotional processes involving physical attractiveness and evaluation of trustworthiness.
Other possible effects of minocycline should be taken into account. Apart from inhibiting microglial activation, minocycline has also been reported to interact with brain glutamate and dopamine neurotransmission35 and to have direct effects on neuronal cells36. Some reports suggest positive links between microglia and glutamate and dopamine interaction37,38. Further research should be performed to clarify the effects of this potential interaction. The dose of minocycline (200 mg/d) used in this experiment was based on previous reports with human subjects4,14 and different doses may have different effects. Therefore, further trials should be conducted to investigate the effect of minocycline dosing.
To date, the biological mechanisms that underlie the honey trap effect remain poorly understood and no drug has been conclusively proven to attenuate honey trap effects during human social decision-making. The results of the present study suggest that minocycline is the first drug to have a novel pharmacologic function in humans—inhibition of honey trap effects. The current findings may shed new light on the mechanism underlying microglial effects on human mental activities and represent a novel psychopharmacologic approach for modulation of microglia.
Methods
This double-blind randomised trial, one of a series of trust game studies with human male subjects9, was approved by the Kyushu University Ethical Committee under the administration of the UMIN Clinical Trials Center (UMIN000004803). After a complete description of the study, all participants provided written informed consent. Either minocycline or placebo was administered to participants for 4 days, after which they participated in a trust game39.
Subjects
Participants were recruited using on-campus advertisements. Therefore, all participants were undergraduate or graduate students at Kyushu University. Healthy adult males (age range, 20–30 years) who were capable of providing informed consent were included. Participants were excluded if they met any of the following 4 criteria: (1) any history of experiencing side effects associated with antibiotics, including minocycline; (2) any history of severe heart, liver, or kidney disease; (3) a history of allergic syndromes; and (4) any history of psychiatric disorders. Their mental and physical health was confirmed via interview with a psychiatrist (TAK). After this screening process, 101 healthy adult males were enrolled in the study.
Drug administration
Participants received a hand-out describing their detailed dosing schedule. They were asked to record the exact time each dose was taken, and to keep and submit all capsule packaging, as evidence of medication administration. Participants began the medication (either minocycline or placebo) on the evening of Day 1 and continued taking the medication twice daily (morning and evening) for 3 additional days. The game experiment was conducted on Day 5. Participants were instructed to take the last capsule 3 h prior to their scheduled appointment time, ensuring that all participants had similar drug levels during the actual experiment. Each capsule contained either 100 mg minocycline (in the treatment group) or 100 mg lactose (in the placebo group). This minocycline dose (200 mg/d) is within the typical range for daily dosing used to treat infections40 and has also been used in recent clinical trials4,6,14. Using a double-blind procedure in advance, participants were randomly assigned to either the treatment group or the placebo group.
Procedure
After 4 days of drug administration, participants were interviewed by a physician regarding drug side effects, other medications, and adherence to the drug administration protocol. Participants then took part in the following trust game.
Trust Game with photographed female partners
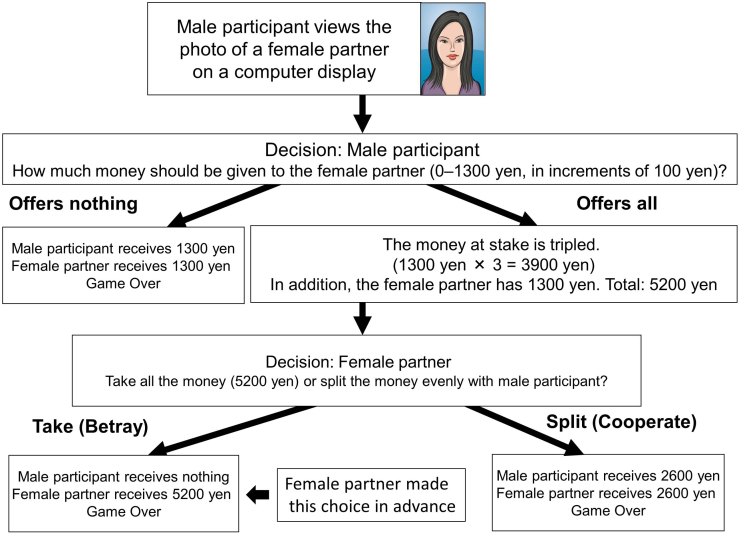
In this 2-player game39, each player was initially given 1300 JPY. The first player (the male participant) then decided how much of the 1300 JPY to give to the second player (the female partner). The amount of money given to the female partner was tripled and the female partner then decided whether to split her money equally with the male participant (namely, cooperate) or to take the entire amount of money (namely, betray). The trust game structure illustrating the most extreme cases is shown in Figure 2. All of the female partners were photographed and had decided in advance to take the entire amount of money. However, the male participants were not aware of this decision.
Figure 2. Trust Game Structure with the Most Extreme Cases.
The male participant's decision regarding how much money to give to the female partner is thought to reflect the level of trust the male participant places in his partner. The amount of money given was expected to function as a behavioural measure of the trust the male participant has in the female partner. In this experiment, male participants had no information about the female partner except for a photograph. Therefore, it is likely that male participants based their decisions regarding how much to trust each female partner, on impressions formed on the basis of the photos. After the experiment, each participant was paid an amount of money corresponding to the result of a randomly selected game from all 8 games.
Photo materials
Prior to the experiment, 61 young females were recruited using on-campus advertisements (mean age, 20.08 years; SD, 1.31 years). Each female participant was asked how they would behave in the role of the female partner in the trust game described above, especially in the case of an anonymous male participant that had chosen to give them the entire amount of money. Eleven participants answered ‘take the entire amount' rather than ‘split equally'. Eight female participants gave permission to use their photos in the experiment (mean age, 19.88 years; SD, 0.93 years). The photographs included the head and shoulders, with a neutral facial expression. During the experiment, each participant was asked if they knew each of the female partners shown in the photographs, in order to avoid confounding effects associated with previous acquaintance. However, there were no acquaintances identified among the participant pairs.
Statistical analyses
Ninety-eight Japanese males, out of 101 initially enrolled, completed all experiments (mean age, 21.49 years; SD, 1.65 years). Of the participants, 3 (1 in the minocycline condition and 2 in the placebo condition) failed to complete the experimental procedure, so the analyses were performed with data from the 98 participants. All data analyses were performed with SPSS (Version 19, IBM Corp., Armonk, NY USA).
Author Contributions
Conceived and designed the experiments: T.A.K., M.W. Performed the experiments: T.A.K., M.W., S.T., K.I. Analysed the data: M.W. Contributed reagents/materials/analysis tools: T.A.K., M.W., K.H., A.M., H.U., S.K. Wrote the paper: M.W., T.A.K.
Acknowledgments
This work was financially supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) to Dr. Kato, Dr. Watabe, and Dr. Kanba. We thank graduate and undergraduate students from Kyushu University and Waseda University for their assistance and are indebted to all our participants.
References
- Buss D. M. & Schmitt D. P. Sexual strategies theory: an evolutionary perspective on human mating. Psychol. Rev. 100 (2), 204–232 (1993). [DOI] [PubMed] [Google Scholar]
- Mulford M., Orbell J., Shatto C. & Stackard J. Attractiveness, Opportunity, and Success in Everyday Exchange. Am. J. Sociol. 103 (6), 1565–1592 (1998). [Google Scholar]
- Takahashi C., Yamagishi T., Tanida S., Kiyonari T. & Kanazawa S. Attractiveness and Cooperation in Social Exchange. Evol. Psychol. 4, 315–329 (2006). [Google Scholar]
- Sofuoglu M., Mooney M., Kosten T., Waters A. & Hashimoto K. Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology (Berl) 213 (1), 61–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T. et al. Minocycline as adjunctive therapy for patients with unipolar psychotic depression: an open-label study. Prog. Neuropsychopharmacol. Biol. Pychiatry 37 (2), 222–226 (2012). [DOI] [PubMed] [Google Scholar]
- Levkovitz Y. et al. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 71 (2), 138–149 (2010). [DOI] [PubMed] [Google Scholar]
- Miyaoka T. et al. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clinical neuropharmacology 31 (5), 287–292 (2008). [DOI] [PubMed] [Google Scholar]
- Watabe M., Kato T. A., Monji A., Horikawa H. & Kanba S. Does minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology (Berl) 220 (3), 551–557 (2012). [DOI] [PubMed] [Google Scholar]
- Kato T. A. et al. Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PLoS ONE 7 (7), e40461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D., Gursuch R. L. & Lushene R. E. Manual for the State-Trait Anxiety Inventory (Self-evaluation Questionnaire) (Consulting Psychologists Press, Palo Alto, CA, 1970). [Google Scholar]
- Yrjanheikki J., Keinanen R., Pellikka M., Hokfelt T. & Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. U. S. A. 95 (26), 15769–15774 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J. et al. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. U. S. A. 96 (23), 13496–13500 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y. et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Nat.l Acad. Sci. U. S. A. 98 (25), 14669–14674 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodel R. et al. Minocycline 1-year therapy in multiple-system-atrophy: effect on clinical symptoms and [(11)C] (R)-PK11195 PET (MEMSA-trial). Mov. Disorders 25 (1), 97–107 (2010). [DOI] [PubMed] [Google Scholar]
- Graeber M. B. & Streit W. J. Microglia: biology and pathology. Acta Neuropathol. 119 (1), 89–105 (2010). [DOI] [PubMed] [Google Scholar]
- Hanisch U. K. & Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10 (11), 1387–1394 (2007). [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. & Cardona A. E. The myeloid cells of the central nervous system parenchyma. Nature 468 (7321), 253–262 (2010). [DOI] [PubMed] [Google Scholar]
- Monji A., Kato T. & Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 63 (3), 257–265 (2009). [DOI] [PubMed] [Google Scholar]
- van Berckel B. N. et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol. Psychiatry 64 (9), 820–822 (2008). [DOI] [PubMed] [Google Scholar]
- Kato T. A. et al. Anti-Inflammatory properties of antipsychotics via microglia modulations: are antipsychotics a 'fire extinguisher' in the brain of schizophrenia? Mini Rev. Med. Chem. 11 (7), 565–574 (2011). [DOI] [PubMed] [Google Scholar]
- Suzuki K. et al. Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry 70 (1), 49–58 (2013). [DOI] [PubMed] [Google Scholar]
- Morgan J. T. et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68 (4), 368–376 (2010). [DOI] [PubMed] [Google Scholar]
- Frank M. G., Baratta M. V., Sprunger D. B., Watkins L. R. & Maier S. F. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 21 (1), 47–59 (2007). [DOI] [PubMed] [Google Scholar]
- Sugama S., Takenouchi T., Fujita M., Conti B. & Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J. Neuroimmunol. 207 (1–2), 24–31 (2009). [DOI] [PubMed] [Google Scholar]
- Tynan R. J. et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav. Immun. 24 (7), 1058–1068 (2010). [DOI] [PubMed] [Google Scholar]
- Neigh G. N. et al. Anxiety after cardiac arrest/cardiopulmonary resuscitation: exacerbated by stress and prevented by minocycline. Stroke 40 (11), 3601–3607 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff R. M. & Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science 333 (6048), 1391–1392 (2011). [DOI] [PubMed] [Google Scholar]
- Graeber M. B. Changing face of microglia. Science 330 (6005), 783–788 (2010). [DOI] [PubMed] [Google Scholar]
- Wake H., Moorhouse A. J., Jinno S., Kohsaka S. & Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29 (13), 3974–3980 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M. E. et al. The role of microglia in the healthy brain. J. Neurosci. 31 (45), 16064–16069 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333 (6048), 1456–1458 (2011). [DOI] [PubMed] [Google Scholar]
- Arakawa S. et al. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol. Biochem. Behav. 100 (3), 601–606 (2012). [DOI] [PubMed] [Google Scholar]
- Winston J. S., Strange B. A., O'Doherty J. & Dolan R. J. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 5 (3), 277–283 (2002). [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann. NY. Acad. Sci. 1191, 42–61 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S. & Suh Y. H. Minocycline and neurodegenerative diseases. Behav. Brain Res. 196 (2), 168–179 (2009). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. & Ishima T. A novel target of action of minocycline in NGF-induced neurite outgrowth in PC12 cells: translation initiation [corrected] factor eIF4AI. PLoS ONE 5 (11), e15430 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka T. M. & Koistinaho J. E. Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J. Immunol. 166 (12), 7527–7533 (2001). [DOI] [PubMed] [Google Scholar]
- Purisai M. G. et al. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol. Dis. 25 (2), 392–400 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J., Dickhaut J. & McCabe K. Trust, Reciprocity, and Social History. Games Econ. Behav. 10 (1), 122–142 (1995). [Google Scholar]
- Jonas M. & Cunha B. A. Minocycline. Ther. Drug Monit. 4 (2), 137–145 (1982). [PubMed] [Google Scholar]